Introduction
Fetal-type posterior cerebral artery (FTP) is a
common anatomic variation observed in the circle of Willis, and
defined as a posterior cerebral artery that originates from the
internal carotid artery (ICA) with or without a small connection
with the basilar artery (BA) (1). In
this condition, instead of the BA, the ICA supplies blood to the
posterior cerebral artery (PCA) (2).
ICA is formed by extending from the paired dorsal
aorta towards the cephalic side on embryonic days 28–30 (4–5.7 mm)
(3). In the embryonic stage of 5–8
mm, pairs of longitudinal nerve arteries appear along the turbid
brain and merge to form basilar arteries. The ICA and the caudal
part of the anastomosed branch of the nerve artery form the PCA
(4). In the stage of 40 mm (8
weeks), the PCA appears as an extension of the posterior
communicating artery (PComA) (5).
The vertebra-basilar system is then formed and contributes to the
blood supply of the PCA through the P1 segment. During this period,
the components of the circle of Willis have the same diameter
(2). During development, the
diameter of the PCA-P1 segment gradually increases, while that of
the PComA gradually decreases. At the time of birth, the PCA-P1
segment diameter is greater than that of the PComA, which
represents the most common type known as the adult PCA (5). Stagnation in the process of embryonic
development results in the following two cases. If the P1 segment
diameter is similar to that of the PComA, the result is
intermediate-type PCA. In the present study, 2.75% of the subjects
had intermediate-type PCA. However, absence of the P1 segment or
the diameter of the P1 segment being less than that of the PComA is
known as FTP. Absence of the P1 segment reflects full-type FTP. If
the diameter of the P1 segment is less than that of the PComA, it
is defined as partial-type FTP (6).
In the present study, 117 strips of FTP in 91 patients were found,
including 40 strips of full-type FTP and 77 strips of partial-type
FTP.
Several studies have described FTP in great detail,
including its incidence, the association between FTP and occipital
lobe infarction, life-threatening headache and white matter
degeneration (7,8). FTP has also been reported to be
associated with the occurrence of PComA aneurysm (9). Thus, it is worthwhile to assess whether
FTP is a risk factor for intracranial aneurysm. To the best of our
knowledge, the characteristics of intracranial aneurysm, including
its incidence, location, association with other variations of the
circle of Willis, subarachnoid hemorrhage, aneurysmal morphology
(the presence or absence of daughter sac) and size (diameter of the
aneurysm neck and aneurysm height), have not been systematically
analyzed. Computed tomography angiography (CTA) is a novel and
effective examination method for the display of intracranial
vessels with the use of three-dimensional reconstruction
technology. Due to its great advantage for the detection and
diagnosis of intracranial aneurysm over other techniques, it is a
preferred choice for diagnosing this condition. In the present
study, CTA data of the intracranial artery of 364 consecutive cases
of patients who were suspected with cerebrovascular disease or
intracranial aneurysm were reviewed, and a detailed analysis was
performed to assess the association between FTP and intracranial
aneurysm.
Patients and methods
Subjects
The CTA data of 364 consecutive Chinese cases of
intracranial artery assessed at Tianjin Hospital (Tianjin, China)
from January 2013 to January 2016 were reviewed. The cohort
comprised 218 male and 146 female patients, with an average age of
61.73±13.33 years. All the patients were initially diagnosed with
cerebral vascular disease or intracranial aneurysm by a
neurologist, and the patients' symptoms included headache,
vomiting, aphasia, difficulty swallowing, limb weakness, gait
instability and hemiplegia. Patients were suggested to undergo CTA
examinations of the intracranial artery. This study was approved by
the Ethics Committee of Tianjin Hospital (Tianjin, China). Prior to
CTA examination, all patients provided written informed consents,
which approved the publication of the images and data in the
present study.
Examination methods
A 16-slice spiral CT (GE Lightspeed; GE Healthcare,
Little Chalfont, UK) and an LF 9000 high-pressure injector
(Libel-Flarsheim Company, Cincinnati, OH, USA) were used in this
study. The patients entered the scanner in the supine position with
the head first. The scan level was from the third cervical vertebra
to the calvaria. Scanning conditions were as follows: Scan
thickness, 5 mm; pitch, 1.375:1; tube voltage, 140 kV; tube
current, 200–250 mA. Iohexol (350 mgI/ml, 50 ml; Yangtze River
Pharmaceutical Group, Taizhou, China) was administered through
either the elbow or dorsal vein at a flow rate of 4 ml/sec. The
scanning delay time was 20–25 sec. The dosage of the contrast agent
was 50 ml in 2013–2014 and 1.0 ml/kg × body weight (kg) in
2014–2016.
Image processing
Image reconstruction, including volume rendering and
the multiplanar reconstructed image (MPR), were obtained on an
AW4.5 workstation (GE Healthcare). The reconstruction thickness was
0.625 mm, the window level was 500 HU and the window width was
1,500 HU.
Interpretation of images
When the diameter of the PComA was greater than that
of the P1 segment of PCA (PCA-P1), partial-type FTP was assumed,
while full-type FTP was identified if the PCA-P1 segment was absent
(Figs. 1 and 2).
