Introduction
Spontaneous hemopneumothorax refers to an
accumulation of >400 ml blood in the pleural cavity without
obvious trauma or other causes. Spontaneous hemopneumothorax is a
rare, potentially life-threatening condition occurring
predominantly in adolescents and adults, with a prevalence ratio of
0.0025%, or 1–12% of all spontaneous pneumothoraces (1). Bleeding at the pleural adhesions is a
major reason for spontaneous hemopneumothorax (2–4), which
can lead to mortality due to acute respiratory and circulatory
dysfunction if the condition becomes aggravated. This condition is
a rare thoracic emergency in the clinical practice, and thus has a
high rate of missed diagnosis (5).
Non-surgical therapy of bleeding at the adhesion bands of the
pleura is not effective and results in relapse; thus, active
surgical treatment is the best therapy available (6,7).
Percutaneous coronary intervention (PCI) for
coronary revascularization has been an extensively used medical
therapy for chronic and acute coronary artery disease (8). More than 50,000 PCI procedures are
performed annually in China (9).
Spontaneous hemopneumothorax is not listed as one of the
complications observed during PCI. Probably due to the low
incidence of spontaneous hemopneumothorax, the occurrence of
spontaneous hemopneumothorax during the PCI therapy is rarely
reported (9).
The present study reports a case of bleeding at
multiple pleural adhesion bands following percutaneous coronary
intervention, causing severe hemothorax. The reason behind
hemothorax occurrence in such cases can be easily neglected, thus
the present study suggests that this condition should be carefully
considered by the clinicians in order to avoid misdiagnosis. The
study was approval by the Ethics Committee of the Institute of
Field Surgery, Daping Hospital of the Third Military Medical
University (Chongqing, China). Written informed consent was
obtained from the patient prior to inclusion in the current
study.
Case report
A 76-year-old male patient was referred to Daping
Hospital due to 3 years of exertional chest distress, accompanied
by chest pain and slight cough that persisted for 1 week. The
patient complained of recurring precordial chest pain resulting
from exhaustion 3 years earlier, which lasted several minutes each
time, but was relieved after rest. The patient did not seek
treatment at that time. Precordial chest pain was not accompanied
by headache, dizziness, cough, sputum, abdominal pain, bloating,
chills or fever. The chest pain occurred repeatedly since the
initial onset, and the patient visited a local hospital three
months prior to the present study, where he received an
electrocardiographic examination indicating myocardial ischemia.
The disease was preliminarily diagnosed as coronary heart disease.
The rest of the examination and treatment details are unknown.
The week before admission, the patient developed a
mild cough and evidently aggravated exertional chest pain that
lasted for >10 min. In order to obtain further diagnosis and
treatment, the patient visited Daping Hospital in July 2011, and
was admitted to the Department of Cardiovascular Medicine as a
patient with coronary heart disease. The patient reported having
suffered from a cold 1 week earlier, low mood, loss of appetite and
sleep, but had normal stool and urine. The patient had a 10-year
history of chronic bronchitis and chronic obstructive emphysema,
15-year history of hypertension and 40-year smoking history (20
cigarettes/day). He had never received systematic blood pressure
monitoring or antihypertensive therapy. Physical examination
demonstrated a good general state of health with a body temperature
of 36.2°C, a pulse rate of 75 bpm, a respiratory rate of 18 bpm,
and blood pressure of 124/72 mmHg, with normal head and face
features. Jugular vein distention was not detected and the thyroid
gland was not enlarged. Weakened breathing movement of both lungs,
widened intercostal space, reduced tactile fremitus and
hyperresonant percussion note were observed. The breath sounds of
the two lungs were diminished, but no dry or moist rales were
detected. Heart auscultation revealed regular rhythm, normal heart
sound without murmur and a heart rate of 75 bpm. However, narrowed
cardiac dullness was found. Liver and spleen were normal, and no
swelling was observed in the lower extremities.
