Introduction
Synovial sarcomas (SS) are clinically aggressive
malignant tumors of mesenchymal origin and patients with SS are
susceptible to early systemic metastases (1,2).
Although the long-term outcomes for patients undergoing surgery for
SS have improved due to the development of systemic chemotherapy,
the overall prognosis of patients with SS remains unsatisfactory
(3,4). SS are characterized by local recurrence
and early lung metastases, and the 5-year survival rate for SS
ranges between 20 and 30% (5).
Therefore, the establishment of efficient therapeutic strategies is
required to improve the prognosis of such patients.
Chemotaxis is involved in many physiological
processes, including stem-cell homing, hematopoiesis, extracellular
matrix remodeling and cell-mediated wound healing (6–11).
Previous studies have identified an association between
tumorigenesis and chemokines (6–12). Thus,
these chemokines such as CXCL may be useful as potential
therapeutic targets to attenuate tumor progression.
Stromal cell-derived factor-1 (SDF-1), also known as
CXCL-12, primarily regulates the progression of chemotaxis and may
promote tumor formation (6–13). SDF-1 has been implicated in almost
all malignant cancers, including breast, lung, and colon cancer, as
well as tumors of hematopoietic origins (14). Furthermore, it has been demonstrated
that SDF-1 increases the recurrence and metastasis of malignant
tumors, as it may enhance the survival of tumor cells by preventing
apoptosis (15,16), resulting in decreased survival rates
and unfavorable clinical outcomes in cancer patients.
Vascular endothelial growth factor (VEGF) is one of
the most important cytokines in the human body and promotes
neovascularization and carcinogenesis via SDF-1 signaling (17). Previous studies have demonstrated
that overexpression of VEGF is essential for the growth and
survival of many types of cancer cells (18). In addition, high levels of VEGF are
associated with unfavorable survival rates in patients with SS
(18,19). The present study aimed to evaluate
the expression patterns of SDF-1 and VEGF in samples from SS tissue
and determine the potential association between SDF-1 and VEGF
expression and patient clinical outcomes.
Materials and methods
Patients and samples
Paraffin-embedded specimens were collected from 54
patients who visited the Fourth Hospital of Hebei Medical
University (Shijiazhuang, China) between January 2004 and December
2010. Clinical and histopathological characteristics, including
sex, age, tumor size, histological grade, distant metastasis, AJCC
staging, and information on patient follow-up and survival, were
collected retrospectively. The specimens were fixed in 10%
neutral-buffered formalin overnight at room temperature and then
were achieved for later use. Furthermore, radiotherapy and
chemotherapy were not administered prior to surgery in any patient.
All of the data were grouped according to the patient's age, sex,
tumor size (<5 vs. ≥5 cm) and histological cancer profile. Each
patient was assigned a histological grade according to the
Fédération Nationale des Centres de Lutte Contre le Cancer
(20), the presence of distant
metastases and American Joint Committee on Cancer (AJCC) staging
(21). The present study was
approved by Ethics Committee of The Fourth Affiliated Hospital of
Hebei Medical University (Shijiazhuang, China) and informed written
consent was obtained from all patients.
Immunohistochemical and
immunofluorescence staining and scoring
For immunohistochemical analysis, a tissue
microarray was produced using 4.0-mm diameter tumor cores with 1
core per case. Antigen retrieval was performed by microwaving the
array in sodium citrate buffer at 95°C for 10 min. Subsequently,
the samples were blocked in normal goat serum (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) at 37°C for 1 h Immunohistochemical
staining was performed according to a routine protocol (18). Samples were incubated at 37°C for 1 h
with rabbit anti-human SDF-1 (cat. no. GTX116092, 1:100; Bethyl
Laboratories, Montgomery, Inc., TX, USA) and rabbit anti-human VEGF
(cat. no. sc-152, 1:50; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). Following this, samples were incubated with horseradish
peroxidase-conjugated secondary antibody (cat. no. A6667,
Sigma-Aldrich; Merck KGaA) at 37°C for 1 h. Stained sections were
analyzed under an optical microscope by three pathologists who were
blinded to the patient data. The mean number of immunopositive
cells in the samples was determined in 5 random fields-of-view at a
magnification of ×400. Furthermore, immunohistochemical results
were evaluated according to the Friedrich' immunoreactivity score
(IRS) (18) based on two categories:
The percentage of stained cells (X): <1% (score=0); 1–25%
(score=1); 25–50% (score=2); 51–80% (score=3); >80% (score=4);
the staining intensity (Y): no staining (score=0); buff (score=1);
darker buff (score=2); tan (score=3). X × Y was calculated as the
final score, and staining was described as low (final score 0–3,
−/+), moderate (final score 4–7, ++) or high (final score >7,
+++). Analysis was performed using ImagePro Plus software (version
6; Media Cybernetics, Rockville, MD, USA).
