Introduction
Paraquat (PQ) has been one of the most effective and
widely used herbicides over the last few decades, particularly in
rural areas of developing countries; however, PQ poisoning has
become a serious problem, with reports of mortality >90%
(1,2). The primary pathological effects of PQ
are observed in the lung, where pulmonary concentrations are 6–10
times higher than in plasma following PQ ingestion (3). Furthermore, PQ accumulates in the lungs
as blood levels begin to decrease (3). The rapid accumulation of PQ damages the
parenchymal cells in the lung and induces the excessive repair of
lung tissues, which results in irreversible and extensive pulmonary
fibrosis (PF) (3) and eventually
leads to high mortality rates. However, the exact mechanism that
leads to toxicity remains unclear, and no specific therapy has been
recommended.
Epithelial-to-mesenchymal transition (EMT) occurs in
multiple contexts, including embryonic development, tissue
fibrosis, and cancer. EMT is defined as the process by which
stationary epithelial cells (identified by high levels of
E-cadherin and zonula occludens-1, which are markers of epithelial
cells) undergo phenotypic changes, including the loss of cell-cell
adhesion and apical-basal polarity, and acquire mesenchymal
characteristics, including high levels of α-smooth muscle actin
(α-SMA) and N-cadherin (markers of mesenchymal cells), that confer
migratory capacity (4,5). According to previous findings, EMT has
an important role in the development of PF. Alveolar epithelial
cells could acquire mesenchyme cell phenotypes through EMT, these
cells could then increase the deposition of extracellular matrix
and further promote the development of PF (5–7).
Furthermore, EMT has been demonstrated to serve an important role
in PQ-induced PF in recent studies by the present authors (8,9).
Hypoxia-inducible factor-1α (HIF-1α) has roles in
tumorigenesis, inflammation, and cell metabolism in hypoxia, and
its expression is correlated with a variety of fibrotic diseases
(10,11). HIF-1α has also been demonstrated to
induce EMT and contribute to PF (12,13).
Previous studies have detected an early increase in HIF-1α
expression following PQ poisoning and revealed that HIF-1α
modulates EMT in cases of PF (9,14).
Lysyl oxidase (LOX) is a secreted copper-dependent
amine oxidase that is important for growth, stabilization,
remodeling and repair. Its primary function is to catalyze the
covalent cross-linking of collagens and elastin in the
extracellular matrix, although it also has intracellular functions
(15). LOX participates in various
fibrosis processes, such as lung, myocardial and renal fibrosis
(16–18). As demonstrated in a previous study by
the present authors, LOX promotes EMT in PQ-induced PF (8). LOX was previously considered a critical
target of HIF-1α (19); however,
HIF-1α and LOX have since been demonstrated to provide
bidirectional regulation of colon and ovarian carcinomas (20,21). The
potential for dual regulation via HIF-1α and LOX remains
controversial, particularly in PQ-induced PF. The present study
investigated the association between HIF-1α and LOX with regard to
PQ-induced PF.
Materials and methods
Reagents
PQ powder was obtained from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Anti-HIF-1α antibodies (cat. no. BS3514)
were purchased from Bioworld Technology, Inc. (St. Louis Park, MN,
USA). Anti-LOX (cat. no. ab174316), anti-E-cadherin (cat. no.
ab184633) and anti-α-SMA (cat. no. ab7817) primary antibodies were
obtained from Abcam (Cambridge, MA, USA). Anti-β-catenin (cat. no.
8480) and anti-GAPDH (cat. no. 5174) antibodies were purchased from
Cell Signaling Technology, Inc. (Boston, MA, USA). Horseradish
peroxidase-conjugated anti-rabbit immunoglobulin (Ig)G (cat. no.
A0208), anti-mouse IgG secondary antibodies (cat. no. A0216),
immunofluorescence staining kits with Alexa Fluor 647-labeled goat
anti-rabbit immunoglobulin G (cat. no. A0468) and kits with Alexa
Fluor 488-labeled goat anti-rabbit IgG (cat. no. A0423) were
obtained from Beyotime Institute of Biotechnology (Shanghai,
China).
Cell culture
Human lung adenocarcinoma epithelial cells (A549)
and rat alveolar type II cells (RLE-6TN) were obtained from the
American Type Culture Collection (Manassas, VA, USA). A549 cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and a 1% antibiotic solution (100 U/ml penicillin
and 0.1 mg/ml streptomycin). RLE-6TN cells were cultured in
DMEM/nutrient mixture F-12 supplemented with 10% FBS and 1%
antibiotic solution. Both cell lines were cultured at 37°C in an
atmosphere containing 5% CO2. Cells were subsequently
treated with PQ (at a concentration of 800 µmol/l for A549 cells
and 160 µmol/l for RLE-6TN cells) for 24 h at 37°C. These
concentrations were used in accordance with a recent study by the
present authors (9). The effect of
HIF-1α or LOX silencing on cells was detected and the expression of
other proteins was subsequently assessed using western
blotting.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). The total RNA
concentration was determined using an ultraviolet
spectrophotometer. Reverse transcription was performed using a
PrimeScript RT Master Mix kit (Takara Biotechnology Co., Ltd.,
Dalian, China), according to the manufacturer's instructions.
Real-time quantitative PCR was performed using a SYBR Premix Ex Taq
kit (Takara Biotechnology Co., Ltd.) in a ViiA 7 PCR system. Sangon
Biotech Co., Ltd. (Shanghai, China) generated the primers for
HIF-1α, LOX, β-catenin and β-actin. Primer sequences are listed in
Table I. The thermocycling
conditions were as follows: 2 min at 95°C for initial denaturation,
followed by 40 amplification cycles consisting of 95°C for 10 sec
(denaturation), 60°C for 30 sec (anneal) and 72°C for 30 sec
(extension). The method of quantification used was the
2−ΔΔCq method (22). Each
assay was performed in triplicate, and β-actin served as a loading
control.
