Introduction
Hepatocellular carcinoma (HCC) is a major malignant
cancer type worldwide. It is estimated that >748,000 new cases
are diagnosed each year, a majority of which are in resource-poor
countries (1). China has a high
disease burden of HCC, with 466,100 new HCC cases and 422,100
HCC-associated mortalities in 2015 (2). The progression of HCC is rapid and the
prognosis is poor. Patients who develop HCC usually die within 12
months and the median survival time after HCC diagnosis is <6
months (3). The understanding of the
molecular mechanisms of the pathogenesis of HCC still requires to
be enhanced.
The most common risk factor for HCC is viral
hepatitis infection, including infection with hepatitis B virus
(HBV) and HCV. Combined HBV and HCV infections are responsible for
85% of HCC cases (4). Activation of
the NOD-like receptor family, pyrin domain containing 3 (NLRP3)
inflammasome is commonly observed in the immune response to viral
infections, including those with HBV and HCV (5–7). The
NLRP3 inflammasome is a multiprotein complex, which is involved in
caspase-1 activation. The major component of the NLRP3 inflammasome
is NLRP3, a pyrin-containing protein that belongs to the
nucleotide-binding oligomerization domain-like receptors. Caspase-1
induced by NLRP3 inflammasome activation is required for the
maturation and secretion of the pro-inflammatory cytokines
interleukin (IL)-18 and IL-1β (8).
NLRP3 inflammasome activation by hepatitis infection finally
results in hepatocyte pyroptosis, apoptosis and fibrosis (9,10).
Post-transcriptional regulation by microRNAs
(miRNAs/miRs) is an important mechanism to control gene expression.
miRNAs have regulatory functions through binding to complementary
sequences in the 3′-untranslated region (3′-UTR) of their target
mRNAs via base pairing to degrade them or inhibit their translation
(11). As indicated in a study by
Guo et al (12), decreases in
protein production in mammalian cells were mainly due to regulatory
interactions with ectopic and endogenous miRNA. In murine
macrophages, NLRP3 transcription is tightly regulated by miR-223
through an evolutionarily conserved binding interaction between the
3′-UTR of NLRP3 mRNA and miR-223-3p, indicating that NLRP3
expression is also regulated by miRNAs (13). miR-223 is a negative regulator of
inflammation and usually upregulated in cells of myeloid lineages
(14). miR-223 is also associated
with HCC development and commonly downregulated in HCC tissues
(15). Based on the abovementioned
previous studies, including those reporting that chronic hepatitis
infection is associated with NLRP3 activation as major cause of
hepatic damage and tumorigenesis, it was deduced that NLRP3 has a
role in HCC development and is regulated by miR-223. Therefore, the
aim of the present study was to assess the influence of NLRP3 on
pathological features of HCC cells and to verify its potential
regulation by miR-223.
Materials and methods
Cell line and culture
The Hep 3B2.1-7 cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA) and used in
all of the experiments. The cell line was derived from a chronic
HBV carrier and suitable for transfection. The cells were cultured
as recommended with Dulbecco's modified Eagle's medium (DMEM;
Gibco®; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS;
Gibco®; Thermo Fisher Scientific, Inc.) in a humidified
atmosphere of 95% air and 5% CO2 at 37°C.
Cell transfection
Hep3B cells were transfected with mature miR-223
mimics (Biomics Biopharma, Nantong, China;
5′-UGUCAGUUUGUCAAAUACCCC-3′) to investigate the regulatory role of
miR-223. All of the transfections were performed using the
Lipofectamine 3000 reagent (Invitrogen®; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Non-homologous miRNA mimics control was used as a negative control
(NC). Cells were trypsinized and collected at 24 h after
transfection for cell proliferation and apoptosis assays. miR-223
mimics and miRNA mimics control were purchased from RiboBio Co.,
Ltd. (Guangzhou, China).
Dual luciferase reporter assay
The predicted miR-223 binding site of the NLRP3
3′-UTR sequence (5′-CGCUAUCUUUCUAUUAACUGACCAUAA-3′) or mutant
sequence(5′-CGCUAUCUUUCUAUUAUGACUCCAUAA-3′) was cloned into the
pMIR-REPORT vector (Ambion®; Thermo Fisher Scientific,
Inc.) to construct the NLRP3-3′UTR-WT plasmid or the
NLRP3-3′UTR-Mut plasmid. HEK293T cells were co-transfected with 40
ng plasmid containing NLRP3 3′-UTR or mutant sites, 40 ng pRL-TK
Renilla luciferase reporter plasmid (Ambion®, Thermo
Fisher Scientific, Austin, TX, USA) and 20 ng miR-223 mimics.