Statistical analyses
The end of the follow-up period was defined as
either the date of patient mortality or the patient's last date of
contact, up to January 2015. Overall survival (OS) was defined as
the period of time from the date of the diagnosis to the date of
last contact or patient mortality. Furthermore, the association of
potential prognostic factors with SEF-1 and VEGF expression was
analyzed using the χ2 test. For the correlation
analysis, the Spearman-rho test was used to compare histological
and clinical variables. Univariate and multivariate analysis for
the potential prognostic factors and the OS was conducted using the
Cox proportional hazards regression analysis. Additionally, the
Kaplan-Meier curve method was used to determine the OS. SPSS
software (version 22.0; IBM SPSS, Armonk, NY, USA) was used for
statistical analysis and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Patient characteristics are presented in Table I. The mean patient age was 56.2±18.4
years (range, 25–74 years) and the median OS was 11 months (range,
3–83 months).
 | Table I.Clinicopathological variables and the
expression of SDF-1 and VEGF. |
Table I.
Clinicopathological variables and the
expression of SDF-1 and VEGF.
|
|
| SDF-1 | VEGF |
|---|
|
|
|
|
|
|---|
| Characteristics |
| −/+ (%) | ++ (%) | +++ (%) | P-value | −/+ (%) | ++ (%) | +++ (%) | P-value |
|---|
| Sex |
|
|
|
| 0.202 |
|
|
| 0.717 |
|
Female | 23 | 6 (26.1) | 10 (43.5) | 7
(30.4) |
| 6
(26.1) | 9
(39.1) | 8
(34.8) |
|
| Male | 31 | 5
(16.1) | 9
(29.0) | 17 (54.8) |