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Species | Gene
(direction) | Sequence
(5′-3′) |
|---|
| Human | HIF-1α (F) | GTC TGA GGG GAC AGG
AGG AT |
|
| HIF-1α (R) | CTC CTC AGG TGG CTT
GTC AG |
|
| LOX (F) | CAA CCT GAG ATG CGC
GG |
|
| LOX (R) | GGT CGG CTG GGT AAG
AAA TC |
|
| β-catenin (F) | CGT TTC GCC TTC ATT
ATG GAC TAC CT |
|
| β-catenin (R) | GCC GCT GGG TGT CCT
GAT GT |
|
| β-actin (F) | CTG GAA CGG TGA AGG
TGA CA |
|
| β-actin (R) | AAG GGA CTT CCT GTA
ACA ATG CA |
| Rat | HIF-1α (F) | AAG TCT AGG GAT GCA
GCA CG |
|
| HIF-1α (R) | AGA TGG GAG CTC ACG
TTG TG |
|
| LOX (F) | CCT ACT ACA TCC AGG
CAT CCA |
|
| LOX (R) | AGT CTC TGA CAT CCG
CCC TA |
|
| β-catenin (F) | GTG CAA TTC CTG AGC
TGA CC |
|
| β-catenin (R) | CGG GCT GTT TCT ACG
TCA TT |
|
| β-actin (F) | CCT CTA TGC CAC ACA
GT |
|
| β-actin (R) | AGC CAC CAA TCC ACA
CAG |
Western blotting
Total proteins were harvested from both cell lines
in each group and lysed using radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology). Protein concentrations were
determined using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Total protein samples (~30 µg per
lane) were separated via 8% SDS-PAGE (Beyotime Institute of
Biotechnology), transferred to polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), blocked with 5%
skimmed milk in Tris-buffered saline containing Tween-20 (TBST) for
90 min at room temperature (RT), and incubated with antibodies
against HIF-1α (1:500), LOX (1:1,000), E-cadherin (1:500), α-SMA
(1:500), β-catenin (1:1,000) or GAPDH (1:500) overnight at 4°C.
Membranes were subsequently incubated with horseradish
peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG secondary
antibodies (1:2,000; Beyotime Institute of Biotechnology) at RT.
Following three washes with TBST, proteins were observed using a
highly sensitive enhanced chemiluminescent agent (Thermo Fisher
Scientific, Inc.). The band intensity was determined using ImageJ
software (version 10.2; National Institutes of Health, Bethesda,
MD, USA).
Immunofluorescence staining
Both cell lines were cultured in confocal dishes for
24 h at 37°C and incubated with PQ for 24 h at 37°C. Cells were
washed with PBS, fixed with 4% paraformaldehyde (Sigma-Aldrich;
Merck KGaA) for 10 min at RT, permeabilized with 0.5% Triton X100
(Sigma-Aldrich; Merck KgaA) for 10 min and blocked with 5% bovine
serum albumin (1 g bovine serum albumin powder and 20 ml
Tris-buffered saline; Beyotime Institute of Biotechnology) for 1 h
at RT. Subsequently, cells were incubated with anti-LOX (1:100) or
anti-HIF-1α (1:50) primary antibodies overnight at 4°C. Following
three washes with TBST, cells were incubated with
immunofluorescence staining kits with Alexa Fluor 647-labeled goat
anti-rabbit IgG (1:200) and kits with Alexa Fluor 488-labeled goat
anti-rabbit IgG (1:200) for 1.5 h at RT. Nuclei were stained with
DAPI (Beyotime Institute of Biotechnology) for 3 min at RT.
Fluorescent signals were detected with a laser confocal scanning
microscope (Leica TCS SP8; Leica Microsystems GmbH, Wetzlar,
Germany) and the cellular morphology was observed with a phase
contrast microscope (AMEX1200, Thermo Fisher Scientific, Inc.) was
used to observe the change of cellular morphology.
Transient transfection
A549 and RLE-6TN cells were cultured in 6-well
culture plates as described above and divided into dimethyl
sulfoxide groups (including the control, sicontrol, siHIF-1α and
siLOX groups) and PQ groups (including the control + PQ, sicontrol
+ PQ, siHIF-1α + PQ and siLOX + PQ groups). HIF-1α and LOX short
interfering (si)RNAs and negative control sequences were purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China) and are listed
in Table II. For transfection of
each siRNA, 4 µl Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) were incubated with 100 pmol siRNA or
negative control sequences in 500 µl Opti-MEM medium (Gino
Biomedical Technology Co., Ltd., Hangzhou, China) for 20 min at RT.
Cells were transfected by replacing the medium with 2 ml Opti-MEM
medium containing the siRNA or negative control sequences and
Lipofectamine® 2000, and then incubating them at 37°C in
a humidified atmosphere of 5% CO2 for 6 h. The Opti-MEM
medium was then replaced with 2 ml fresh culture medium.
Subsequently, the cells in the PQ groups were incubated with PQ for
24 h and the other cells were treated with phosphate buffered
saline. The total time from the start of transfection to subsequent
experimentation was 48 h.
 | Table II.Sequences of siRNAs used for
transfection. |
Table II.