Renilla luciferase activity was measured 48 h after transfection by
using the dual luciferase reporter assay system (Promega Corp.,
Madison, WI, USA).
Cell proliferation assay and apoptosis
assay
Cell proliferation was determined by the MTT method.
Cells were seeded in a 96-well plate (3,000 cells/well) with 200 µl
medium and cultured for 48 h. For the proliferation assay, 20 µl
MTT solution (5 mg/ml) was added to each well, followed by
incubation for 2 h. The cell density was calculated according to
the optical density at 450 nm determined using a microplate reader.
For the apoptosis assay, collected cells were stained with Annexin
V-fluorescein isothiocyanate (FITC) and propidium iodide solution
(included in the Annexin V-FITC Apoptosis Detection kit (cat no.
C1062; Beyotime Institute of Biotechnology, Haimen, China) and
incubated in the dark for 20 min. The apoptotic rate was then
measured by flow cytometry. For each sample, signals of
105 cells were detected.
RNA isolation and mRNA detection
Total RNA was extracted from harvested cells by
using the RNAiso Plus kit (cat no. 9180; Takara Biotechnology,
Dalian, China). First-strand complementary (c)DNA synthesis was
performed by using the PrimeScript 1st Strand cDNA Synthesis kit
(cat no. 6110A; Takara Biotechnology). To determine the gene
expression levels, quantitative polymerase chain reaction (qPCR)
was performed using the QuantiTect SYBR Green PCR kit (cat no.
204145; Qiagen, Hilden, Germany). The primers used for reverse
transcription and qPCR are summarized in Table I. U6 small nuclear RNA was used as an
internal control for miR-223. β-actin was used as an internal
control for the other genes. The relative mRNA levels of the target
genes were normalized to β-actin by using the 2−ΔΔCq
method (16).
 | Table I.Primers used for RT-qPCR. |
Table I.
Primers used for RT-qPCR.
| A, Primers used for
RT |
|---|
|
|---|
| RNA | Primer sequence |
|---|
| miR-223 |
5′-GTCGTATCCAGTGCAGGGTCCGAGGT |
|
|
ATTCGCACTGGATACGACTGGGGT-3′ |
| U6 |
5′-GTCGTATCCAGTGCAGGGTCCGAGGT |
|
|
ATTCGCACTGGATACGACAAAATATGGA |
|
| AC-3′ |
|
| B, Primers used
for qPCR |
|
|
Gene/direction | Primer
sequence |
|
| U6 |
|
|
Forward |
5′-CTCGCTTCGGCAGCACA-3′ |
|
Reverse |
5′-AACGCTTCACGAATTTGCGT-3′ |
| miR-223 |
|
|
Forward |
5′-GTGCAGGGTCCGAGGT-3′ |
|
Reverse |
5′-CGGGCTGTCAGTTTGTCA-3′ |
| NLRP3 |
|
|
Forward |
5′-GCAGCAAACTGGAAAGGAAG-3′ |
|
Reverse |
5′-CTTCTCTGATGAGGCCCAAG-3′ |
| Caspase-1 |
|
|
Forward |
5′-CCGAAGGTGATCATCATCCA-3′ |
|
Reverse |
5′-ATAGCATCATCCTCAAACTCTTCTG-3′ |
| β-actin |
|
|
Forward |
5′-AGGGGCCGGACTCGTCATACT-3′ |
|
Reverse |
5′-GGCGGCACCACCATGTACCCT-3′ |
Western blot analysis
Total cell lysates were prepared in SDS lysis buffer
(Beyotime Institute of Biotechnology). The protein concentration
was quantified by using the BCA Protein Assay kit (Beyotime
Institute of Biotechnology). Protein lysates (30 µg/lane) were
separated by 10% SDS-PAGE and electro-transferred onto a
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). The
membrane was blocked with 5% skimmed milk and then incubated
overnight at 4°C with the following primary antibodies: Rabbit
polyclonal antibody to NLRP3 (1:2,000 dilution; cat. no. ab98151),
rabbit polyclonal antibody to caspase-1 (1:2,000 dilution; cat. no.
ab1872) and rabbit polyclonal antibody to β-actin (1:2,000
dilution; cat. no. ab8227). Blots were then incubated with
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G
(1:3,000 dilution; cat. no. ab205718) at room temperature for 1 h.
The protein bands were visualized by using the BeyoECL Plus kit
(cat no. P0018; Beyotime Institute of Biotechnology). All of the
antibodies were purchased from Abcam Inc. (Cambridge, MA, USA).
β-actin was used as an internal control.