Following hospitalization, electrocardiography (ECG)
results indicated a sinus rhythm with a heart rate of 75 bpm and a
slightly-depressed ST segment on lead V2-6 of ECG with inverted T
wave. Echocardiography demonstrated minor effusion in the heart
sac, some reflux across aortic valves, and reduction in the left
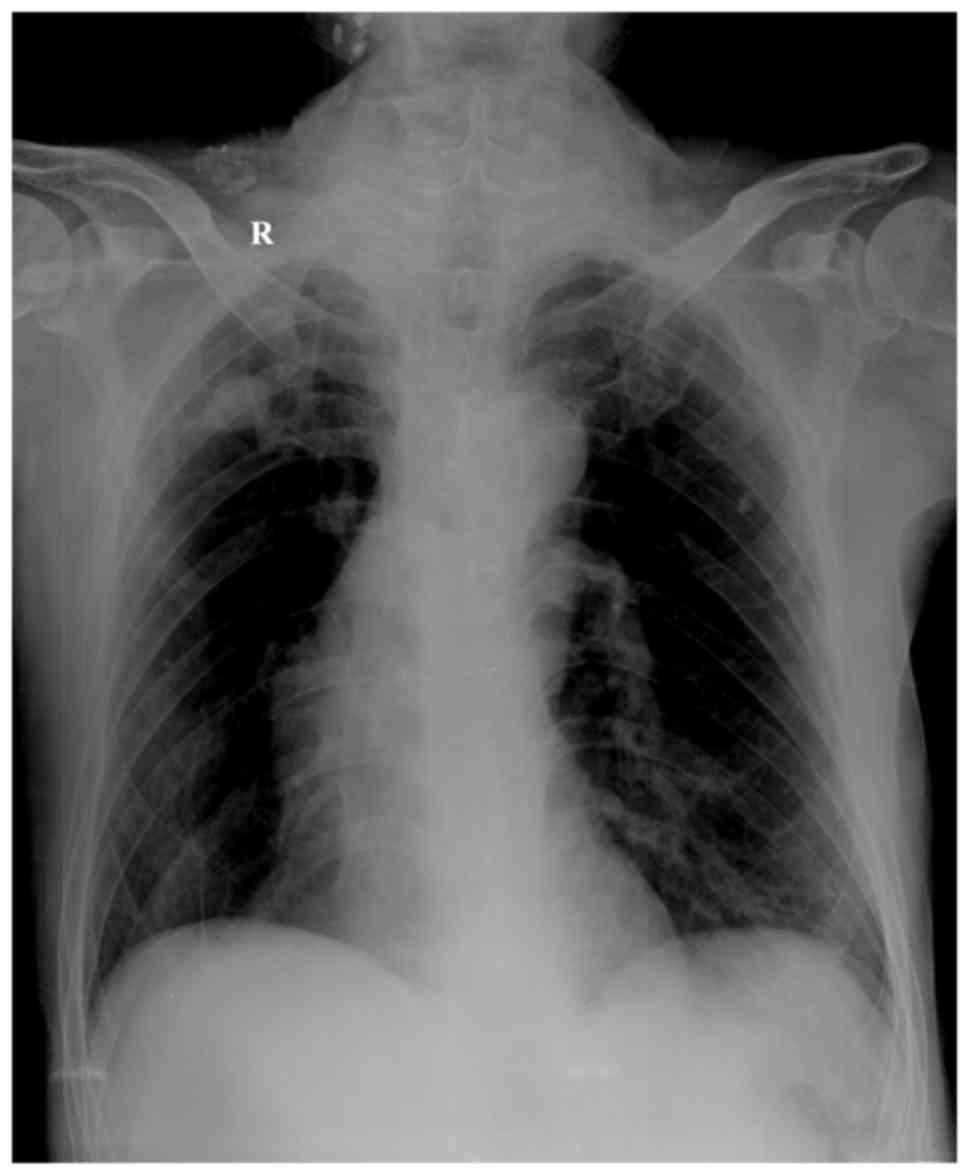
ventricular diastolic function. Chest X-ray examination revealed
increased texture and light transmittance in both lungs, dense
nodule shadows in bilateral upper lungs, a long and narrow heart
shadow, as well as tortuous and widened aortic arch with arc
calcification at its edge (Fig. 1).