Sequences of siRNAs used for
transfection.
| Species | siRNA | Sequence
(5′-3′) |
|---|
| Human | HIF-1α | F: GCC GAG GAA GAA
CUA UGA ATT |
|
|
| R: UUC AUA GUU CUU
CCU CGG CTT |
|
| LOX | F: CAG GCG AUU UGC
AUG UAC UTT |
|
|
| R: AGU ACA UGC AAA
UCG CCU GTT |
| Rat | HIF-1α | F: GGG CCG UUC AAU
UUA UGA ATT |
|
|
| R: UUC AUA AAU UGA
ACG GCC CTT |
|
| LOX | F: CCG GAU GUU AUG
AUA CUU ATT |
|
|
| R: UAA GUA UCA UAA
CAU CCG GTT |
Statistical analyses
Data were analyzed using SPSS (version 16.0; SPSS,
Inc., Chicago, IL, USA) and expressed as the mean + standard
deviation of triplicate experiments. Comparisons between two groups
were performed using a Student's t-test and comparisons of multiple
groups were performed using one-way analysis of variance and
Dunnett's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
HIF-1α and LOX may regulate PQ-induced
EMT
PQ treatment induced a significant decrease in
E-cadherin expression and significantly increased α-SMA expression
as determined by western blotting (Fig.
1A), which confirmed that EMT participated in PQ-induced PF.
Protein levels of HIF-1α and LOX were significantly increased in
the PQ groups compared with the control groups (Fig. 1B). Based on the immunofluorescence
staining, the levels of HIF-1α and LOX were markedly increased with
24 h of treatment with PQ compared with control groups (Fig. 1C). These results suggested that EMT
served an important role in PQ-induced PF, and that HIF-1α and LOX
may regulate EMT following PQ poisoning.
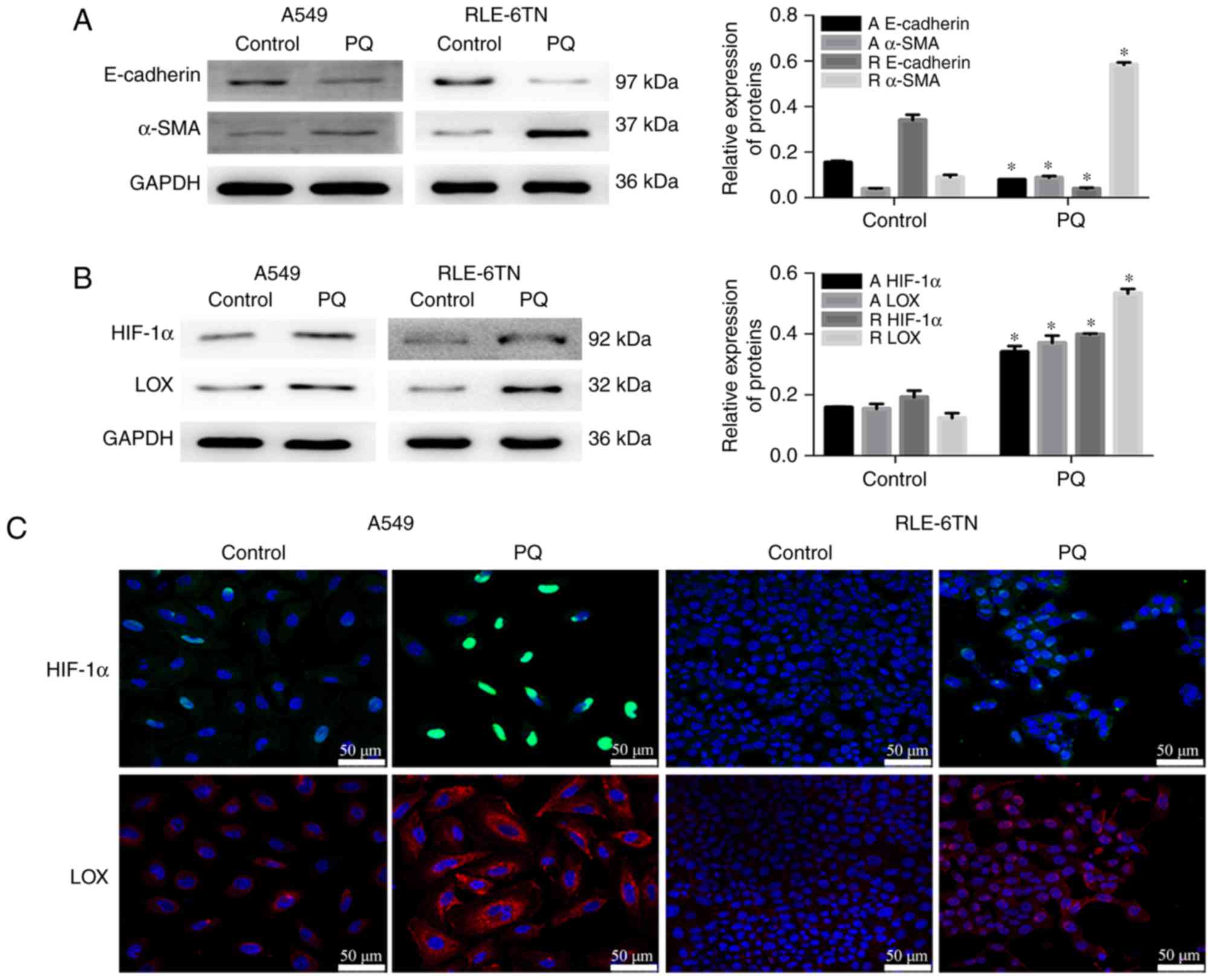 | Figure 1.Levels of the
epithelial-to-mesenchymal transition-associated proteins, HIF-1α
and LOX, increase during PQ-induced pulmonary fibrosis. (A)
E-cadherin, α-SMA and GAPDH levels were detected by western
blotting. GAPDH was used as a loading control. (B) HIF-1α, LOX and
GAPDH levels were detected by western blotting. (C) Levels of
HIF-1α and LOX proteins in A549 and RLE-6TN cells were detected by
immunofluorescence staining. Scale bars, 50 µm. Data are presented
as the mean + standard deviation (n=3). *P<0.05 vs. control.