Cytokine detection
The inflammatory cytokines IL-1β and IL-18 were
detected in the cell culture supernatants. The Human IL-1 Beta
PicoKine ELISA kit (cat no. EK0392) and the Human IL-18 PicoKine
ELISA kit (cat no. EK0864; Boster Biological Technology, Wuhan,
China) were used to measure the concentrations of the
cytokines.
Statistical analysis
All data were analyzed by using SPSS software
version 19.0 (IBM Corp., Armonk, NY, USA). All experiments were
repeated at least 3 times and representative images of one
experiment are displayed. Values are expressed as the mean ±
standard deviation (SD). Student's t-test was performed to analyze
differences between two groups. One-way analysis of variance was
used to compare between multiple groups followed by a Newman-Keuls
multiple comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-223 interacts with the 3′-UTR of
NLRP3 mRNA
Bases 406–412 of the 3′-UTR of NLRP3 mRNA were
predicted to be a binding site for miR-223-3p using TargetScanHuman
(http://www.targetscan.org/vert_71/)
(Fig. 1A). To confirm the presence
of a direct interaction between the two molecules, a
dual-luciferase reporter assay was performed. As presented in
Fig. 1B, co-transfection with
miR-223 reduced the luciferase activity of the plasmid containing
the wild-type of the respective fragment of NLRP3 3′-UTR. However,
the luciferase activity of the plasmid containing the mutant NLRP3
3′-UTR fragment was not affected by co-transfection with miR-223
mimics or negative control. These results indicated that miR-223
directly interacted with the 3′-UTR of NLRP3 mRNA.
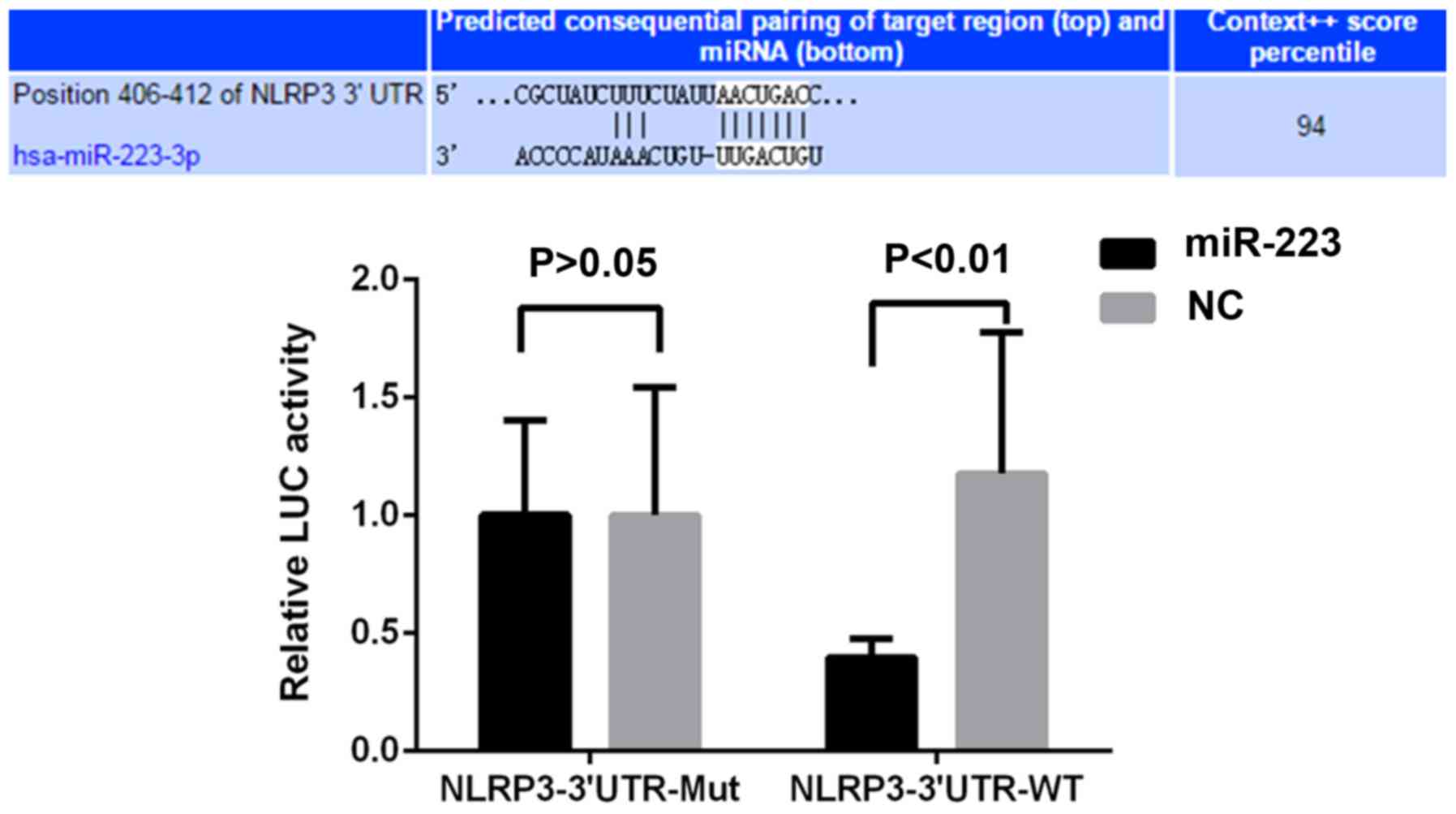 | Figure 1.miR-223 regulates NLRP3 expression by