| 6
(19.4) | 11 (35.5) | 14 (45.2) |
|
| Age (years) |
|
|
|
| 0.217 |
|
|
| 0.490 |
| ≥30 | 25 | 5
(20.0) | 6
(24.0) | 14 (56.0) |
| 4
(16.0) | 11 (44.0) | 10 (40.0) |
|
|
<30 | 29 | 6
(20.7) | 13 (44.8) | 10 (34.5) |
| 8
(27.6) | 9
(31.0) | 12 (41.4) |
|
| Tumor size (cm) |
|
|
|
| 0.609 |
|
|
| 0.787 |
|
<5 | 32 | 6
(18.8) | 10 (31.3) | 16 (50.0) |
| 7
(21.9) | 13 (40.6) | 12 (37.5) |
|
| ≥5 | 22 | 5
(22.7) | 9
(40.9) | 8
(36.4) |
| 5
(22.7) | 7
(31.8) | 10 (45.5) |
|
| Histological
gradea |
|
|
|
| 0.004 |
|
|
| 0.042 |
| I | 12 | 5
(41.7) | 2
(16.7) | 5
(41.7) |
| 6
(50.0) | 4
(33.3) | 2
(16.7) |
|
| II | 20 | 2
(10.0) | 13 (65.0) | 5
(25.0) |
| 2
(10.0) | 10 (50.0) | 8
(40.0) |
|
|
III | 22 | 4
(18.2) | 4
(18.2) | 14 (63.6) |
| 4
(18.2) | 6
(27.3) | 12 (54.5) |
|
| Distant
metastatisa |
|
|
|
| 0.009 |
|
|
| 0.028 |
| No | 34 | 11 (32.4) | 12 (35.3) | 11 (32.4) |
| 11 (32.4) | 13 (38.2) | 10 (29.4) |
|
|
Yes | 20 | 0 (0.0) | 7
(35.0) | 13 (65.0) |
| 1 (5.0) | 7
(35.0) | 12 (60.0) |
|
| AJCC
staginga |
|
|
|
| <0.001 |
|
|
| 0.003 |
|
I/II | 25 | 11 (44.0) | 11 (44.0) | 3
(12.0) |
| 10 (40.0) | 10 (40.0) | 5
(20.0) |
|
|
III/IV | 29 | 0 (0.0) | 8
(27.6) | 21 (72.4) |
| 2 (6. 9) | 10 (34.5) | 17 (58.6) |
|
Association between SDF-1 and VEGF
expression levels and clinicopathological characteristics
Associations between SDF-1 and VEGF and
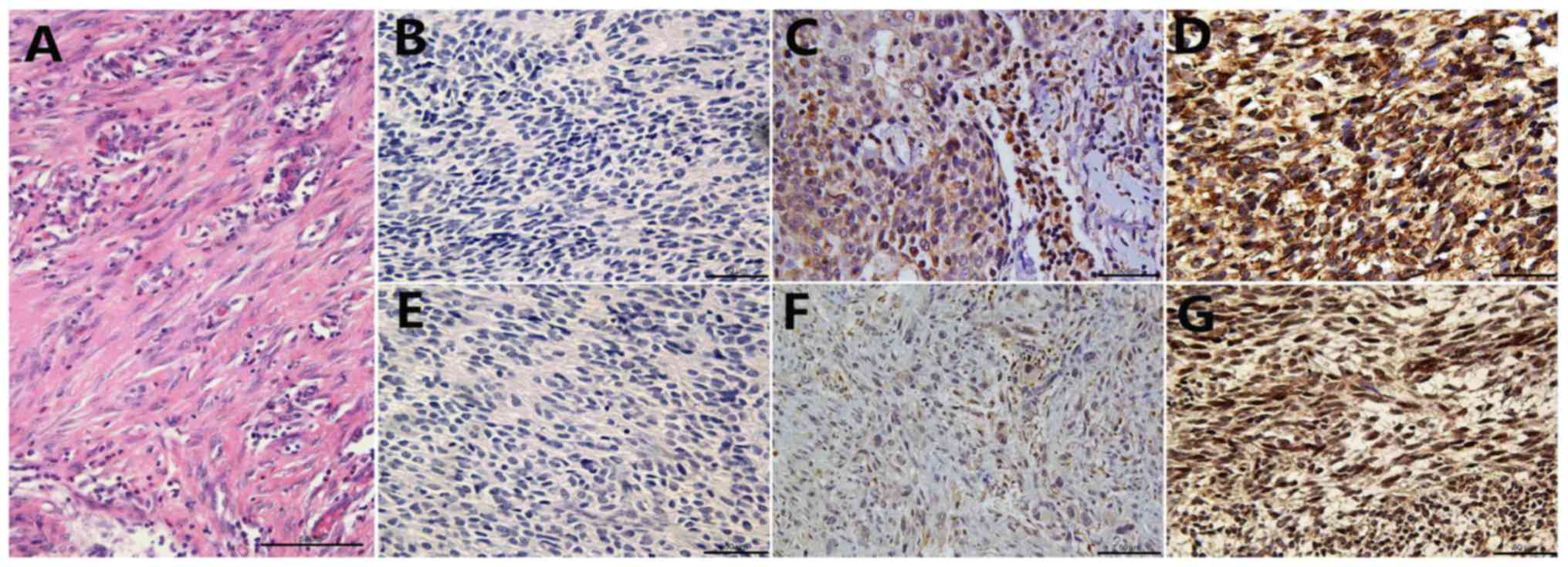
clinicopathological characteristics are summarized in Table I. Typical SDF-1 and VEGF staining in
SS tissues are presented in Fig. 1.