HIF-1α, hypoxia-inducible factor-1α; LOX, lysyl oxidase; PQ,
paraquat; α-SMA, α-smooth muscle actin; A, levels in A549 cells; R,
levels in RLE-6TN cells. |
HIF-1α may promote EMT by upregulating
LOX expression
The levels of HIF-1α, LOX- and EMT-related markers
in PQ-poisoned A549 and RLE-6TN cells were measured following
HIF-1α silencing to determine the potential roles of HIF-1α and LOX
in PQ-induced EMT. HIF-1α mRNA expression was significantly
decreased in the siHIF-1α + PQ group compared with the sicontrol +
PQ group (Fig. 2A). The expression
of EMT markers was reversed following HIF-1α silencing, as α-SMA
expression decreased and E-cadherin expression increased (Fig. 2B). In addition, phase-contrast
microscopy revealed that the morphology of cells in the PQ groups
changed from a polygon to fusiform morphology compared with the
control group. However, these changes were alleviated following
HIF-1α silencing (Fig. 2C). The
level of LOX mRNA was significantly decreased in the siHIF-1α + PQ
group compared with the sicontrol + PQ group (Fig. 2D). The protein expression of LOX was
reduced in the siHIF-1α + PQ group compared with the sicontrol + PQ
group (Fig. 2E). Therefore, HIF-1α
may have an important function in modulating PQ-induced EMT by
inducing LOX expression.
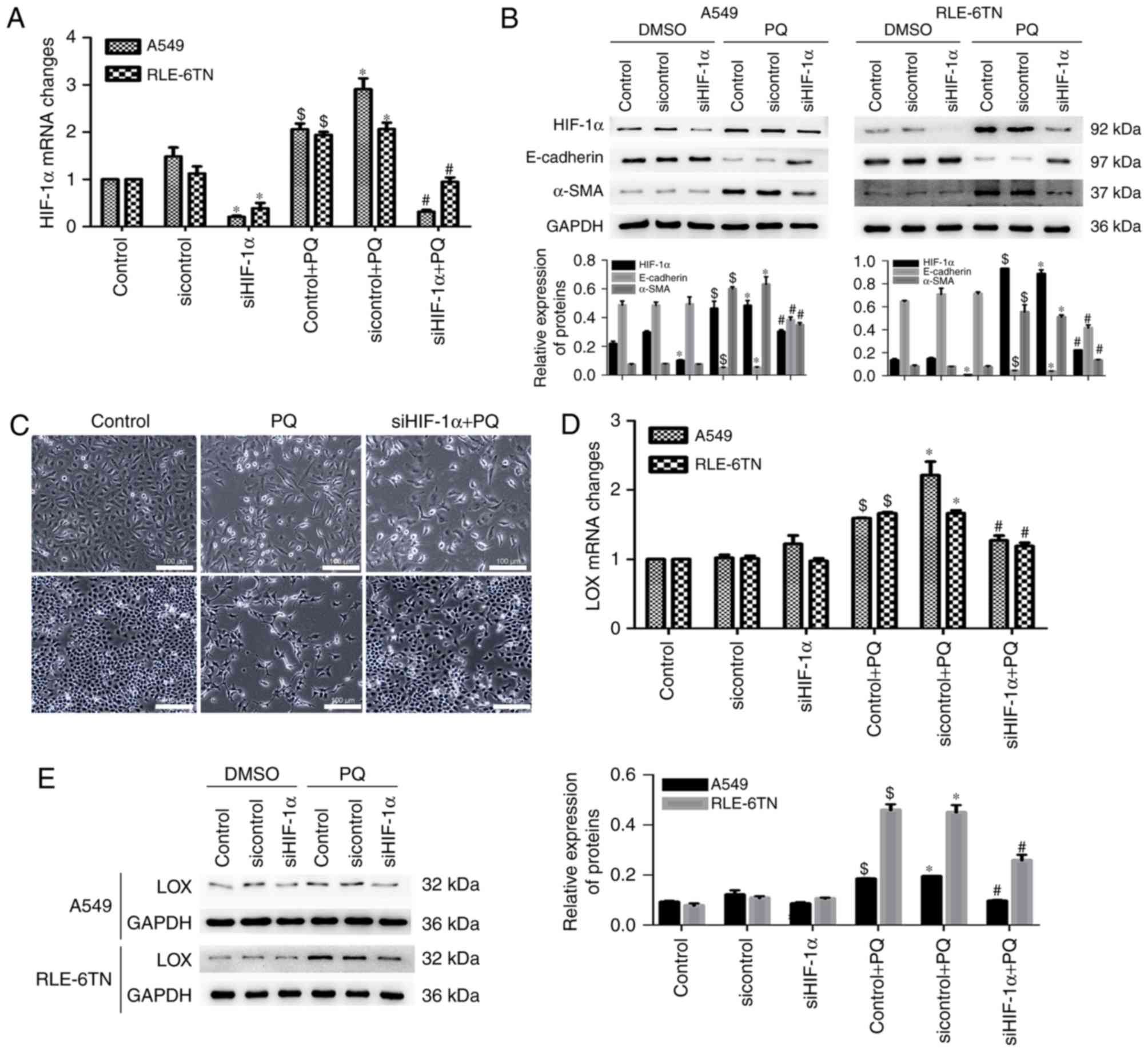 | Figure 2.HIF-1α ameliorated the degree of
PQ-induced epithelial-to-mesenchymal transition and LOX expression.
(A) HIF-1α mRNA levels in HIF-1α-silenced cell lines was detected
by RT-qPCR. (B) Protein levels of HIF-1α, E-cadherin, α-SMA and
GAPDH were detected by western blotting. GAPDH served as a loading
control. (C) Morphological changes were detected using a
phase-contrast microscope. Scale bars, 100 µm. (D) The level of LOX
mRNA in both HIF-1α-silenced cell lines was detected using RT-qPCR.