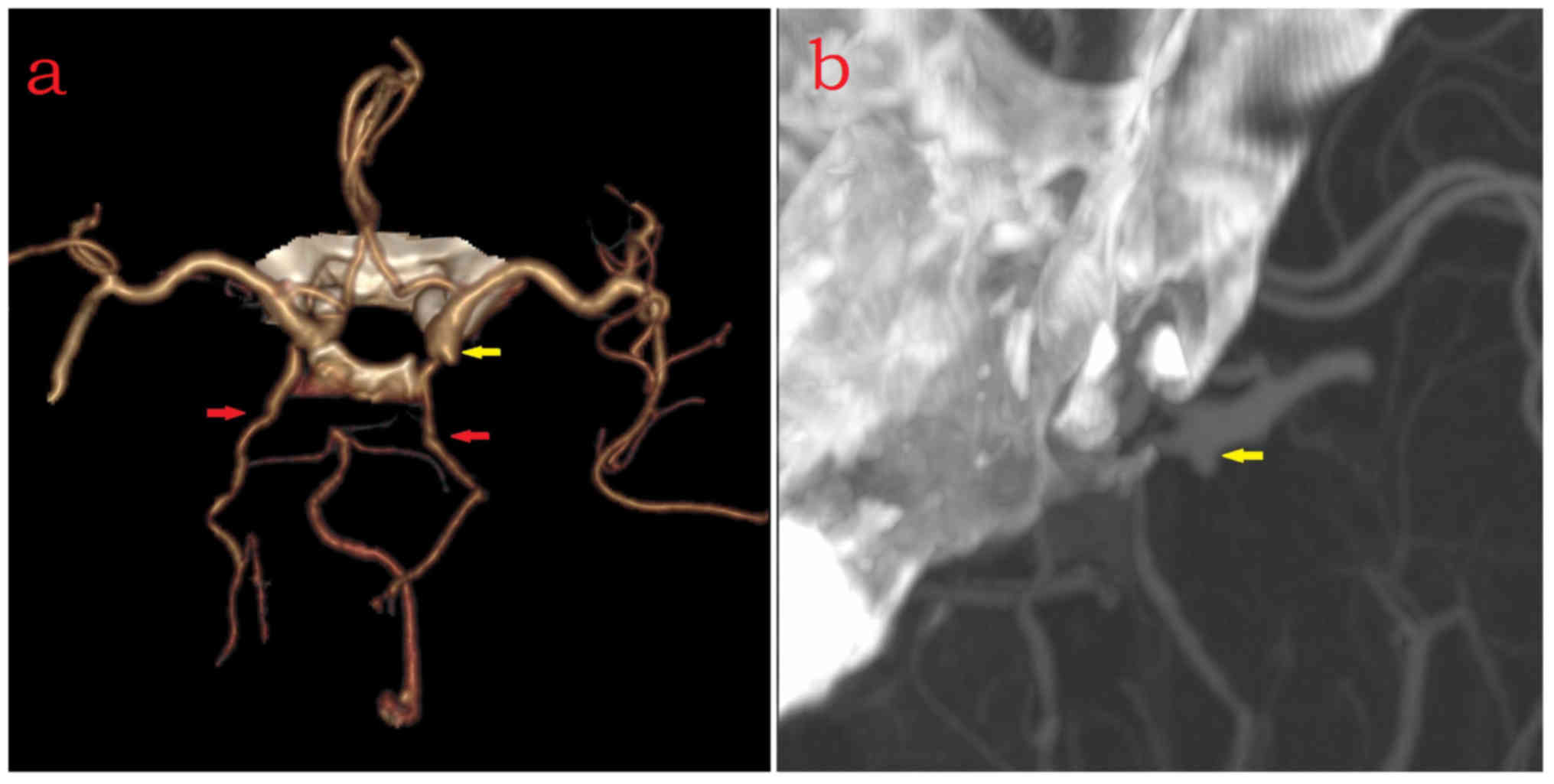
 | Figure 1.Right full or partial FTP. (a and b)
Female (age, 75 years) with right full-type FTP (red arrow),
saccular aneurysm with daughter sac located at bifurcation of
bilateral ACA-A2 (yellow arrow) and absence of right ACA-A1. (c-f)
Female (age, 75 years) with right partial-type FTP (red arrow),
saccular aneurysm located at bifurcation of MCA-M1 and MCA-M2
(yellow arrow), absence of left posterior communicating artery, and
subarachnoid hemorrhage. ACA-A1, A1 segment of anterior cerebral
artery; MCA, middle cerebral artery; M1, M1 segment; FTP, fetal
type of posterior cerebral artery. |
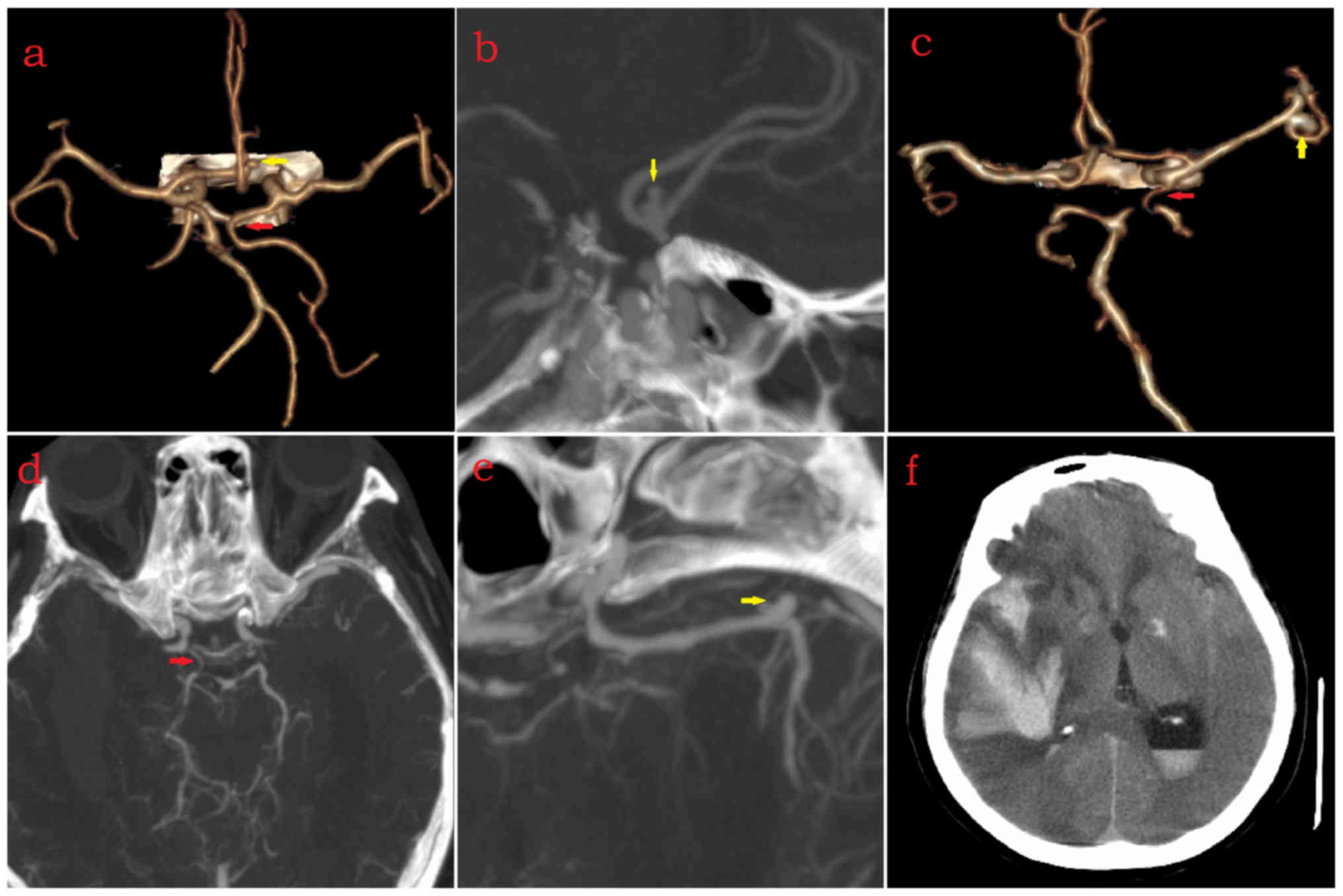
 | Figure 2.Left full or partial FTP. (a-c) Male
(age, 64 years) with left full FTP (red arrow), saccular ICA-PComA
aneurysm (ICA type, yellow arrow), left ACA-A1 hyposplasia, absence
of anterior communicating artery, absence of right PComA, and
subarachnoid hemorrhage. (d and e) Female (age, 75 years) with left
partial-type FTP (red arrow), saccular aneurysm located at bottom
of BA (yellow arrow), left heubner recurrent artery (blue arrows
head) and absence of right PComA. (f-k) Female (age, 70 years) with
left partial-type FTP (red arrow), fusiform aneurysm located at
transition between BA and right posterior cerebral artery (yellow
arrow), saccular aneurysm located at bifurcation of bilateral
ACA-A2 (blue arrow) (neck diameter of aneurysm, 5.16 mm; height of
aneurysm, 3.89 mm), absence of right ACA-A1 and absence of right
ICA (blue arrow heads). ICA, internal carotid artery; ACA-A1, A1
segment of anterior cerebral artery; PComA, posterior communicating
artery; BA, basilar artery; ICA, internal carotid artery; FTP,
fetal type of posterior cerebral artery. |
Intracranial aneurysm was defined as abnormal
expansion of the artery with congenital, infectious or traumatic
causes. According to their shape, aneurysms were divided into
saccular and fusiform types (10).
Based on their location, saccular aneurysms were divided into