directly targeting the 3′-UTR of its mRNA. Predicted potential
binding sites of miR-223 and NLRP3 mRNA are displayed in the upper
panel. A dual luciferase reporter assay was performed. Luciferase
activities of plasmids with WT or Mut sequences were assessed in
HEK293T cells co-transfected with miR-223 mimics or non-homologous
NC. NLRP3-3′UTR-WT, luciferase reporter plasmid containing the WT
3′UTR sequence of NLRP3; BL, blank control; UTR, untranslated
region; miR, microRNA; WT, wild-type; Mut, mutant; NLRP3, NACHT,
LRR and PYD domains-containing protein 3; hsa, Homo sapiens; NC,
negative control; LUC, luciferase. |
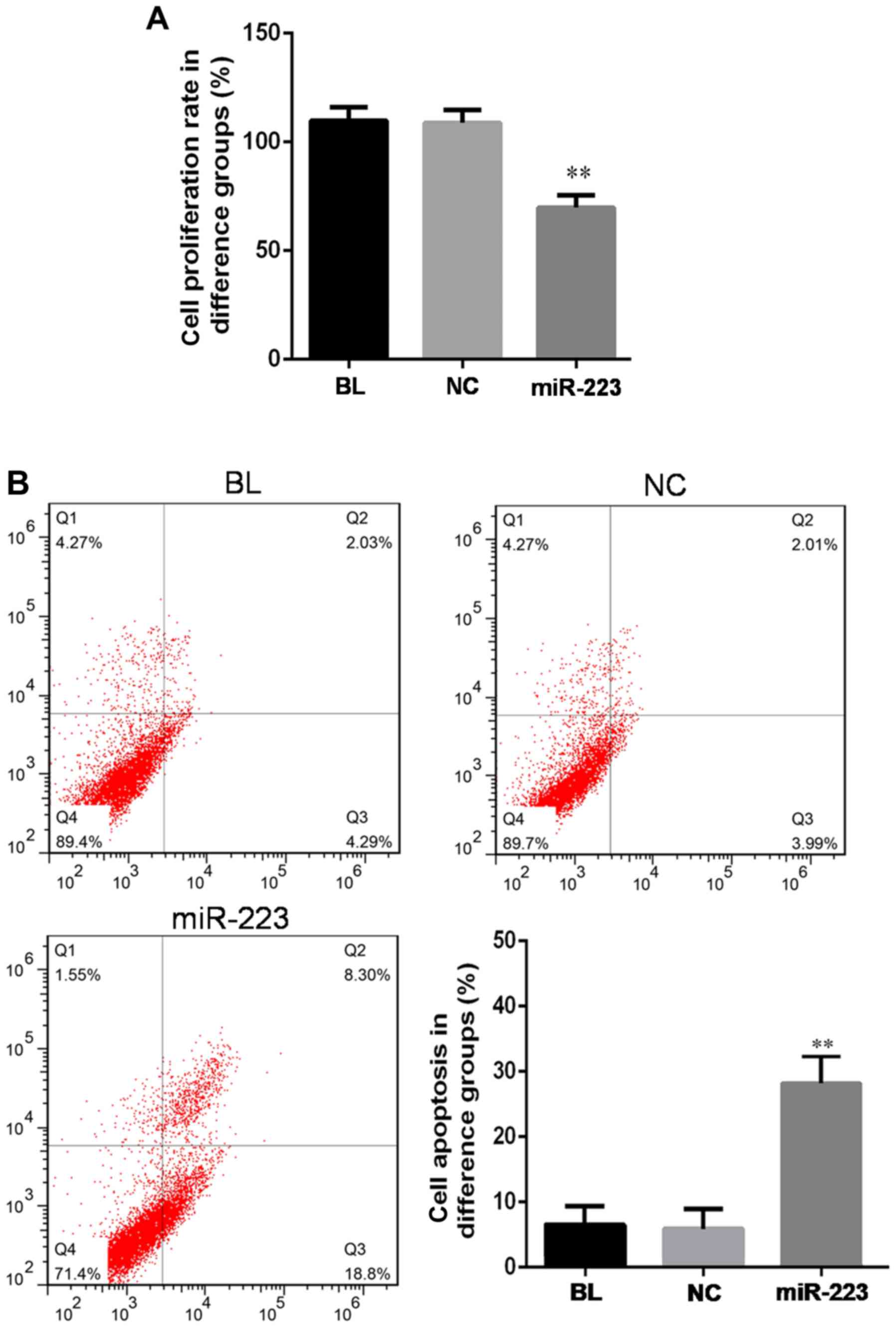
miR-223 influences the proliferation
and apoptosis of hep3B cells
Next, the effect of miR-223 overexpression on the
proliferation or apoptosis of hep3B cells was assessed. When hep3B
cells were transfected with miR-223, a significant decrease of cell
proliferation was detected by using the MMT method (Fig. 2A). The replication rate of the cells
transfected with miR-223 declined to ~65% of that of the cells
transfected with mimics control (P<0.05). At the same time,
miR-223 increased the apoptotic rate (Fig. 2B). The apoptotic rate increased to
27.1% following transfection with miR-223, which was almost 4-fold
of the rate in the blank control group or the negative control
group (P<0.01). These results indicated that miR-223 has a tumor
suppressor role in hep3B cells.
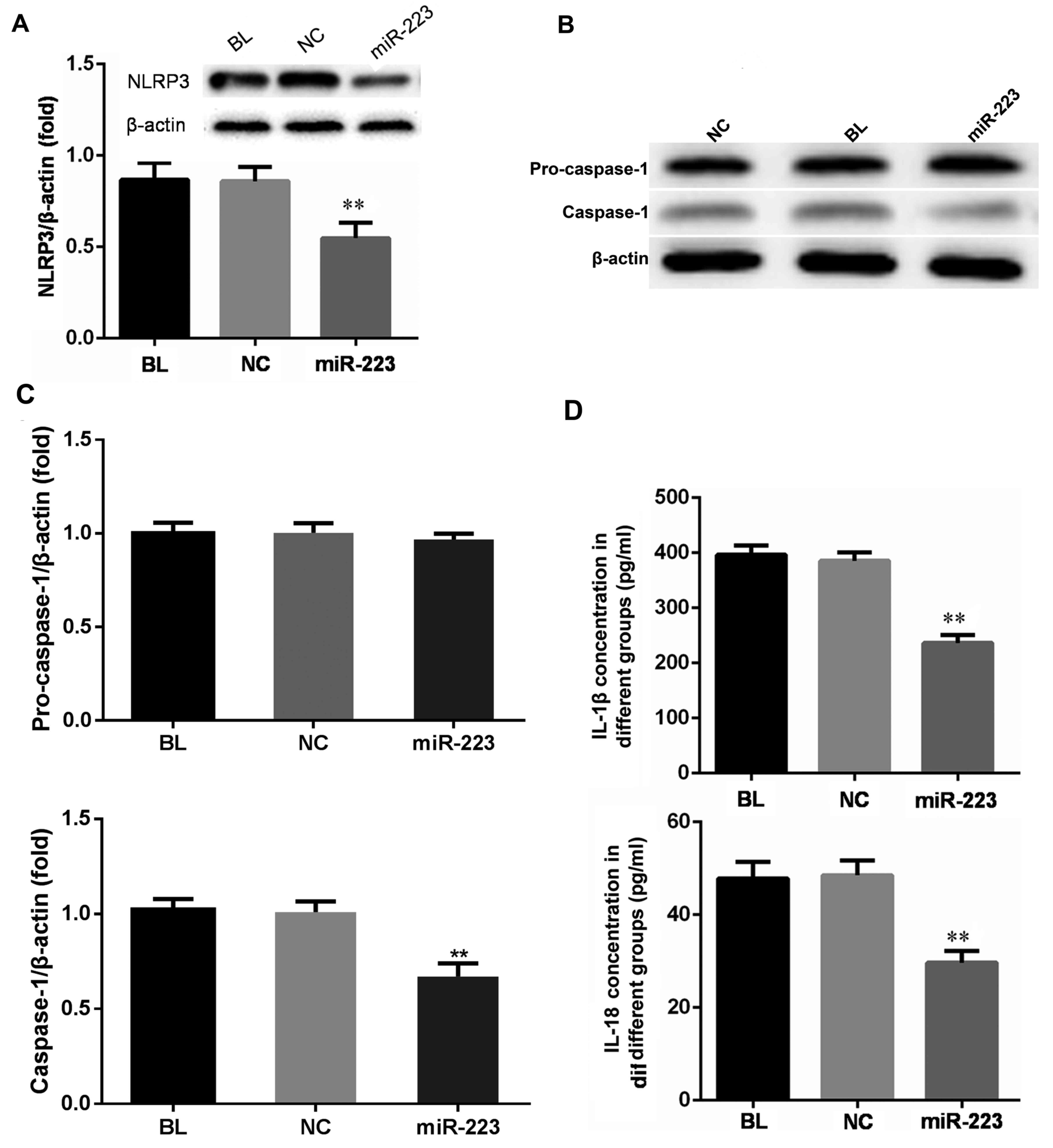
miR-223 regulates the expression of
NLRP3 inflammasome components and downstream cytokines
As the 3′-UTR of NLRP3 mRNA was demonstrated to
contain a direct target region of miR-223, it was further assessed
whether the miR-223 levels correlated with the expression of the