The bilateral diaphragm was at a lower level than the chest, the
intercostal space was widened, and the costophrenic angle was
sharp. All these observations suggested chronic bronchitis and
chronic obstructive emphysema (Fig.
1). No other abnormalities were present in other examinations,
including routine blood, urine and fecal samples, liver and kidney
function, blood electrolyte levels, blood glucose, blood lipids,
coagulogram, myocardial damage markers, thyroid gland function and
abdominal B-mode ultrasound. The patient was preliminarily
diagnosed with coronary heart disease, unstable angina and cardiac
function of grade 2 (NYHA II) (10),
hypertension of grade 3 (11),
chronic non-obstructive bronchitis (acute phase) and chronic
obstructive emphysema. The patient was administered
low-molecular-weight heparin (enoxaparin sodium, 0.4 ml i.p.,
once/12 h), rosuvastatin calcium tablets (10 mg, once/day),
clopidogrel hydrogen sulfate (75 mg, once/day), aspirin (100 mg,
once/day), intravenous infusion with nitroglycerin, and other
relevant therapies.
Due to persistent and frequent chest tightness,
coronary angiography through the right radial artery was performed
on the day of hospitalization after the patient was administered
300 mg clopidogrel hydrogen sulfate and 300 mg aspirin. Angiography
revealed a dominant right coronary artery, intimal flap at the left
main coronary artery, 30–80% stenosis of the left main coronary
artery opening to the middle anterior descending branch, and nearly
total occlusion of the distal segment of the anterior descending
branch and the third diagonal branch. In addition, ~90% proximal
circumflex artery stenosis was observed with a TIMI grade 3, as
well as ~30% right coronary opening stenosis and ~80% distal right
coronary stenosis. Following a balloon angioplasty, the coronary
artery stenosis was eliminated following the implantation of two
Maverick stents in the anterior descending branch, one in the
circumflex artery and another in the right coronary artery. During
surgery, the patient experienced two episodes of severe cough,
without any other discomfort. Percutaneous coronary intervention
(PCI) was conducted within ~2 h, and then the patient was returned
to the critical care unit. Following surgery, the aforementioned
medications were continued.
At 5 h after the surgery, the patient suddenly
presented symptoms of palpitations, shortness of breath, dizziness,
sweating, clammy and pale skin, tachypnea, narrowed pulse beating
at a faster speed, reduced blood pressure (65–90/35–58 mmHg), left
shift of the trachea, decreased right chest breathing mobility,
further widened intercostal space, tactile fremitus disappearance,
percussive flatness, and disappearance of lower right lung breath
sounds during auscultation. The symptoms of the left chest were