(E) LOX and GAPDH protein levels were detected by western blotting.
$P<0.05 vs. control; *P<0.05 vs. sicontrol;
#P<0.05 vs. sicontrol + PQ. HIF-1α, hypoxia-inducible
factor-1α; LOX, lysyl oxidase; PQ, paraquat; LOX, lysyl oxidase;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; α-SMA, α-smooth muscle actin; DMSO, dimethyl
sulfoxide. |
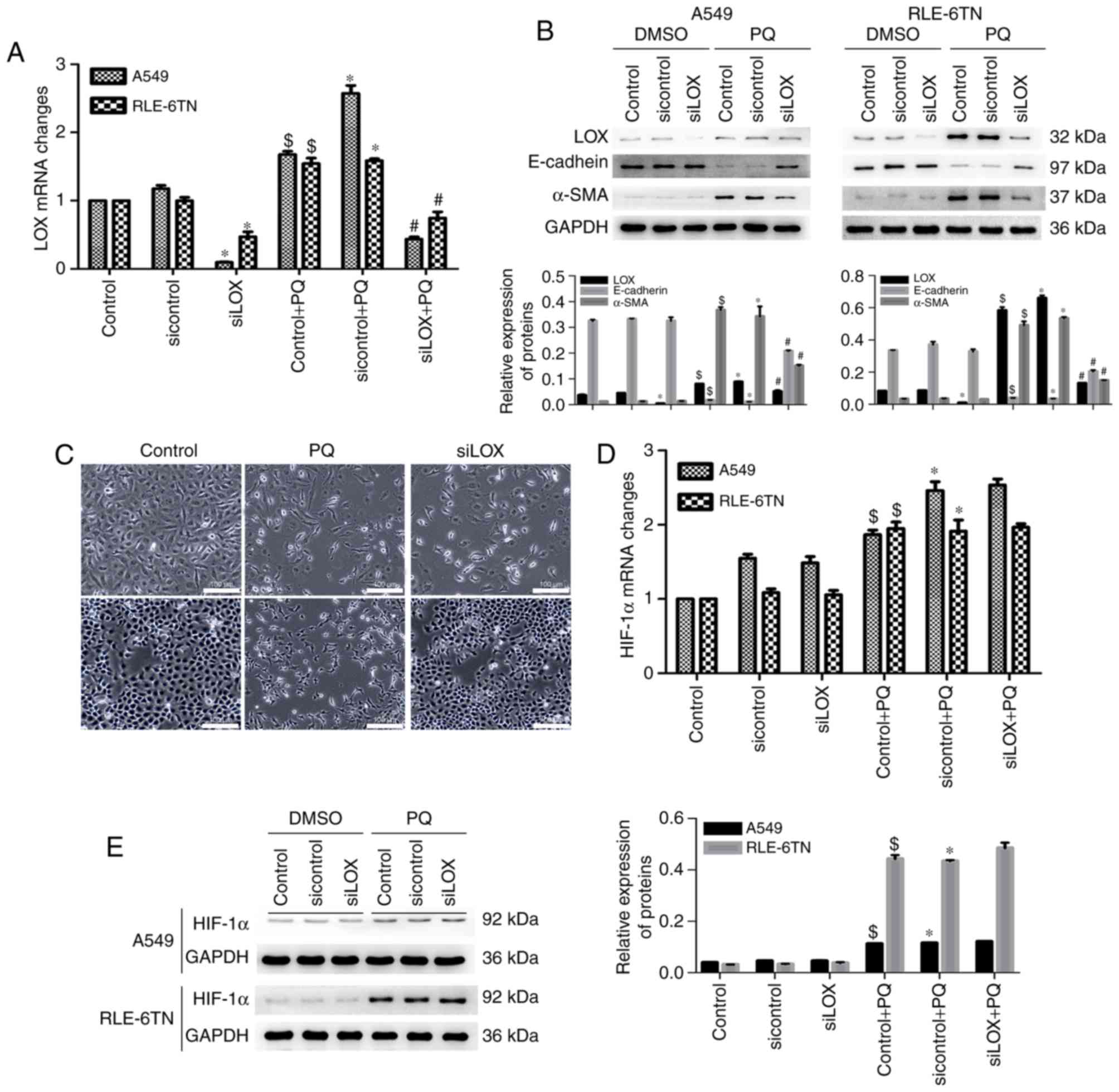
LOX promotes PQ-induced EMT
independently from HIF-1α
Levels of HIF-1α, LOX and EMT markers in PQ-poisoned
cells following LOX silencing were subsequently determined. The
level of LOX mRNA was significantly decreased in the siLOX group
compared with the sicontrol group and in the siLOX + PQ group
compared with the sicontrol + PQ group (Fig. 3A). The expression of EMT markers was
also reversed following LOX silencing, as α-SMA expression
decreased, and E-cadherin increased (Fig. 3B). In addition, phase-contrast
microscopy revealed that the morphological changes (the degree of
fusiformity was reduced) observed in cells in the PQ groups were
alleviated following LOX silencing (Fig.
3C). However, the expression of HIF-1α mRNA was not
significantly changed in the siLOX + PQ group compared with the
sicontrol + PQ group (Fig. 3D).
Levels of HIF-1α protein were also not significantly decreased
following LOX expression inhibition (Fig. 3E). These findings suggest that LOX
may promote PQ-induced EMT, but it does not regulate HIF-1α
expression.