NLRP3 inflammasome regulation pathway. As presented in Fig. 3A and B, NLRP3 expression in hep3B
cells was inhibited at the mRNA as well as the protein level
following transfection with miR-223. The NLRP3 inflammasome
contains a caspase-recruitment domain. Upon activation of the NLRP3
inflammasome, pro-caspase-1 is recruited to the inflammasome
complex and cleaved to generate caspase-1. Consistent with the
decrease of NLRP3 expression, caspase-1 expression was also
downregulated at the mRNA and protein level following
overexpression of miR-223, while the expression of pro-caspase-1
remained unchanged (Fig. 3A-C).
Activation of caspase-1 subsequently cleaves pro-IL-1β and
pro-IL-18 into mature IL-1β and IL-18, so the secretion of these
two cytokines was also measured. The concentration of IL-1β and
IL-18 in the miR-223 transfection group was lower than that in the
mimics and the blank control group (Fig.
3D). The results indicated that miR-223 suppressed NLRP3
expression and subsequently reduced the levels of caspase-1, IL-1β
and IL-18.
Discussion
The role of the NLRP3 inflammasome in cancer remains
controversial. The present study observed that downregulation of
NLRP3, caspase-1, IL-1β and IL-18 favored apoptosis of the HCC cell
line hep3B, indicating that activation of the NLRP3 inflammasome
pathway has a procarcinogenic effect. This type of role was also
reported in colitis-associated cancer (17). During chemotherapy, the NLRP3
inflammasome in dendritic cells was reported to be activated to
induce IL-1β-dependent adaptive immunity against thymoma (18). The NLRP3 inflammasome was also
revealed to be activated by chemotherapy-triggered cathepsin B