It was determined that in SS tissues, SDF-1 expression was low in
20.4% (11/54), moderate in 35.2% (19/54) and high in 44.4% (24/54)
of cases, whereas VEGF expression was low in 22.2% (12/54),
moderate in 37.0% (20/54) and high in 40.7% (22/54) of cases.
Additionally, both SDF-1 and VEGF expression were significantly
associated with histological grade (P<0.05), distant metastasis
(P<0.05) and AJCC staging (P<0.05). No significant
associations were identified between SDF-1 and VEGF expression
levels and other clinicopathological features (Table I).
SDF-1 expression is positively
correlated with VEGF expression
The expression of SDF-1 was significantly correlated
with VEGF expression (P<0.001), with a correlation coefficient
of 0.618 (Table II). Furthermore,
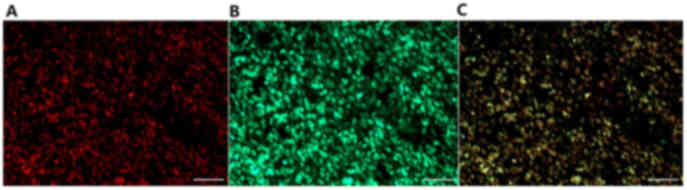
immunofluorescence analysis of paraffin-embedded specimens
determined that SDF-1 and VEGF were located at the appropriate
sections in SS cells (Fig. 2).
 | Table II.Correlation between SDF-1 and VEGF
expression. |
Table II.
Correlation between SDF-1 and VEGF
expression.
|
|
| SDF-1 |
|---|
|
|