bifurcation and lateral wall subtypes (11). ICA-PComA aneurysm is an aneurysm with
the neck located in the ICA-PComA. ICA-PComA aneurysms were divided
into 5 types according to the location of the aneurysm neck
(12). In the present study, 3 types
were considered, including the bifurcation (aneurysm neck occupying
both ICA and PCA), ICA (aneurysm neck located mainly in the ICA)
and PComA (aneurysm neck mainly located in the PComA) types.
Observation
CTA images of the intracranial artery were reviewed
to further define FTP occurrence, location (side), type, potential
combination with other variations of the circle of Willis (13) and potential combination with
intracranial aneurysm.
Regarding aneurysms, the type (fusiform or saccular,
bifurcation or lateral wall), location, presence or absence of
daughter sac, presence or absence of subarachnoid hemorrhage and
potential combination with FTP or other variations of the circle of
Willis were assessed. ICA-PComA aneurysms were then classified. The
neck of the aneurysm was displayed on an MPR image and the neck
diameter and height of the aneurysm were measured.
Statistical analysis
All statistical analyses were performed with SPSS
21.0 software (IBM Corp., Armonk, NY, USA). The incidence of FTP,
intracranial aneurysm and FTP with intracranial aneurysm was
calculated. The chi-square test was used to assess the influence of
FTP and gender on the above items, as well as on aneurysm daughter
sac and subarachnoid hemorrhage. The chi-square correction test was
performed with a total sample size of >40 and a minimum
theoretical frequency between 1 and 5. Two independent sample
Student's t-tests was used to compare the aneurysm neck diameter
and aneurysm height between the FTP and non-FTP patients. Binary
logistic regression analysis was performed to assess whether FTP
and gender were risk factors for intracranial aneurysm and
ICA-PComA aneurysm and the association was evaluated by the
standards shown in Table I.
 | Table I.Standards for the association
evaluation of binary logistic regression analysis. |
Table I.