release in myeloid-derived suppressor cells and blunted the
anticancer efficacy of the chemotherapy by IL-1β induction
(19). However, other studies also
reported a protective role of the NLRP3 inflammasome in cancer. The
NLRP3 inflammasome activated the tumoricidal function of natural
killing cells and suppressed colorectal cancer metastasis in the
liver (20). A study by Wei et
al (21) indicated that
expression of NLRP3 inflammasome components was upregulated in
hepatic parenchymal cells with hepatitis infection or cirrhosis but
downregulated in HCC tissues when compared with adjacent normal
tissues. A further study by the same group indicated that treatment
with 17β-estradiol inhibited the invasion and migration of HCC
cells through estrogen/mitogen-activated protein kinase-mediated
upregulation of the NLRP3 inflammasome (22). These two studies revealed a dynamic
expression pattern of NLRP3 during multiple stages of HCC
progression; however, the authors did not perform a stratified
analysis by the hepatitis infection status in HCC patients. NLRP3
expression may also be cell linage-associated. The HCC cell lines
used by Wei et al (21,22) are
all non-B or non-C associated (BEL7402, SMMC7721 and HepG2), while
the hep3B cell line used in the present study is derived from an
HBV-positive patient. The role of the NLRP3 inflammasome in HCC or
other human cancers has yet to be elucidated by further studies.
Furthermore, previous research has demonstrated that NF-kB may
induce the inhibition of apoptosis-associated gene expression
through TRAF1 (TNFR-associated factor 1), TRAF2, c-IAP1
(Inhibitor-of-apoptosis) and c-IAP2, which results in the
suppression of caspase-1 expression in the inflammatory environment
(23).