|
|
|---|
|
Characteristics |
| −/+ (%) | ++ (%) | +++ (%) | P-value
(Spearman) |
|---|
| VEGFa |
|
|
|
| <0.001 |
|
−/+ | 12 | 8 (66.7) | 3 (25.0) | 1 (8.3) |
|
| ++ | 20 | 2 (10.0) | 11 (55.0) | 7 (35.0) |
|
|
+++ | 22 | 1 (4.5) | 5 (22.7) | 16 (72.7) |
|
| Total | 54 | 11 | 19 | 24 |
|
High expression of SDF-1 and VEGF in
patients with SS correlates with poor OS
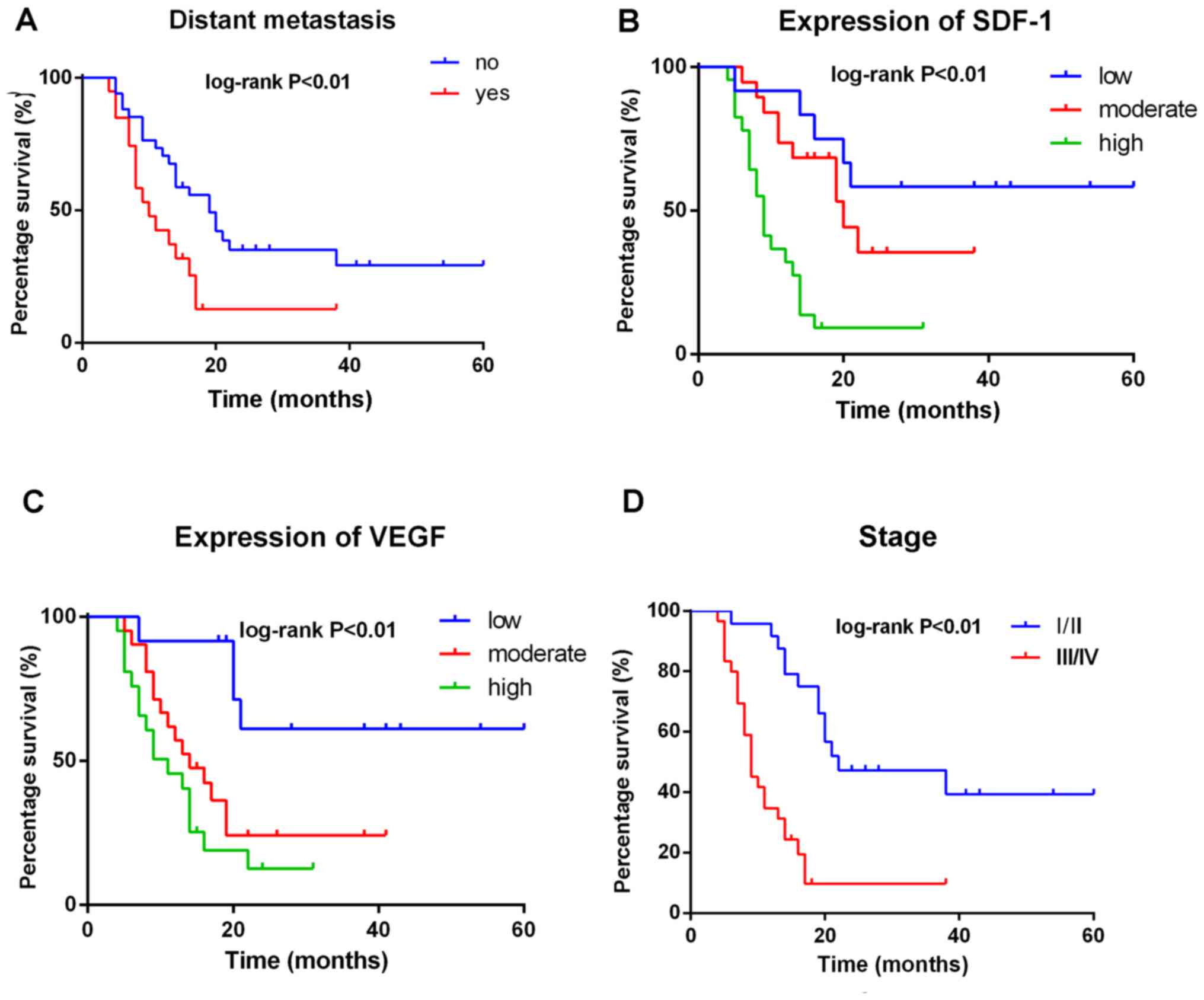
Univariate Cox proportional hazard analyzes for OS
are summarized in Table III and
Kaplan-Meier curves are presented in Fig. 3. Sex, age, tumor size and
histological grade were not significant in predicting OS. However,
distant metastasis (P<0.01) and higher AJCC staging (P<0.01)
predicted shorter OS (Fig. 3). In
addition, univariate analysis revealed that SDF-1 (P<0.05) and
VEGF (P<0.01) expression were significantly associated with
shorter OS (Table III; Fig. 3).
 | Table III.Univariate Cox proportional
regression analysis of clinicopathological factors associated with
OS. |
Table III.
Univariate Cox proportional
regression analysis of clinicopathological factors associated with
OS.