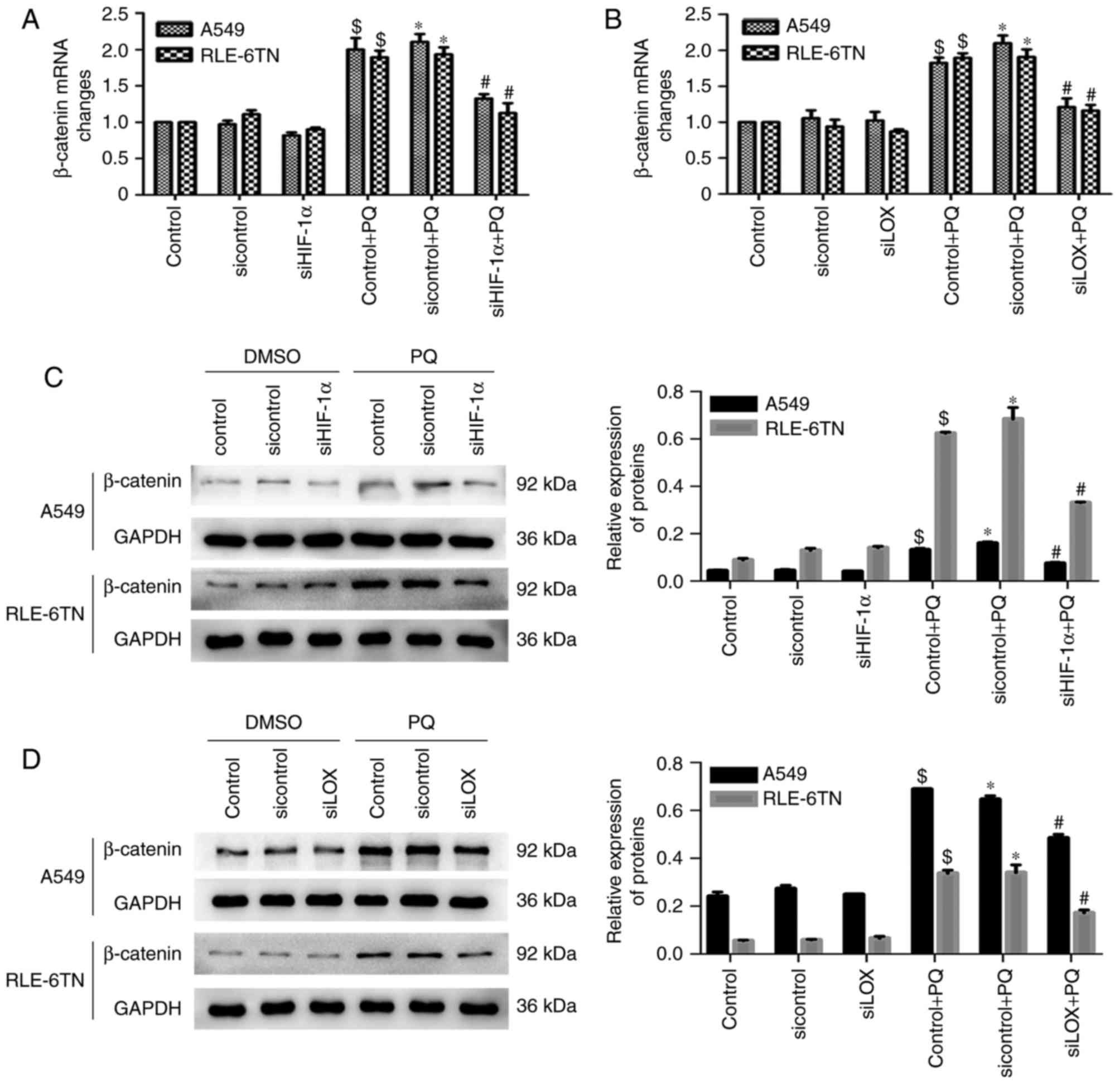
HIF-1α may regulate PQ-induced EMT
through the LOX/β-catenin pathway
Changes in the levels of β-catenin were detected
following HIF-1α (LOX) inhibition in vitro to further reveal
the interactions between HIF-1α, LOX and β-catenin. β-catenin mRNA
levels in the PQ groups were significantly decreased following
HIF-1α (LOX) silencing (Fig. 4A and
B). Similar results were observed for the protein expression of
β-catenin (Fig. 4C and D). These
findings suggest that HIF-1α may modulate PQ-induced EMT via the
LOX/β-catenin pathway.
Discussion
PQ accumulates in the lungs and eventually leads to
PF; however, its molecular mechanisms are complex and remain
unclear (2–4,7). EMT is
known to have an important function in PF (4,7,23). As demonstrated in recent studies by
the present authors, EMT occurs in PQ-induced PF and may be
modulated by HIF-1α or LOX (8,9).
However, the interaction between HIF-1α and LOX remains unclear.
Therefore, the association between HIF-1α and LOX was investigated,
as was the pathway that regulates PQ-induced EMT. It was
demonstrated that HIF-1α may modulate PQ-induced EMT via the
LOX/β-catenin pathway.
LOX is a downstream target gene of HIF-1α, and a
number of previous gene profiling studies have confirmed that LOX
expression is upregulated by HIF-1α (24–27). LOX
is also an important regulator of hypoxia-induced tumor progression
in a variety of cancers via a HIF-1α-dependent mechanism (19,28).
However, the correlation between HIF-1α and LOX in fibrosis remains
unexplored. In the present study, HIF-1α and LOX expression were
significantly increased in the model of PQ-induced PF. E-cadherin
expression was decreased, and α-SMA expression was significantly
increased following PQ treatment, which confirmed that HIF-1α, LOX
and EMT are associated with PQ-induced PF. Furthermore, HIF-1α
silencing downregulated the expression of LOX mRNA and protein. The
expression of EMT markers was also reversed following HIF-1α
silencing, as α-SMA expression decreased, and E-cadherin expression
increased. In addition, changes in cellular morphology were
alleviated following HIF-1α silencing, which indicated that the
degree of PQ-induced EMT was alleviated following HIF-1α silencing.