The results of the present study demonstrated that
miR-223 promoted apoptosis and inhibited the proliferation of HCC
cells by directly regulating NLRP3. The regulation of NLRP3 by
miR-223 was also reported in other diseases. miR-223 was revealed
to downregulate NLRP3 to inhibit inflammation, reduce brain edema
and improve neurological functions after intracerebral hemorrhage
(24). During Epstein-Barr virus
(EBV) infection, decreased miR-223 expression led to overexpression
of NLRP3 and IL-1β-associated inflammation. EBV miR-BART15 was
reported to target the miR-223 binding site in the 3′-UTR of NLRP3
mRNA and thereby inhibited NLRP3 expression (25).
In addition to NLRP3, miR-223 has other targets in
various solid tumor types. In HCC, downregulation of miR-223 was
also correlated with an upregulation of stathmin1 (15). Insulin-like growth factor-1 receptor
(IGF-1R) and cyclin-dependent kinase 2 were identified as direct
targets of miR-223 in non-small cell lung cancer (26). Suppression of IGF-1R by miR-223 was
also observed in HeLa cells and the osteosarcoma cell line MG63
(27,28). In MG63 cells, heat-shock protein 90B1
was another target of miR-223 (28).
Artemin was reported to be a target of miR-223 in human esophageal
carcinoma (29). miR-223 was also
revealed to promote the invasion and metastasis of gastric cancer
by regulating erythrocyte membrane protein band 4.1 like 3
(30). miR-223 also regulates
transcription factor forkhead box O1 in multiple cancer cell lines,
including the colorectal cancer cell line HCT116, the cervical
cancer cell line HeLa and the hepatoma cell HuH-7 (31). These studies comprehensively
indicated multiple roles of miR-223 during tumorigenesis.
In conclusion, the present study demonstrated a
regulatory effect of miR-223 on NLRP3 inflammasome components in
HCC cells. These results provided insight into the association
between the innate immune system and the genesis of HCC. Of note,
the present study had certain limitations. Only one cell line, HCC,
was used in all of the experiments. Considering the different
biological features of HCC cell lines, replication of the
experiments is required to fully reveal the link between the NLRP3
inflammasome and HCC development. Furthermore, in vivo tests
are considered for further studies to confirm the in vitro
results of the present study.
Acknowledgements
The study was financially supported by the ‘Six
Talent Projects’ of Jiangsu Province (grant no. 2015-WSN-047), a
Research Project of the Affiliated Hospital of Nanjing University
of Traditional Chinese Medicine (grant no. Y16019) and the Priority
Academic Program Development of Jiangsu Higher Education
Institutions.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kew MC: Hepatocellular carcinoma:
Epidemiology and risk factors. J Hepatocell Carcinoma. 1:115–125.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perz JF, Armstrong GL, Farrington LA,
Hutin YJ and Bell BP: The contributions of hepatitis B virus and
hepatitis C virus infections to cirrhosis and primary liver cancer
worldwide. J Hepatol. 45:529–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanneganti T: Central roles of NLRs and
inflammasomes in viral infection. Nat Rev Immunol. 10:688–698.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen W, Xu Y, Li H, Tao W, Xiang Y, Huang
B, Niu J, Zhong J and Meng G: HCV genomic RNA activates the NLRP3
inflammasome in human myeloid cells. PLoS One. 9:e849532014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Szabo G and Petrasek J: Inflammasome
activation and function in liver disease. Nat Rev Gastro Hepat.
12:387–400. 2015. View Article : Google Scholar
|
|
8
|
Franchi L, Eigenbrod T, Muñoz-Planillo R
and Nuñez G: The inflammasome: A caspase-1-activation platform that
regulates immune responses and disease pathogenesis. Nat Immunol.
10:241–247. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wree A, Eguchi A, Mcgeough MD, Pena CA,
Johnson CD, Canbay A, Hoffman HM and Feldstein AE: NLRP3
inflammasome activation results in hepatocyte pyroptosis, liver
inflammation, and fibrosis in mice. Hepatology. 59:898–910. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aachoui Y, Sagulenko V, Miao EA and Stacey
KJ: Inflammasome-mediated pyroptotic and apoptotic cell death, and
defense against infection. Curr Opin Microbiol. 16:319–326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bauernfeind F, Rieger A, Schildberg FA,
Knolle PA, Schmid-Burgk JL and Hornung V: NLRP3 inflammasome
activity is negatively controlled by miR-223. J Immunol.