Standards for the association
evaluation of binary logistic regression analysis.
| OR (lower odds) | OR (higher odds) | Association
degree |
|---|
| 0.9–1.0 | 1.0–1.1 | None |
| 0.7–0.8 | 1.2–1.4 | Low |
| 0.4–0.6 | 1.5–2.9 | Moderate |
| 0.1–0.3 | 3.0–9.0 | Strong |
| <0.1 | >10.0 | Very strong |
Results
Incidence of FTP
The total incidence of FTP and bilateral FTP
(Fig. 3) was 25.00 and 7.14%,
respectively (Table II). There was
no statistical difference between the total incidence of FTP and
bilateral FTP between males and females (χ2=2.577,
P=0.108). A total of 117 strips of FTP were identified, including
77 strips of partial-type FTP and 40 strips of full-type FTP. In
addition, 10 patients (2.75% in total) with intermediate-type PCA
were identified.
 | Table II.Incidence of FTP, bilateral FTP,
intracranial aneurysm and intracranial aneurysm associated with
FTP/bilateral FTP and influence of gender. |
Table II.
Incidence of FTP, bilateral FTP,
intracranial aneurysm and intracranial aneurysm associated with
FTP/bilateral FTP and influence of gender.
|
| FTP (N1) | Bilateral FTP
(N1) | Intracranial aneurysm
(N1) | Intracranial aneurysm
(N2) | Intracranial aneurysm
(N2) |
|---|
| Variable | Yes (%) | No | Yes (%) | No | Yes (%) | No | With FTP (%) | Without FTP | With bilateral FTP
(%) | Without bilateral
FTP |
|---|
| Gender |
|
|
|
|
|
|
|
|
|
|
| Male | 48 | 170 | 12 | 206 | 18 | 200 | 4
(22.22) | 14 | 1 (5.56) | 17 |
|
Female | 43 | 103 | 14 | 132 | 32 | 114 | 12 (37.5) | 20 | 4 (12.5) | 28 |
| Total incidence | 25.00 |
| 7.14 |
| 13.74a |
| 4.40 |
| 1.37 |
|
| χ2 | 2.577 |
| 2.199 |
| 16.524 |
|
0.633b |
|
0.087b |
|
| P-value | 0.108 |
|
0.159 |
| <0.001 |
| 0.426 |
| 0.768 |
|
Other variations of the circle of Willis in FTP and
non-FTP patients are presented in Table III. The percentage of other
variations of the circle of Willis in patients with and without FTP
was 49.45 and 7.69%, respectively (Table IV). There was a statistical
difference on other variations of the circle of Willis between FTP
and non-FTP patients (χ2=80.173, P<0.001).
 | Table III.Comparison of other variations of the
circle of Willis between FTP and non-FTP patients. |
Table III.
Comparison of other variations of the
circle of Willis between FTP and non-FTP patients.
| Other variations of
circle of Willis | FTP (N) | Non-FTP (N) |
|---|
| Variations of
anterior part of circle of Willis |
|
|
| ACA-A1
hypoplasia | 20 | 5 |
| ACA-A1
absence | 10 | 2 |
| Azygos
ACA | 1 | 1 |
|
Trifurcation of ACA | 1 | 1 |
| ACA-A1
fenestration | 0 | 2 |
| Common
trunk of ACA-A2 | 1 | 0 |
| AComA
fenestration | 0 | 1 |
| AComA
absence | 6 | 3 |
|
Duplication MCA | 2 | 1 |
| Early
bifurcation of MCA | 2 | 1 |
| Variations of
posterior part of circle of Willis |
|
|
| PComA
absence | 25 | 31 |
|
Duplication PCA | 4 | 0 |
|
Hyperplastic anterior
choroidal artery | 2 | 0 |
| BA
fenestration | 2 | 0 |
| VA
fenestration | 0 | 1 |
| Total (N/n) | 45/76a | 21/49b |
 | Table IV.Influence of FTP on other variations
of circle of Willis, intracranial aneurysm, ICA-PComA aneurysm,
other type of aneurysm in the anterior part of the circle of
Willis, BA aneurysm, aneurysm in the posterior part of the circle
of Willis, daughter sac of saccular aneurysm and subarachnoid
hemorrhage analyzed by χ2 test. |
Table IV.
Influence of FTP on other variations
of circle of Willis, intracranial aneurysm, ICA-PComA aneurysm,
other type of aneurysm in the anterior part of the circle of
Willis, BA aneurysm, aneurysm in the posterior part of the circle
of Willis, daughter sac of saccular aneurysm and subarachnoid
hemorrhage analyzed by χ2 test.