unchanged. The suspected diagnosis was massive hemothorax in the
right thoracic cavity and hemorrhagic shock. Thus, anticoagulant
and antiplatelet therapies were immediately terminated, and the
patient was subjected to blood transfusion and was administered
dopamine to increase the blood pressure. Blood examination
suggested that hemoglobin level was decreased from the preoperative
level of 150 g/l to a postoperative level of 65 g/l (normal range
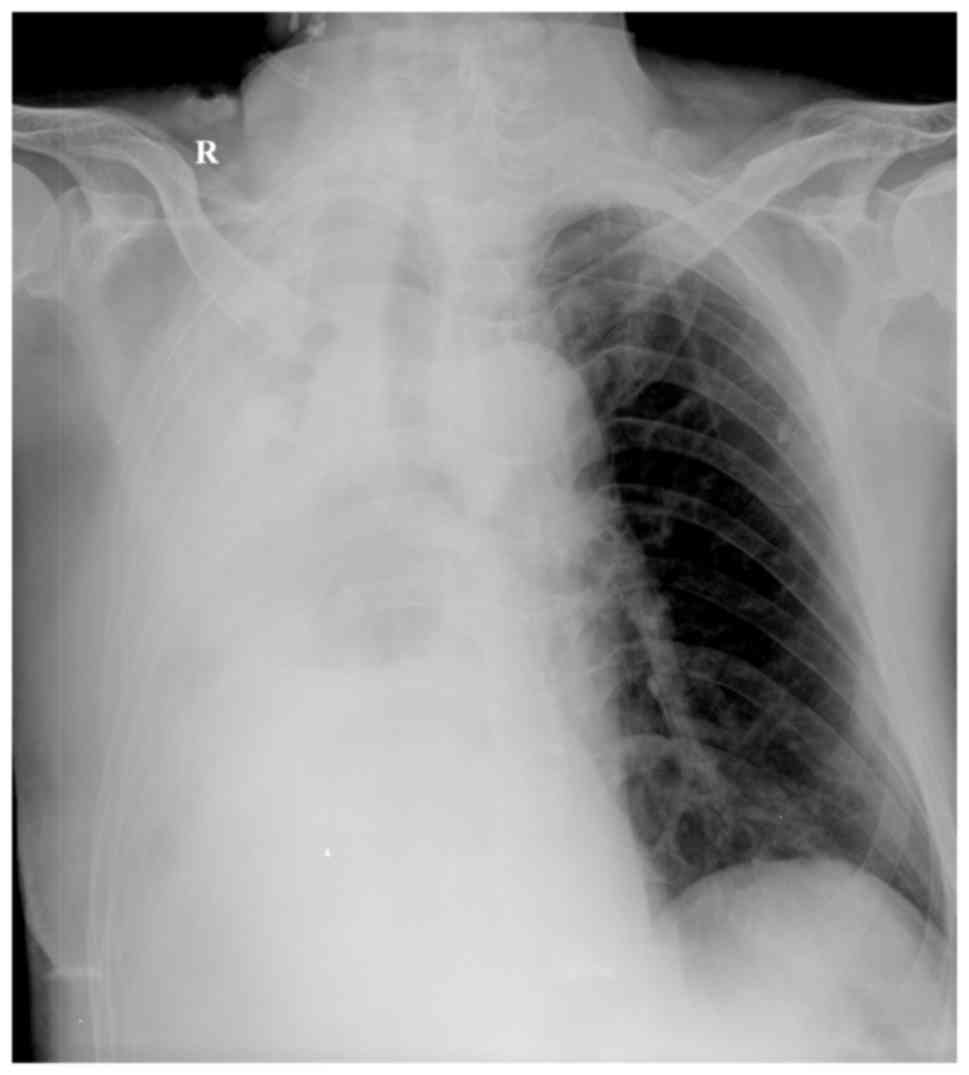
115–156 g/l). A chest X-ray examination suggested that the right
thoracic cavity of the patient had a large number of effusions and
the mediastinum was broader compared with that on admission
(Fig. 2). Ultrasound examination
indicated low effusion in the heart sac (as observed earlier), and
high effusion and blood clotting (verified as bright red and
non-condensing blood through puncture) in the right thoracic
cavity. The reason of hemothorax was suspected to be one of the
following: i) Vascular injury in the right subclavian artery and
brachiocephalic artery resulting in openings to the thoracic cavity
and bleeding into the right thoracic cavity; ii) bleeding through
the perforated coronary artery into the pericardial cavity, the
impaired epicardium and finally the right thorax; iii) aortic
dissection leading to bleeding into the right thoracic cavity. Due
to the continuous decrease in blood pressure, the loss of
consciousness and 70–80% oxygen saturation with mask inhalation of
oxygen, norepinephrine (1 µg/kg/min) was administered to maintain
the blood pressure (approximately 80/40 mmHg) and artificial
assisted respiration with tracheal intubation was applied.
Simultaneously, an emergency diagnosis by angiography of the
coronary artery, aorta, right subclavian artery and truncus
brachiocephalicus was conducted once again. Following careful
examination, no artery injury, puncture or aorta dissection was
observed. Considering his chronic obstructive pulmonary disease
history and intense coughs during the surgery, it was suspected
that the hemothorax was caused by rupture of the pleural blood
vessels. Therefore, the patient was urgently subjected to right
chest examination with a video-assisted thoracoscope after thoracic
investigation. Approximately 3,000 ml blood was observed in the
right thoracic cavity, where a number of blood clots existed, and
the right lung was found to be compressed. After removal of the
blood and clots, tearing of the pleural adhesions, with band sizes
of 2.1×2.0, 3.5×2.9 and 1.8×2.5 cm was observed at the top of the
right chest, right upper chest and right upper mediastinum,
respectively. Hemorrhage was also evident in certain areas. Thus,
pleural adhesion band solidification and suture hemostasis were
performed by an open chest surgery and adhesion band lysis.