189:4175–4181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramkissoon SH, Mainwaring LA, Ogasawara Y,
Keyvanfar K, McCoy JP Jr, Sloand EM, Kajigaya S and Young NS:
Hematopoietic-specific microRNA expression in human cells. Leuk
Res. 30:643–647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong QW, Lung RW, Law PT, Lai PB, Chan KY,
To KF and Wong N: MicroRNA-223 is commonly repressed in
hepatocellular carcinoma and potentiatesexpression of stathmin1.
Gastroenterology. 135:257–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allen IC, Tekippe EM, Woodford RM, Uronis
JM, Holl EK, Rogers AB, Herfarth HH, Jobin C and Ting JP: The NLRP3
inflammasome functions as a negative regulator of tumorigenesis
during colitis-associated cancer. J Exp Med. 207:1045–1056. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghiringhelli F, Apetoh L, Tesniere A,
Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G,
Ullrich E, et al: Activation of the NLRP3 inflammasome in dendritic
cells induces IL-1beta-dependent adaptive immunity against tumors.
Nat Med. 15:1170–1178. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bruchard M, Mignot G, Derangère V, Chalmin
F, Chevriaux A, Végran F, Boireau W, Simon B, Ryffel B, Connat JL,
et al: Chemotherapy-triggered cathepsin B release in
myeloid-derived suppressor cells activates the Nlrp3 inflammasome
and promotes tumor growth. Nat Med. 19:57–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dupaul-Chicoine J, Arabzadeh A, Dagenais
M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton
V, Colpitts SL, Beauchemin N and Saleh M: The Nlrp3 inflammasome
suppresses colorectal cancer metastatic growth in the liver by
promoting natural killer cell tumoricidal activity. Immunity.
43:751–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X,
Zhao W, Huai W, Guo P and Han L: Deregulation of the NLRP3
inflammasome in hepatic parenchymal cells during liver cancer
progression. Lab Invest. 94:52–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei Q, Guo P, Mu K, Zhang Y, Zhao W, Huai
W, Qiu Y, Li T, Ma X, Liu Y, et al: Estrogen suppresses
hepatocellular carcinoma cells through ERβ-mediated upregulation of
the NLRP3 inflammasome. Lab Invest. 95:804–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen K, Mi MT and Yu XP: Effects of
taurine on apoptosis of photoreceptors and NF-kB/Caspase-1 pathway
in photochemical damage. Acta Nutrimenta Sinica. 8:296–299.
2006.(In Chinese).
|
|
24
|
Yang N, Ekanem NR, Sakyi CA and Ray SD:
Hepatocellular carcinoma and microRNA: New perspectives on
therapeutics and diagnostics. Adv Drug Deliv Rev. 81:62–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haneklaus M, Gerlic M, Kurowska Stolarska
M, Rainey AA, Pich D, Mcinnes IB, Hammerschmidt W, Neill LA and
Masters SL: Cutting edge: miR-223 and EBV miR-BART15 regulate the
NLRP3 inflammasome and IL-1β production. J Immunol. 189:3795–3799.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nian W, Ao X, Wu Y, Huang Y, Shao J, Wang
Y, Chen Z, Chen F and Wang D: miR-223 functions as a potent tumor
suppressor of the Lewis lung carcinoma cell line by targeting
insulin-like growth factor-1 receptor and cyclin-dependent kinase
2. Oncol Lett. 6:359–366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia CY, Li HH, Zhu XC, Dong YW, Fu D, Zhao
QL, Wu W and Wu XZ: MiR-223 suppresses cell proliferation by
targeting IGF-1R. PLoS One. 6:e270082011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li G, Cai M, Fu D, Chen K, Sun M, Cai Z
and Cheng B: Heat shock protein 90B1 plays an oncogenic role and is
a target of microRNA-223 in human osteosarcoma. Cell Physiol
Bioche. 30:1481–1490. 2012. View Article : Google Scholar
|
|
29
|
Li S, Li Z, Guo F, Qin X, Liu B, Lei Z,
Song Z, Sun L, Zhang HT, You J and Zhou Q: miR-223 regulates
migration and invasion by targeting Artemin in human esophageal
carcinoma. J Biomed Sci. 18:242011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li
M, Sun L, Ji G, Shi Y, Han Z, et al: miRNA-223 promotes gastric
cancer invasion and metastasis by targeting tumor suppressor
EPB41L3. Mol Cancer Res. 9:824–833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu L, Li H, Jia CY, Cheng W, Yu M, Peng M,
Zhu Y, Zhao Q, Dong YW, Shao K, et al: MicroRNA-223 regulates FOXO1
expression and cell proliferation. FEBS Lett. 586:1038–1043. 2012.
View Article : Google Scholar : PubMed/NCBI
|