|
| Other variations of
circle of Willis (N) | Intracranial
aneurysm (N) | ICA-PComA aneurysm
(n) | Other aneurysm in
anterior part of circle of Willis (n) | BA aneurysm
(n) | Aneurysm in
posterior part of circle of Willis (n) | Daughter sac of
saccular aneurysm (n) | Subarachnoid
hemorrhage (n) |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| FTP | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No |
|---|
| FTP
(%)a | 45
(49.45%) | 46 | 16 (17.58%) | 75 | 13 (72.22%) | 5 | 3
(16.67%) | 15 | 2 (11.11%) | 16 | 3 (16.67%) | 15 | 3 (23.08%) | 10 | 1 (7.69%) | 12 |
| Non-FTP
(%)b | 21 (7.69%) | 252 | 34 (12.45%) | 239 | 16 (42.11%) | 22 | 13 (34.21%) | 25 | 4
(10.53%) | 34 | 9 (23.68%) | 29 | 9 (27.27%) | 24 | 8
(24.24%) | 25 |
| χ2 | 80.173 | | 1.285 | | 4.437 |
|
1.842c |
|
0.004c |
|
0.357c |
|
<0.001c |
| 0.74c |
|
| P-value | <0.001 | | 0.257 |
| 0.035 | | 0.175 | | 0.947 |
| 0.550 |
|
1.000 |
| 0.389 |
|
Incidence of intracranial
aneurysms
Within the cohort (n=364), 50 patients (13.74%; 18
males and 32 females; mean age, 61.66±14.03 years) with 56
intracranial aneurysms were identified, including 11.54% (42/364;
48 aneurysms in 42 patients) saccular aneurysms and 2.20% (8/364; 8
aneurysms in 8 patients) fusiform aneurysms (Table II). Among them, multiple aneurysms
accounted for 1.37% (5/364; 11 aneurysms in 5 patients). The
dimensions of the intracranial saccular aneurysms were 2.93±1.6 mm
(aneurysm neck diameter) ×3.52±2.47 mm (aneurysm height).
Of the intracranial aneurysms, 62% (31/50) were
associated with variations of the circle of Willis, particularly
the FTP variation (Table V). Among
the 48 saccular aneurysms, 12 were accompanied with a daughter sac
and 9 with subarachnoid hemorrhage. In addition, 6 saccular
aneurysms had an aneurysm daughter sac combined with subarachnoid
hemorrhage. The probability of subarachnoid hemorrhage in
intracranial saccular aneurysm with daughter sac was significantly
higher than that in saccular aneurysm without daughter sac, as
analyzed by Continuity Correction χ2 test
(χ2=7.704, P=0.006; Table
VI). In addition, the presence of an aneurysm daughter sac was
closely associated with subarachnoid hemorrhage as analyzed by
binary logistic regression, with a strong association (OR=11.000;
Table VII).
 | Table V.Variations in circle of Willis
associated with intracranial aneurysm in a total of 50 patients
with intracranial aneurysms. |
Table V.
Variations in circle of Willis
associated with intracranial aneurysm in a total of 50 patients
with intracranial aneurysms.
| Variations in
circle of Willis | Patients associated
with intracranial aneurysm (N/%) |
|---|
| FTP | 15 (10 of 15
accompanied with other variations of circle of Willis, 30) |
| ACA-A1 absence | 7 (3 of 7
accompanied with FTP, 14) |
| ACA-A1
hypoplasia | 5 (3 of 7
accompanied with FTP, 10) |
| Azygos ACA | 1 (2) |
| ACmoA
fenestration | 1 (2) |
| ACmoA absence | 3 (6) |
| Duplication
MCA | 2 (4) |
| Early bifurcation
of MCA | 1 (accompanied with
FTP, 2) |
| PComA | 6 (3 of 6
accompanied with FTP, 12) |
| Total | 31 (62) |
 | Table VI.Influence of daughter sac associated
with subarachnoid hemorrhage in saccular aneurysms. |
Table VI.
Influence of daughter sac associated
with subarachnoid hemorrhage in saccular aneurysms.
| Daughter sac of
saccular aneurysm | With Subarachnoid
hemorrhage (n) | Without
Subarachnoid hemorrhage (n) |
|---|
| Yes | 6 | 6 |
| No | 3 | 33 |
| Continuity
correction χ2 | 7.704 |
|
| P-value | 0.006 |
|
 | Table VII.Influence of daughter sac on
subarachnoid hemorrhage in saccular aneurysms. |
Table VII.
Influence of daughter sac on
subarachnoid hemorrhage in saccular aneurysms.
| Risk factor | B | SE | Wald (χ2) | df | P-value | Exp(B) (OR) | 95.0% CI for
Exp(B) |
|---|
| Daughter sac | 2.398 | 0.835 | 8.250 | 1 | 0.004 | 11.000 | 2.142–56.496 |
Intracranial aneurysms in FTP
The incidence of intracranial aneurysm with FTP in
all patients was 4.40% (16/364) (Table
II). The rates of intracranial aneurysm combined with
unilateral or bilateral FTP in female patients (37.5% for
unilateral and 12.5% for bilateral FTP) were higher compared with
those in males (22.22% for unilateral and 5.56% for bilateral)
(Table II). The incidence of FTP in
intracranial aneurysm patients was 32% (16/50), including 10%
(5/50) of bilateral FTP cases. The incidence rate of intracranial
aneurysm in FTP patients was 17.58% (16/91), which was slightly
higher than that in non-FTP patients with 12.45% (34/273), but the
difference was not statistically significant (χ2=1.285,
P=0.257; Table IV). Table VIII presents the location of
intracranial aneurysm in FTP and non-FTP patients.
 | Table VIII.Comparison of the location of
intracranial aneurysms between FTP and non-FTP patients. |
Table VIII.