Subsequent to treatments including intravenous
dopamine/dobutamine administration and continuous blood
transfusion, increase of blood pressure, prevention of infection,
cough relief and closed thoracic drainage, the patient recovered to
a normal state 2–3 days following the thoracic surgery, with a
blood pressure of 110–130/60–70 mmHg and hemoglobin levels of
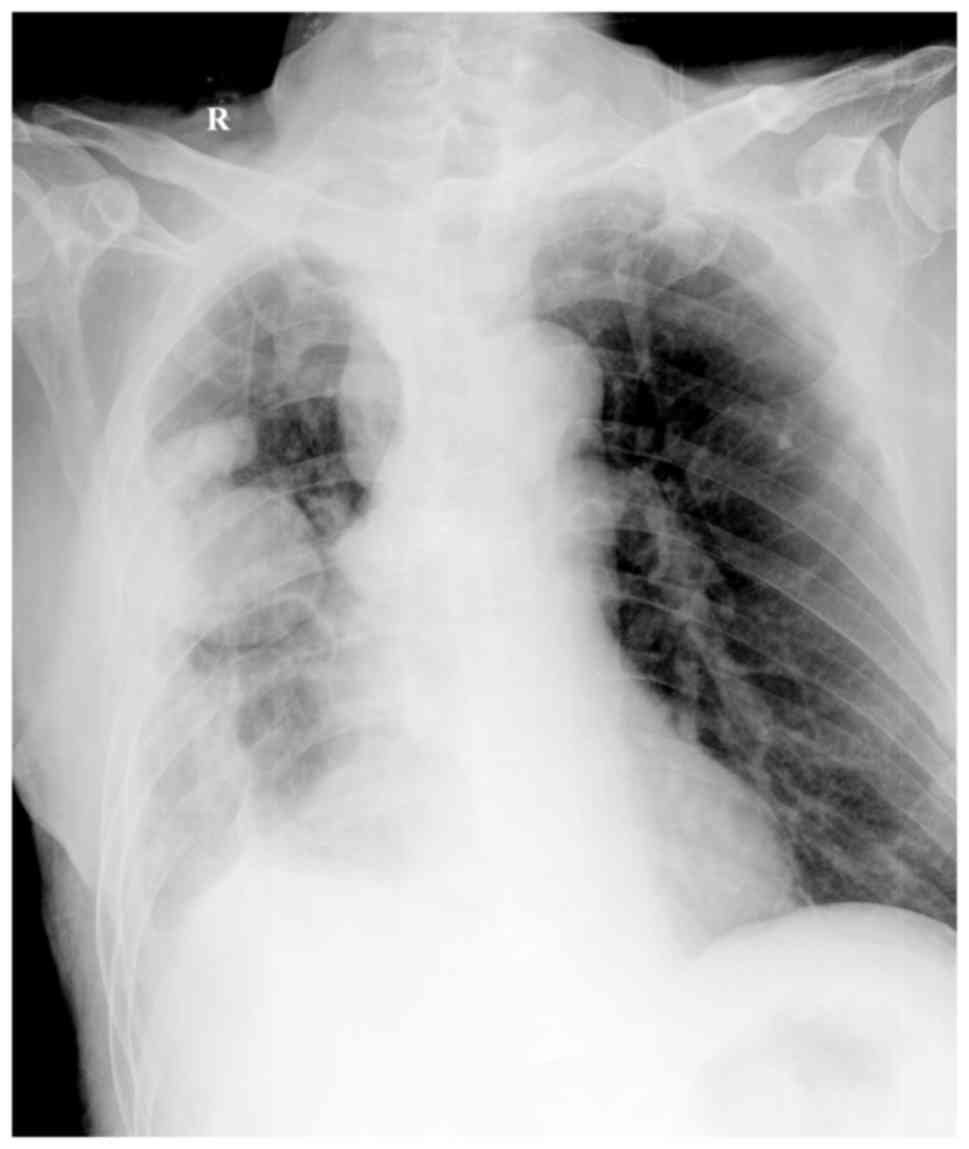
130–150 g/l. A chest ultrasound revealed encapsulated effusion,
while the volume of fluid did not increase after thoracic puncture.