Therefore, HIF-1α serves an important role in modulating EMT by
activating LOX in PQ-induced PF. This result is consistent with
findings from previous studies, which demonstrated that HIF-1α
promotes EMT by upregulating LOX expression in ovarian and renal
cancers (27,29). It was also confirmed that LOX may be
a target of HIF-1α in PQ-induced PF and in tumors by modulating
EMT.
In addition to acting as a HIF-1α-responsive gene,
LOX may have more complex functions. According to a previous study
by Pez et al (21), LOX and
HIF-1α act synergistically to promote colon cancer cell
proliferation and tumor formation. As previously demonstrated by Ji
et al (20), LOX silencing
downregulates the protein expression of HIF-1α in epithelial
ovarian cancer cells. These findings indicated that LOX and HIF-1α
may bidirectionally regulate PQ-induced EMT. However, in the
present study, LOX silencing did not induce changes in the protein
and mRNA levels of HIF-1α. However, the expression of EMT markers
was ameliorated following LOX silencing. In addition, changes in
cellular morphology were alleviated following LOX silencing.
Therefore, the degree of PQ-induced EMT was alleviated following
LOX silencing in vitro. This finding is consistent with
other previously published results (27,29)
which reported that LOX inhibition did not prevent HIF-1α
upregulation. Furthermore, LOX is only an intermediate signaling
molecule that mediates HIF-1α-promoted PQ-induced EMT.
β-catenin is a protein located in cytoplasmic
plaques that serves a major role in EMT. β-catenin has been used as
a marker of EMT in a number of studies of embryonic development,
cancer, and fibrosis (30–33). According to previous studies,
β-catenin is associated with EMT during renal fibrosis (34) and fibrosis in other organs (35,36). In
addition, β-catenin participates in the development of PF by
transforming A549 cells into fibroblasts (23,37). As
demonstrated previously, HIF-1α is positively correlated with
β-catenin in rat models, and HIF-1α regulates EMT through the
β-catenin pathway (9,38). β-catenin mRNA and protein levels were
significantly decreased when HIF-1α and LOX were silenced in the
present study, which suggests that HIF-1α regulates PQ-induced EMT
through the LOX/β-catenin pathway.
The present study aimed to research the role of EMT
in the development of PQ-induced pulmonary fibrosis. A549 cells
retain the feature of type II alveolar epithelial cells even though
they are a type of cancer cell. RLE-6TN cells were type II rat
alveolar epithelial cells. These two cell types are widely used to
study the mechanism of pulmonary fibrosis, therefore they were each
selected for use within the present study to give a more
comprehensive investigation. In the present study it was confirmed
that EMT served a role in PQ-induced pulmonary fibrosis and may be
modulated by HIF-1α or LOX. HIF-1α may modulate PQ-induced EMT via
the LOX/β-catenin pathway.
In conclusion, HIF-1α unidirectionally upregulates
LOX expression in PQ-induced EMT. The mechanism may be associated
with HIF-1α-induced LOX expression, which subsequently increases
β-catenin levels, induces EMT and ultimately leads to the
development of PQ-induced PF. Therefore, HIF-1α may be a potential
target for restraining the development and exacerbation of PF
induced by PQ.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81602873
and 81502829) and the Key and Weak Subject Construction Project of
the Shanghai Health and Family Planning System (grant no.
2016ZB0205).
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
LOX
|
lysyl oxidase
|
|
PF
|
pulmonary fibrosis
|
|
PQ
|
paraquat
|
|
α-SMA
|
α-smooth muscle actin
|
References
|
1
|
Gil HW, Hong JR, Jang SH and Hong SY:
Diagnostic and therapeutic approach for acute paraquat
intoxication. J Korean Med Sci. 29:1441–1449. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu L, Xu J and Wang Z: Molecular
mechanisms of paraquat-induced acute lung injury: A current review.
Drug Chem Toxicol. 37:130–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dinis-Oliveira RJ, Duarte JA,
Sanchez-Navarro A, Remiao F, Bastos ML and Carvalho F: Paraquat
poisonings: Mechanisms of lung toxicity, clinical features, and
treatment. Crit Rev Toxicol. 38:13–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stone RC, Pastar I, Ojeh N, Chen V, Liu S,
Garzon KI and Tomic-Canic M: Epithelial-mesenchymal transition in
tissue repair and fibrosis. Cell Tissue Res. 365:495–506. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartis D, Mise N, Mahida RY, Eickelberg O
and Thickett DR: Epithelial-mesenchymal transition in lung
development and disease: Does it exist and is it important? Thorax.
69:760–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kage H and Borok Z: EMT and interstitial
lung disease: A mysterious relationship. Curr Opin Pulm Med.
18:517–523. 2012.PubMed/NCBI
|
|
8
|
Wang J, Zhu Y, Tan J, Meng X, Xie H and
Wang R: Lysyl oxidase promotes epithelial-to-mesenchymal transition
during paraquat-induced pulmonary fibrosis. Mol Biosyst.
12:499–507. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu Y, Tan J, Xie H, Wang J, Meng X and
Wang R: HIF-1alpha regulates EMT via the Snail and beta-catenin
pathways in paraquat poisoning-induced early pulmonary fibrosis. J
Cell Mol Med. 20:688–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masoud GN and Li W: HIF-1alpha pathway:
Role, regulation and intervention for cancer therapy. Acta Pharm
Sin B. 5:378–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. 138:1058–1066.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou G, Dada LA, Wu M, Kelly A, Trejo H,
Zhou Q, Varga J and Sznajder JI: Hypoxia-induced alveolar
epithelial-mesenchymal transition requires mitochondrial ROS and
hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol.