|
|
| OS |
|---|
|
|
|
|
|---|
|
Characteristics |
| HR | 95% CI | P-value |
|---|
| Sex |
|
|
|
|
|
Female | 23 | 1 | – | – |
|
Male | 31 | 1.543 | 0.810–2.936 | 0.187 |
| Age (years) |
|
|
|
|
|
≥30 | 25 | 1 | – | – |
|
<30 | 29 | 1.062 | 0.569–1.982 | 0.851 |
| Tumor size
(cm) |
|
|
|
|
|
<5 | 32 | 1 | – | – |
| ≥5 | 22 | 0.924 | 0.492–1.736 | 0.806 |
| Histological
grade |
|
|
|
|
| I | 12 | 1 | – | 0.251 |
| II | 20 | 1.105 | 0.471–2.589 | 0.819 |
|
III | 22 | 1.802 | 0.802–4.048 | 0.154 |
| Distant
metastasisa |
|
|
|
|
| No | 56 | 1 | – | – |
|
Yes | 33 | 2.452 | 1.247–4.820 | 0.009 |
| AJCC
staginga |
|
|
|
|
| I | 9 | 1 | – | <0.001 |
| II | 16 | 3.517 | 1.013–12.210 | 0.048 |
|
III | 11 | 22.10 | 5.371–90.908 | <0.001 |
| IV | 18 | 33.33 | 6.599–139.440 | <0.001 |
| SDF-1a |
|
|
|
|
| Low
(−/+) | 11 | 1 | – | <0.001 |
|
Moderate (++) | 19 | 3.165 | 1.042–9.610 | 0.042 |
| High
(+++) | 24 | 18.73 | 5.496–63.880 | <0.001 |
| VEGFa |
|
|
|
|
| Low
(−/+) | 12 | 1 | – | <0.001 |
|
Moderate (++) | 20 | 3.305 | 1.265–8.632 | 0.015 |
| High
(+++) | 22 | 4.826 | 1.825–12.760 | 0.002 |
SDF-1 expression is an independent
prognostic factor for poor overall survival of SS patients
Univariate factors associated with OS were
identified by a multivariate Cox proportional hazard (Table IV). Distant metastasis [P=0.017,
HR=0.185 (0.046–0.737)], AJCC staging [P=0.04, HR=3.680
(1.519–8.914)] and SDF-1 expression [P=0.026, HR=2.640
(1.124–6.200)] were independent prognostic factors for OS.
Furthermore, SS patients with higher SDF-1 expression exhibited a
significantly greater risk of mortality (Plog-rank
<0.01) than patients with lower SDF-1 expression (Fig. 3). Therefore, the expression of SDF-1
appears to be a potentially significant clinical prognostic factor
in patients with SS.
 | Table IV.Multivariate Cox regression analysis
of clinicopathological factors associated with OS. |
Table IV.
Multivariate Cox regression analysis
of clinicopathological factors associated with OS.
|
| OS |
|---|
|
|
|
|---|
| Factors | HR | 95% CI | P-value |
|---|
| AJCC
staginga | 3.680 | 1.519–8.914 | 0.004 |
| Distant
metastasisa | 0.185 | 0.046–0.737 | 0.017 |
| SDF-1a | 2.640 | 1.124–6.200 | 0.026 |
Discussion
To the best of our knowledge, this is the first
study to demonstrate that levels of SDF-1 and VEGF expression are
correlated with the occurrence of SS. In addition, it was
determined that high SDF-1 and VEGF expression was significantly
associated with unfavorable clinical variables. Furthermore, SDF-1
expression was positively correlated with VEGF expression, and
SDF-1 expression alone was sufficient to be an independent
prognostic indicator of OS in multivariate Cox regression analysis.
Overall, the results of the present study demonstrate that both
SDF-1 and VEGF expression may be significant prognostic factors for
SS and result in unfavorable clinical outcomes in patients.
SDF-1 is one of the CXCs family chemokines and is
important in chemotaxis, stem cell homing, self-renewal and
differentiation, hematopoiesis and wound healing (3,12,22–24).
Tumor cells are capable of overexpressing chemokine receptors and
chemokines may be important in cancer progression and
organ-selective metastasis (14,25). It
has been hypothesized that disseminated tumor cells expressing
chemokine receptors can invade the circulation and are subsequently
attracted and arrested by their corresponding ligands. The
local/original and specific metastatic sites initiate an
inflammatory response in nearby tissues, resulting in the
expression of additional chemokines (26). These chemokines are then able to
induce the procession, recurrence, and migration of tumor cells. A
number of studies have demonstrated that SDF-1 is important in
various types of cancers, including prostate, ovarian and breast
tumors (12,13,27). The
observations of the present study are consistent with those from
previous studies and confirm that SDF-1 expression is associated
with poor clinical outcomes.