However, encapsulated effusion and pleural adhesion were still
observed in the right chest in an X-ray scan (Fig. 3). At 5 days after surgery, the
patient was treated with low-molecular-weight heparin (enoxaparin
sodium, 0.4 ml, subcutaneous injection, once/12 h), clopidogrel
hydrogen sulfate (75 mg once/day) and aspirin (100 mg once/day). No
further bleeding in relation to the disease was developed. The
patient was discharged from the hospital on day 12 after the
surgery. During the nearly 1 year of follow-up, the patient was
maintained in a normal condition, without severe discomfort, and no
hemothorax was detected by ultrasound. Pleural adhesion was
detected but no encapsulated effusion was observed.
Discussion
PCI is an effective therapy for treating coronary
artery disease, and hemothorax during PCI is rare (12). The possible causes of hemothorax are
vascular injuries along the catheter passage (subclavian artery,
brachiocephalic artery, and connected to the thoracic cavity),
coronary artery perforation resulting in bleeding into the
pericardial cavity and then into the thoracic cavity through the
injured epicardium, or spontaneous hemorrhage induced by poor blood
coagulation (6). By comprehensively
analyzing the conditions, spontaneous hemorrhage resulting from
poor blood coagulation was excluded in the present case. In
addition, the injury of blood vessels (including coronary artery)
was also excluded through an angiogram examination. It was
eventually verified by an open chest surgery that the complication
was caused by multiple-site bleeding at the adhesion bands of the
pleura.
Spontaneous and progressive hemothorax is rare in
the clinical practice, and is predominantly observed in
20–40-year-old males (13). The
occurrence ratio of spontaneous and progressive hemothorax is ~25:1
for males:females (7), this may
partly ascribe to tobacco smoking among young males that induces
the release and formation of inflammatory factors (7). Spontaneous and progressive hemothorax
seldom appears in the elderly, and early diagnosis is difficult.
Bleeding at the adhesion band of the pleura due to laceration is a
cause of spontaneous and progressive hemothorax (2–4).
However, the patient in the present study experienced multiple-site
bleeding at the adhesion bands of the apex of the right lung, which
is very rare. The pleural adhesion band is a localized adhesion
occurring between the visceral pleura and the parietal pleura
following pleural inflammation (14). The unbalance of the ventilation/blood
flow ratio due to the special anatomic structure of the lung apex
and easily lead to inflammation in this region and to pleural
adhesions when the immune system is weakened (15). It is uncommon for pleural adhesions
to be torn or cause hemothorax. Nevertheless, the lung may
instantly change from overexpansion to rapid retraction due to
abrupt alterations in intrathoracic pressure after coughing,
sneezing, breath-holding, deep breathing or sudden changes in body
posture. Under such occasions, the adhesion band can be torn or
broken by outbursts of tractive force and twisting force, resulting
in bleeding. The range of motion of the diaphragm may serve an
important role in the onset of this condition.