Comparison of the location of
intracranial aneurysms between FTP and non-FTP patients.
| Location of
intracranial aneurysm | Intracranial
aneurysm with FTP (N/%) | Intracranial
aneurysm without FTP (N/%) |
|---|
| Anterior part of
circle of Willis |
|
|
|
ACA | 2a (12.5) |
4b (11.76) |
|
AComA | 0 | 1 (2.94) |
|
Trifurcation of
ICA-ACA-MCA | 0 | 3 (8.82) |
|
ICA-PcomA | 11c (13 aneurysms, 2 fusiform
aneurysms among them) (68.75) | 13d (16 aneurysms) (38.24) |
|
MCA | 1 (6.25) | 5 (1 fusiform
aneurysm) (14.71) |
| Posterior part of
circle of Willis |
|
|
|
PCA | 1 (6.25) | 1 (2.94%) |
| BA | 2 (1 fusiform
aneurysm) (12.5) | 4 (2 fusiform
aneurysms) (11.76) |
| VA | 0 | 4 (2 fusiform
aneurysms) (11.76) |
| Total | 16 (18
aneurysms) | 34 (38
aneurysms) |
Incidence of FTP and ICA-PComA
aneurysm
In the present study, 29 ICA-PComA aneurysms (24
patients) were identified. Of the patients with FTP, 12.09% (11/91)
presented with ICA-PComA aneurysm and 2.20% (2/91) of cases
occurred in the initial part of PComA (Table IX), and were predominantly
identified in females. Table IX
displayed the ICA-PComA aneurysm type in FTP and non-FTP patients.
The ICA type was predominant type in FTP or non-FTP patients. With
the exception of ICA-PComA aneurysm, there was no statistical
difference regarding intracranial aneurysms at other positions
between FTP and no-FTP patients (Table
IV).
 | Table IX.Comparison of ICA-PComA aneurysm type
between FTP and non-FTP patients. |
Table IX.
Comparison of ICA-PComA aneurysm type
between FTP and non-FTP patients.
| Type of ICA-PComA
aneurysm | ICA-PComA aneurysm
with FTP (N/n)(%a) | ICA-PComA aneurysm
without FTP (N/n)(%b) |
|---|
| Bifurcation | 1/1 (1.10) | 0/0 |
| ICA | 8/10a (8.79) | 12/15b (4.56) |
| PComA | 2/2a (2.20) | 1/1 (0.38) |
| Total | 11/13 (12.09) | 13/16 (4.94) |
Influences of FTP and gender on
intracranial aneurysm and ICA-PComA aneurysm
Table II indicates
the incidence of FTP, bilateral FTP, intracranial aneurysm,
intracranial aneurysm with FTP and intracranial aneurysm with
bilateral FTP between females and males by the χ2 test.
A statistically significant difference in the incidence of
intracranial aneurysm between females and males was identified
(χ2=16.524, P<0.001). More females than males had
intracranial aneurysm with FTP and bilateral FTP; however, the
difference was not significant. No statistically significant
differences in the incidence of any of the other conditions
mentioned above were noted between females and males.
Table IV displays
the influence of FTP on other variations of the circle of Willis,
intracranial aneurysm, ICA-PComA aneurysm, other aneurysms in the
anterior part of the circle of Willis, BA aneurysm, aneurysm in the
posterior part of the circle of Willis, daughter sac of saccular
aneurysm and subarachnoid hemorrhage as analyzed by the
χ2 test. Statistically significant differences in the
incidence of other variations of the circle of Willis
(χ2=80.173, P<0.001) and ICA-PComA aneurysm
(χ2=4.437, P=0.035) were identified between FTP and
non-FTP patients (Table IV). No
statistically significant differences in the incidence of any of
the other conditions mentioned above were noted between FTP and
non-FTP patients.
Table X presents the
association of FTP and gender with intracranial aneurysm. A weak
association was identified between FTP and intracranial aneurysm
(OR=1.365), while there was a stronger association between gender
and intracranial aneurysm (OR=0.328).
 | Table X.Influence of FTP and gender on
intracranial aneurysm by binary logistic regression analysis. |
Table X.
Influence of FTP and gender on
intracranial aneurysm by binary logistic regression analysis.
| Risk factor | B | SE | Wald (χ2) | df | P-value | Exp(B) (OR) | 95.0% CI for
Exp(B) |
|---|
| FTP | 0.311 | 0.338 | 0.846 | 1 | 0.358 | 1.365 | 0.703–2.649 |
| Gender | −1.116 | 0.318 | 12.296 | 1 | 0.000 | 0.328 | 0.176–0.611 |
Table XI displays
the association of FTP and gender with ICA-PComA aneurysm. A
moderate association was identified between FTP and ICA-PComA
aneurysm (OR=2.762). In addition, a moderate association was
present between gender and ICA-PComA aneurysm (OR=0.357).
 | Table XI.Influence of FTP and gender on
ICA-PComA aneurysm by binary logistic regression analysis. |
Table XI.
Influence of FTP and gender on
ICA-PComA aneurysm by binary logistic regression analysis.
| Risk factor | B | SE | Wald
(χ2) | df | P-value | Exp(B) (OR) | 95.0% CI for
Exp(B) |
|---|
| FTP | 1.016 | 0.442 | 5.292 | 1 | 0.021 | 2.762 | 1.162–6.563 |
| Gender | −1.029 | 0.456 | 5.091 | 1 | 0.024 | 0.357 | 0.146–0.874 |
Discussion
FTP is a posterior cyclic variation of the circle of
Willis. Blood supply of the PCA on the FTP side is exclusively from
the ipsilateral ICA, or from both the ipsilateral ICA and the BA,
but predominantly from the ICA. Under normal circumstances,
intracranial blood supply on both sides simultaneously relies on
the cervical and vertebral basilar system, and the cerebral blood
flow pressure remains similar between both sides. In the case of
FTP, the blood flow of the ICA and vertebral basilar system is
unbalanced, leading to a series of hemodynamic changes in circle of
Willis components (14). First,
blood flow is increased in the ICA-PComA and the blood pressure is
enhanced, leading to increased impact on the vessel wall (15). Furthermore, the membrane lacks the
muscle layer in the blood vessel wall of the arterial bifurcation
and the blood vessel wall appears to be thinning (16).