297:L1120–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Darby IA and Hewitson TD: Hypoxia in
tissue repair and fibrosis. Cell Tissue Res. 365:553–562. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang RL, Tang X, Wu X, Xu R, Yu KL and Xu
K: The relationship between HIF-1α expression and the early lung
fibrosis in rats with acute paraquat poisoning. Zhonghua Lao Dong
Wei Sheng Zhi Ye Bing Za Zhi. 30:273–277. 2012.(In Chinese).
PubMed/NCBI
|
|
15
|
Cox TR, Bird D, Baker AM, Barker HE, Ho
MW, Lang G and Erler JT: LOX-mediated collagen crosslinking is
responsible for fibrosis-enhanced metastasis. Cancer Res.
73:1721–1732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopez B, Gonzalez A, Hermida N, Valencia
F, de Teresa E and Diez J: Role of lysyl oxidase in myocardial
fibrosis: From basic science to clinical aspects. Am J Physiol
Heart Circ Physiol. 299:H1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng S, Jin T, Zhang L, Bu H and Zhang P:
Mechanism of tacrolimus-induced chronic renal fibrosis following
transplantation is regulated by ox-LDL and its receptor, LOX-1. Mol
Med Rep. 14:4124–4134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng T, Liu Q, Zhang R, Zhang Y, Chen J,
Yu R and Ge G: Lysyl oxidase promotes bleomycin-induced lung
fibrosis through modulating inflammation. J Mol Cell Biol.
6:506–515. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Erler JT, Bennewith KL, Nicolau M,
Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS and Giaccia AJ:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji F, Wang Y, Qiu L, Li S, Zhu J, Liang Z,
Wan Y and Di W: Hypoxia inducible factor 1α-mediated LOX expression
correlates with migration and invasion in epithelial ovarian
cancer. Int J Oncol. 42:1578–1588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pez F, Dayan F, Durivault J, Kaniewski B,
Aimond G, Le Provost GS, Deux B, Clézardin P, Sommer P, Pouysségur
J and Reynaud C: The HIF-1-inducible lysyl oxidase activates HIF-1
via the Akt pathway in a positive regulation loop and synergizes
with HIF-1 in promoting tumor cell growth. Cancer Res.
71:1647–1657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elvidge GP, Glenny L, Appelhoff RJ,
Ratcliffe PJ, Ragoussis J and Gleadle JM: Concordant regulation of
gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase
inhibition: The role of HIF-1alpha, HIF-2alpha, and other pathways.
J Biol Chem. 281:15215–15226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goto TM, Arima Y, Nagano O and Saya H:
Lysyl oxidase is induced by cell density-mediated cell cycle
suppression via RB-E2F1-HIF-1α axis. Cell Struct Funct. 38:9–14.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang X, Li S, Li W, Chen J, Xiao X, Wang
Y, Yan G and Chen L: Inactivation of lysyl oxidase by
β-aminopropionitrile inhibits hypoxia-induced invasion and
migration of cervical cancer cells. Oncol Rep. 29:541–548. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Ma J, Shen H, Wang C, Sun Y,
Howell SB and Lin X: Reactive oxygen species promote ovarian cancer
progression via the HIF-1α/LOX/E-cadherin pathway. Oncol Rep.
32:2150–2158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reynaud C, Ferreras L, Di Mauro P, Kan C,
Croset M, Bonnelye E, Pez F, Thomas C, Aimond G, Karnoub AE, et al:
Lysyl oxidase is a strong determinant of tumor cell colonization in
bone. Cancer Res. 77:268–278. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schietke R, Warnecke C, Wacker I, Schödel
J, Mole DR, Campean V, Amann K, Goppelt-Struebe M, Behrens J,
Eckardt KU and Wiesener MS: The lysyl oxidases LOX and LOXL2 are
necessary and sufficient to repress E-cadherin in hypoxia: Insights
into cellular transformation processes mediated by HIF-1. J Biol
Chem. 285:6658–6669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu L, Cui WH, Zhou WC, Li DL, Li LC, Zhao
P, Mo XT, Zhang Z and Gao J: Activation of Wnt/β-catenin signalling
is required for TGF-β/Smad2/3 signalling during myofibroblast
proliferation. J Cell Mol Med. 21:1545–1554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ji S, Deng H, Jin W, Yan P, Wang R, Pang
L, Zhou J, Zhang J, Chen X, Zhao X and Shen J: Beta-catenin
participates in dialysate-induced peritoneal fibrosis via enhanced
peritoneal cell epithelial-to-mesenchymal transition. FEBS Open
Bio. 7:265–273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Dai W, Wang Y, Gu Q, Yang D and
Zhang M: Blocking the Wnt/β-catenin pathway by lentivirus-mediated
short hairpin RNA targeting β-catenin gene suppresses
silica-induced lung fibrosis in mice. Int J Environ Res Public
Health. 12:10739–10754. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martinez-Martinez E, Ibarrola J, Calvier
L, Fernandez-Celis A, Leroy C, Cachofeiro V, Rossignol P and
Lopez-Andres N: Galectin-3 blockade reduces renal fibrosis in two
normotensive experimental models of renal damage. PLoS One.
11:e01662722016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu BL, Shi C, Lei RE, Lu DH, Luo W, Qin
SY, Zhou Y and Jianga HX: Interleukin-22 ameliorates liver fibrosis
through miR-200a/beta-catenin. Sci Rep. 6:364362016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin JC, Kuo WW, Baskaran R, Chen MC, Ho
TJ, Chen RJ, Chen YF, Vijaya Padma V, Lay IS and Huang CY:
Enhancement of beta-catenin in cardiomyocytes suppresses survival
protein expression but promotes apoptosis and fibrosis. Cardiol J.
24:195–205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie H, Tan JT, Wang RL, Meng XX, Tang X
and Gao S: Expression and significance of HIF-1alpha in pulmonary
fibrosis induced by paraquat. Exp Biol Med (Maywood).
238:1062–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|