In original and/or metastatic tumor sites,
reconstruction of local microvascular environment is one of the
most important steps facilitating the survival of new mitotic tumor
cells (28). It is thought that
SDF-1 may upregulate the expression of VEGF via the SDF-1/CXCR-4
(chemokine receptor-4, the specific receptor of SDF-1) pathway
(29). Furthermore, overexpression
of VEGF in tumor cells may lead to aggressive tumorigenesis and
distant metastasis, resulting in poor clinical outcomes (30,31). The
present study revealed that there is a significant association
between the levels of SDF-1 and VEGF expression in SS. A strong
association between levels of SDF-1 and VEGF expression and lower
histological grade, higher stage, increased distant metastasis and
poor prognosis in patients with SS was also identified. To the best
of our knowledge, these results provide the first evidence that
SDF-1 and VEGF are involved in SS, which is consistent with
previous studies (32). Therefore,
SDF-1 is not only a potential prognostic marker, but also a novel
target for therapeutic intervention in patients with SS. However,
the exact role of SDF-1 and VEGF in SS has not been fully
elucidated, and additional in vivo and in vitro
investigations of the molecular mechanisms are required.
There were a number of limitations in the present
study. For example, immunohistochemistry and immunofluorescence
were semi-quantitative and not as accurate as reverse
transcription-quantitative polymerase chain reaction or western
blot analysis would have been. Therefore, some bias may have been
introduced. However, the samples were analyzed in a blinded fashion
by three pathologists, and a consensus was reached by discussion if
disagreements occurred. The sample size of the present study was
also relatively small, meaning that the results would need to be
confirmed in a larger population.
In conclusion, a significant proportion of patients
with SS exhibited high expression of SDF-1 and VEGF. Expression of
SDF-1 and VEGF was associated with unfavorable clinical
characteristics and poor prognosis of SS patients. Although the
role of SDF-1 and VEGF in SS remains unclear, SDF-1 appears to be a
significant potential clinical prognostic factor in patients with
SS.
References
|
1
|
Nielsen TO, Poulin NM and Ladanyi M:
Synovial sarcoma: Recent discoveries as a roadmap to new avenues
for therapy. Cancer Discov. 5:124–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spillane AJ, A'Hern R, Judson IR, Fisher C
and Thomas JM: Synovial sarcoma: A clinicopathologic, staging, and
prognostic assessment. J Clin Oncol. 18:3794–3803. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wunder JS, Nielsen TO, Maki RG, O'Sullivan
B and Alman BA: Opportunities for improving the therapeutic ratio
for patients with sarcoma. Lancet Oncol. 8:513–524. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thway K and Fisher C: Synovial sarcoma:
Defining features and diagnostic evolution. Ann Diagn Pathol.
18:369–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palmerini E, Paioli A and Ferrari S:
Emerging therapeutic targets for synovial sarcoma. Expert Rev
Anticancer Ther. 14:791–806. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murphy PM: Chemokines and the molecular
basis of cancer metastasis. N Engl J Med. 345:833–835. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuil J, Buckle T and van Leeuwen FW:
Imaging agents for the chemokine receptor 4 (CXCR4). Chem Soc Rev.
41:5239–5261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruiz de Almodovar C, Luttun A and
Carmeliet P: An SDF-1 trap for myeloid cells stimulates
angiogenesis. Cell. 124:18–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kiel MJ and Morrison SJ: Maintaining
hematopoietic stem cells in the vascular niche. Immunity.