In addition, the present study identified some other
variations of the circle of Willis in FTP and non-FTP patients. The
percentage of other variations of the circle of Willis in patients
with and without FTP was 49.45 and 7.69%, respectively. The former
was identified to be significantly higher compared with the latter.
In theory, the hemodynamic changes of the circle of Willis would be
more complex if FTP was combined with other variations (17). Previous studies have reported that
anatomical variations of the circle of Willis, including persistent
trigeminal artery, arterial window and anterior cerebral artery
(ACA) -A1 dysplasia or absence, are associated with the occurrence
of intracranial aneurysm (18,19). In
fact, the present study also indicated that the incidence of
intracranial aneurysm in FTP with other variations of the circle of
Willis was higher than that in non-FTP patients; however, there was
no significant difference between them. In addition, some
variations in the circle of Willis were demonstrated to be
associated with intracranial aneurysm in the present study. Among
them, FTP was the most common variation associated with
intracranial aneurysm. The incidence of FTP in intracranial
aneurysm patients was 30%, including 10% for bilateral FTP cases,
which was in line with the results of a previous study (20).
In 50 patients with intracranial aneurysm, there
were 18 males and 32 females. There was significant difference
between females and males who had intracranial aneurysms. Of the 48
intracranial saccular aneurysms identified in the present study, 12
had a daughter sac, 9 occurred with subarachnoid hemorrhage and 6
simultaneously occurred with both aneurysm daughter sac and
subarachnoid hemorrhage. Analysis by Continuity Correction
χ2 test revealed that saccular aneurysms with daughter
sacs demonstrated a higher chance of subarachnoid hemorrhage, which
was consistent with previous study (21). Furthermore, 3 saccular aneurysms were
present with the daughter sac and FTP, and 1 saccular aneurysm was
indicated with subarachnoid hemorrhage and FTP. However, no
association between FTP and subarachnoid hemorrhage was identified
in the present study. Results demonstrated the location of
intracranial aneurysm, including the ACA, AComA, ICA-PComA, MCA,
PCA and BA between FTP and non-FTP patients. Regardless of patients
with FTP and patients without FTP, ICA-PComA aneurysm accounted for
the largest proportion.
As mentioned above, there was no statistically
significant difference between FTP and non-FTP patients regarding
the incidence of intracranial aneurysm. However, a statistical
difference was identified between FTP and non-FTP with ICA-PComA
aneurysms. No significant differences were determined between FTP
and non-FTP in intracranial aneurysms located elsewhere. In the
present study, the ICA-PComA aneurysms were divided into 3 types,
including the bifurcation type (aneurysm neck occupying both ICA
and PCA), the ICA type and the PComA type. The ICA type was the
predominant type in FTP and non-FTP patients. These results
corroborated with the findings of Zada et al (22). Of note, ICA-PcomA aneurysm require
distinguishing from the PComA funnel due to differences in
treatment (12); the PComA funnel is
a variation which does not require surgical treatment.
Binary logistic regression analysis revealed that
gender was a risk factor for intracranial aneurysm and ICA-PComA
aneurysm. A strong association was identified between gender and
intracranial aneurysm (OR=0.328), and a moderate association
between gender and ICA-PComA aneurysm (OR=0.357). Among patients
with unilateral and bilateral FTP, more female than male patients
with intracranial aneurysm were identified. This result was in
accordance with that of a previous study, which proved that the
prevalence of unruptured intracranial aneurysms in women was higher
than that in men (23). The
significant difference in the prevalence between males and females
may be due to estrogen levels (24),
which are also easily influenced by age, and the interplay among
these factors deserves further research.
In conclusion, the present study indicated that
female is an independent risk factor for intracranial aneurysm, and
FTP and female are independent risk factors for ICA-PcomA aneurysm.
It is known that age, gender, smoking, alcohol consumption,
hypertension, coronary heart disease and diabetes are risk factors
for intracranial aneurysm (25,26).
Therefore, clinicians should pay sufficient attention to female
patients with FTP, and a comprehensive follow-up program combined
with risk factors of other aneurysms should be designed for the
early prevention and treatment of intracranial aneurysm.
References
|
1
|
Arjal RK, Zhu T and Zhou Y: The study of
fetal-type posterior cerebral circulation on multislice CT
angiography and its influence on cerebral ischemic strokes. Clin
Imaging. 38:221–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lv X, Li Y, Yang X, Jiang C and Wu Z:
Potential proneness of fetal-type posterior cerebral artery to
vascular insufficiency in parent vessel occlusion of distal
posterior cerebral artery aneurysms. J Neurosurg. 117:284–287.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alexandre AM, Visconti E, Schiarelli C,
Frassanito P and Pedicelli A: Bilateral internal carotid artery
segmental agenesis: Embryology, common collateral pathways,
clinical presentation and clinical importance of a rare condition.
World Neurosurg. 95:620.e9–620.e15. 2016. View Article : Google Scholar
|
|
4
|
Xu J, Xu L, Wu Z, Chen X, Yu J and Zhang
J: Fetal-type posterior cerebral artery: The pitfall of parent
artery occlusion for ruptured P2 segment and distal
aneurysms. J Neurosurg. 123:906–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu T and Wang D: Association between
anatomical variations of the posterior communicating artery and the
presence of aneurysms. Neurol Res. 1-7:2016.(Epub ahead of
print).
|
|
6
|
Tocco P, Fenzi F, Cerini R and Monaco S:
Adult-onset migraine-related ophthalmoplegia and omolateral
fetal-type posterior cerebral artery. BMJ Case Rep. 2011:pii:
bcr10201149302011. View Article : Google Scholar
|
|
7
|
Diogo MC, Fragata I, Dias SP, Nunes J,
Pamplona J and Reis J: Low prevalence of fetal-type posterior
cerebral artery in patients with basilar tip aneurysms. J
Neurointerv Surg. 9:698–701. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolukisa M, Gursoy AE, Kocaman G, Dürüyen
H, Toprak H and Asil T: Carotid endarterectomy in a patient with
posterior cerebral artery infarction: Influence of Fetal Type PCA
on atypical clinical course. Case Rep Neurol Med.