Pleural pressure is negative due to a suction effect
caused by lung recoil. Negative pressure within the pleural cavity
may cause bleeding in the pleura, which is difficult to be stopped
by normal hemostasis, and thus a large amount and long duration of
bleeding may be observed, causing severe bleeding and shock
(14,16). Due to quick bleeding,
defibrinogenation is not complete and coagulation occurs. Blood
clots are frequently formed at the early stages in up to 84% of
patients (2). In the present study,
the 76-year-old patient suffered from massive hemorrhage in the
chest soon after PCI, thus, pleural hemorrhage was initially
suggested to be associated with surgical injury prior to further
angiography examination. Eventually, it was observed that pleural
bleeding was due to the tear of pleural adhesion bands. A
reasonable explanation for the occurrence of pleural bleeding can
be provided, considering the patient's chronic obstructive
pulmonary disease history and that the angina pectoris of coronary
artery disease occurred with cough. More specifically, coughing
possibly resulted in the laceration of preexisting adhesion bands,
and the bleeding continued at a faster pace due to the lack of
vascular smooth muscle and thus contraction of blood vessels in the
pleura, as well as due to high blood pressure at the top of the
parietal layer of the thoracic cavity as part of the systemic
circulation, and the effect of negative pressure in the thoracic
cavity. Massive hemothorax was further promoted by the use of
large-dose anticoagulant and antiplatelet agents.
The case reported in the present study suggests
that, in clinical practice, it should be considered that the
bleeding may be caused by laceration of preexisting pleural
adhesion bands, following exclusion of other common reasons for
hemothorax. Early diagnosis of this condition may be established
according to the following features: i) hemothorax occurring
suddenly in a healthy young patients; ii) forceful chest movements
and strenuous exercise prior to onset; iii) progressive bleeding
and shock; iv) physical signs of effusion on one side of the
thoracic cavity with corresponding X-ray features; and v) blood
sampling through thoracentesis.
For non-progressively aggravated spontaneous
hemothorax in which the bleeding is slow and at a small amount,
conservative treatments can be applied in patients with light
compression of lung tissue, good general condition, unchanged
breathing, pulse and blood pressure. These treatments include rest,
oxygen inhalation, and prevention of infection, blood volume
supplement and application of hemostatic drugs. If necessary,
thoracentesis or placement of a thoracic closed drainage tube can
be used, which not only helps the reengagement of previously
compressed lungs, but also stops the bleeding and provides a method
to observe whether progressive bleeding appears in the thoracic
cavity (17,18). With a growing number of patients
receiving PCI therapy, the rare but potentially fatal complications
should receive worthy attention during the perioperative period.
Chest contrast-enhanced computed tomography (CT) to examine the
leural adhesions may help to improve the evaluation of the risk of
hemothorax. The use of antitussive drugs for the patients with
heart disease, especially the elderly, prior to PCI may reduce the
occurrence of the severe cough and thereby avoiding its caused
hemothorax during the therapy. Recently, electrical hemostasis,
suture at the bleeding point and removal of blood clots in the
thoracic cavity with video-assisted thoracoscopic surgery have been
observed to have certain advantages, including reduced trauma, good
therapeutic effect, quick recovery and easily accepted by the
patient (19). However, special and
expensive equipment with demanding skills are required for such
treatment, thus it is difficult to perform these procedures at
local hospitals. Patients presenting shock or progressively
aggravated bleeding, or when no lung expansion after thoracentesis
or closed drainage of the thoracic cavity is observed in a patient
with or without suspected active bleeding, then surgery should be
performed as soon as possible. In fact, the majority of such
patients eventually require surgical intervention (20–22).
In conclusion, spontaneous and progressive
hemothorax caused by bleeding at the adhesion band of the pleura is
a life-threatening complication during the perioperative period of
PCI. The current study reported the case of an elderly male patient
with coronary heart disease who presented multiple-site bleeding at
pleural adhesions following PCI. Patients with a history of lung
disease are at a higher risk of hemothorax. Early diagnosis and
effective treatment including surgical intervention may
significantly improve the prognosis for these patients.
References
|
1
|
Hentel K, Brill PW and Winchester P:
Spontaneous hemopneumothorax. Pediatr Radiol. 32:457–459. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsu NY, Hsieh MJ, Liu HP, Kao CL, Chang
JP, Lin PJ and Chang CH: Video-assisted thoracoscopic surgery for
spontaneous hemopneumothorax. World J Surg. 22:23–27. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Homma T, Sugiyama S, Kotoh K, Doki Y,
Tsuda M and Misaki T: Early surgery for treatment of spontaneous
hemopneumothorax. Scand J Surg. 98:160–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohmori K, Ohata M, Narata M, Iida M,
Nakaoka Y, Irako M, Kitamura K, Nakamura S, Natori H and Sezaki Y:
28 cases of spontaneous hemopneumothorax. Nihon Kyobu Geka Gakkai
Zasshi. 36:1059–1064. 1998.(In Japanese).
|
|
5
|
Tulay CM and Aygün M: Emergency surgery
for spontaneous hemopneumothorax. J Coll Physicians Surg Pak.