25:862–864. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ratajczak MZ, Kim CH, Abdel-Latif A,
Schneider G, Kucia M, Morris AJ, Laughlin MJ and Ratajczak J: A
novel perspective on stem cell homing and mobilization: Review on
bioactive lipids as potent chemoattractants and cationic peptides
as underappreciated modulators of responsiveness to SDF-1
gradients. Leukemia. 26:63–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klein RS and Rubin JB: Immune and nervous
system CXCL12 and CXCR4: Parallel roles in patterning and
plasticity. Trends Immunol. 25:306–314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Epstein RJ: The CXCL12-CXCR4 chemotactic
pathway as a target of adjuvant breast cancer therapies. Nat Rev
Cancer. 4:901–909. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burger JA and Kipps TJ: CXCR4: A key
receptor in the crosstalk between tumor cells and their
microenvironment. Blood. 107:1761–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12/CXCR4/CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao C, Li P, Song H, Song F, Qu Y, Ma X,
Shi R and Wu J: CXCL12/CXCR4 axis upregulates twist to induce EMT
in human glioblastoma. Mol Neurobiol. 53:3948–3953. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang P, Wang G, Huo H, Li Q, Zhao Y and
Liu Y: SDF-1/CXCR4 signaling up-regulates survivin to regulate
human sacral chondrosarcoma cell cycle and epithelial-mesenchymal
transition via ERK and PI3K/AKT pathway. Med Oncol. 32:3772015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Gou X, Kong DK, Wang X, Wang J,
Chen Z, Huang C and Zhou J: EMMPRIN regulates tumor growth and
metastasis by recruiting bone marrow-derived cells through
paracrine signaling of SDF-1 and VEGF. Oncotarget. 6:32575–32585.
2015.PubMed/NCBI
|
|
18
|
Hassan S, Buchanan M, Jahan K,
Aguilar-Mahecha A, Gaboury L, Muller WJ, Alsawafi Y, Mourskaia AA,
Siegel PM, Salvucci O and Basik M: CXCR4 peptide antagonist
inhibits primary breast tumor growth, metastasis and enhances the
efficacy of anti-VEGF treatment or docetaxel in a transgenic mouse
model. Int J Cancer. 129:225–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eswarappa SM and Fox PL: Antiangiogenic
VEGF-Ax: A new participant in tumor angiogenesis. Cancer Res.
75:2765–2769. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sápi Z: Pathology of soft tissue sarcomas.
Magy Onkol. 58:11–23. 2014.(In Hungarian). PubMed/NCBI
|
|
21
|
Kneisl JS, Coleman MM and Raut CP:
Outcomes in the management of adult soft tissue sarcomas. J Surg
Oncol. 110:527–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sánchez-Martín L, Sánchez-Mateos P and
Cabañas C: CXCR7 impact on CXCL12 biology and disease. Trends Mol
Med. 19:12–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Woodard LE and Nimmagadda S: CXCR4-based
imaging agents. J Nucl Med. 52:1665–1669. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barbieri F, Thellung S, Würth R, Gatto F,
Corsaro A, Villa V, Nizzari M, Albertelli M, Ferone D and Florio T:
Emerging targets in pituitary adenomas: Role of the CXCL12/CXCR4-R7
system. Int J Endocrinol. 2014:7535242014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chatterjee S, Behnam Azad B and Nimmagadda
S: The intricate role of CXCR4 in cancer. Adv Cancer Res.
124:31–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao AW, Jiang TH, Sun XJ and Peng J:
Application of chemokine receptor antagonist with stents reduces
local inflammation and suppresses cancer growth. Tumour Biol.
36:8637–8643. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee HJ and Jo DY: The role of the
CXCR4/CXCL12 axis and its clinical implications in gastric cancer.
Histol Histopathol. 27:1155–1161. 2012.PubMed/NCBI
|
|
28
|
García-Román J and Zentella-Dehesa A:
Vascular permeability changes involved in tumor metastasis. Cancer
Lett. 335:259–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kampmann E, Altendorf-Hofmann A, Gibis S,
Lindner LH, Issels R, Kirchner T and Knösel T: VEGFR2 predicts
decreased patients survival in soft tissue sarcomas. Pathol Res
Pract. 211:726–730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ben-Baruch A: The multifaceted roles of
chemokines in malignancy. Cancer Metastasis Rev. 25:357–371. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawauchi S, Fukuda T and Tsuneyoshi M:
Angiogenesis does not correlate with prognosis or expression of
vascular endothelial growth factor in synovial sarcomas. Oncol Rep.
6:959–964. 1999.PubMed/NCBI
|