2015:1912022015.PubMed/NCBI
|
|
9
|
Lv N, Feng Z, Wang C, Cao W, Fang Y,
Karmonik C, Liu J and Huang Q: Morphological risk factors for
rupture of small (<7 mm) posterior communicating artery
aneurysms. World Neurosurg. 87:311–315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cron DC, Coleman DM, Sheetz KH, Englesbe
MJ and Waits SA: Aneurysms in abdominal organ transplant
recipients. J Vasc Surg. 59:594–598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang DZ, Jiang B, He W, Wang YH and Wang
ZG: Risk factors for the recurrence of an intracranial saccular
aneurysm following endovascular treatment. Oncotarget.
8:33676–33682. 2017.PubMed/NCBI
|
|
12
|
González-Darder JM, Quilis-Quesada V,
Talamantes-Escribá F, Botella-Maciá L and Verdú-López F:
Microsurgical relations between internal carotid artery-posterior
communicating artery (ICA-PComA) segment aneurysms and skull base:
An anatomoclinical study. J Neurol Surg B Skull Base. 73:337–341.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim KM, Kang HS, Lee WJ, Cho YD, Kim JE
and Han MH: Clinical significance of the circle of Willis in
intracranial atherosclerotic stenosis. J Neurointerv Surg.
8:251–255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lochner P, Golaszewski S, Caleri F,
Ladurner G, Tezzon F, Zuccoli G and Nardone R: Posterior
circulation ischemia in patients with fetal-type circle of Willis
and hypoplastic vertebrobasilar system. Neurol Sci. 32:1143–1146.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu J, Yu Y, Wu X, Wu Y, Jiang C, Wang S,
Huang Q and Liu J: Morphological and hemodynamic analysis of mirror
posterior communicating artery aneurysms. PLoS One. 8:e554132013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin W, Ma X, Deng D and Li Y: Hemodynamics
in the circle of Willis with internal carotid artery stenosis under
cervical rotatory manipulation: A Finite element analysis. Med Sci
Monit. 21:1820–1826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Law-Ye B, Geerts B, Galanaud D, Dormont D
and Pyatigorskaya N: Pseudo-asymmetry of cerebral blood flow in
arterial spin labeling caused by unilateral fetal-type circle of
Willis: Technical limitation or a way to better understanding
physiological variations of cerebral perfusion and improving
arterial spin labeling acquisition? J Cereb Blood Flow Metab 36:
1641–1643, 2016? J Cereb Blood Flow Metab 36: 1641–1643, 2016. 36:
1641–1643, 2016:1641-1643, 2016–1643, 2016. 2016.
|
|
18
|
Patel MA, Caplan JM, Yang W, Colby GP,
Coon AL, Tamargo RJ and Huang J: Arterial fenestrations and their
association with cerebral aneurysms. J Clin Neurosci. 21:2184–2188.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Orakdöğen M, Emon ST, Somay H, Engin T, Is
M and Hakan T: Vascular variations associated with intracranial
aneurysms. Turk Neurosurg. 27:853–862. 2017.PubMed/NCBI
|
|
20
|
Ilbay K, Ismailoglu O and Albayrak BS:
Co-existence of bilateral fetal type posterior cerebral artery and
the bilateral giant internal carotid artery aneurysms in an ataxic
patient. Eur J Radiol. 81:1388–1389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hao M, Ma J, Huang QJ, He SX, Liang Z and
Wang CB: Morphological parameters of digital subtraction
angiography 2D image in rupture risk profile of small intracranial
Aneurysms: A Pilot Study. J Neurol Surg A Cent Eur Neurosurg.
77:25–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zada G, Breault J, Liu CY, Khalessi AA,
Larsen DW, Teitelbaum GP and Giannotta SL: Internal carotid artery
aneurysms occurring at the origin of fetal variant posterior
cerebral arteries: surgical and endovascular experience.
Neurosurgery. 63 1 Suppl 1:ONS55–ONS62. 2008.PubMed/NCBI
|
|
23
|
Harada K, Fukuyama K, Shirouzu T, Ichinose
M, Fujimura H, Kakumoto K and Yamanaga Y: Prevalence of unruptured
intracranial aneurysms in healthy asymptomatic Japanese adults:
Differences in gender and age. Acta Neurochir (Wien).
155:2037–2043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tada Y, Wada K, Shimada K, Makino H, Liang
EI, Murakami S, Kudo M, Shikata F, Pena Silva RA, Kitazato KT, et
al: Estrogen protects against intracranial aneurysm rupture in
ovariectomized mice. Hypertension. 63:1339–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brinjikji W, Zhu YQ, Lanzino G, Cloft HJ,
Murad MH, Wang Z and Kallmes DF: Risk factors for growth of
intracranial aneurysms: A systematic review and meta-analysis. AJNR
Am J Neuroradiol. 37:615–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang HG, Kim BJ, Lee J, Kim MJ, Kang DW,
Kim JS and Kwon SU: Risk factors associated with the presence of
unruptured intracranial aneurysms. Stroke. 46:3093–3098. 2015.
View Article : Google Scholar : PubMed/NCBI
|