24:435–447. 2014.PubMed/NCBI
|
|
6
|
Levine GN, Bates ER, Blankenship JC,
Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA,
Hollenberg SM, et al: 2011 ACCF/AHA/SCAI Guideline for Percutaneous
Coronary Intervention: A report of the American College of
Cardiology Foundation/American Heart association task force on
practice guidelines and the society for cardiovascular angiography
and interventions. J Am Coll Cardiol. 58:e44–e122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Onuki T, Goto Y, Kuramochi M, Inagaki M
and Sato Y: Spontaneous hemopneumothorax: Epidemiological details
and clinical features. Surg Today. 44:2022–2027. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel MR, Calhoon JH, Dehmer GJ, Grantham
JA, Maddox TM, Maron DJ and Smith PK:
ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate use criteria
for coronary revascularization in patients with stable ischemic
heart disease: A report of the American College of Cardiology
Appropriate use criteria task force, American Association for
Thoracic Surgery, American Heart Association, American Society of
Echocardiography, American society of nuclear cardiology, society
for cardiovascular angiography and interventions, society of
cardiovascular computed tomography, and society of thoracic
surgeons. J Am Coll Cardiol. 69:2212–2241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng X, Curtis JP, Hu S, Wang Y, Yang Y,
Masoudi FA, Spertus JA, Li X, Li J, Dharmarajan K, et al: Coronary
catheterization and percutaneous coronary intervention in China:
10-year results from the China PEACE-retrospective CathPCI study.
JAMA Intern Med. 176:512–521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
St John Sutton M, Ghio S, Plappert T,
Tavazzi L, Scelsi L, Daubert C, Abraham WT, Gold MR, Hassager C,
Herre JM, et al: Cardiac resynchronization induces major structural
and functional reverse remodeling in patients with New York Heart
Association class I/II heart failure. Circulation. 120:1858–1865.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Touyz RM and Dominiczak AF: Hypertension
guidelines: Is it time to reappraise blood pressure thresholds and
targets? Hypertension. 67:688–689. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robinson NM, Thomas MR and Jewitt DE:
Spontaneous haemothorax as a complication of anti-coagulation
following coronary angioplasty. Respir Med. 89:629–630. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Azfar Ali H, Lippmann M, Mundathaje U and
Khaleeq G: Spontaneous hemothorax: A comprehensive review. Chest.
134:1056–1065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim ES, Kang JY, Pyo CH, Jeon EY and Lee
WB: 12-year experience of spontaneous hemopneumothorax. Ann Thorac
Cardiovasc Surg. 14:149–153. 2008.PubMed/NCBI
|
|
15
|
De Smedt A, Vanderlinden E, Demanet C, De
Waele M, Goossens A and Noppen M: Characterisation of pleural
inflammation occurring after primary spontaneous pneumothorax. Eur
Respir J. 23:896–900. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang YT, Dai ZK, Kao EL, Chuang HY, Cheng
YJ, Chou SH and Huang MF: Early video-assisted thoracic surgery for
primary spontaneous hemopneumothorax. World J Surg. 31:19–25. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kakaris S, Athanassiadi K, Vassilikos K
and Skottis I: Spontaneous hemopneumothorax: A rare but
life-threatening entity. Eur J Cardiothorac Surg. 25:856–858. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ng CSh and Yim AP: Spontaneous
hemopneumothorax. Curr Opin Pulm Med. 12:273–277. 2006.PubMed/NCBI
|
|
19
|
Wu YC, Lu MS, Yeh CH, Liu YH, Hsieh MJ, Lu
HI and Liu HP: Justifying video-assisted thoracic surgery for
spontaneous hemopneumothorax. Chest. 122:1844–1847. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Inafuku K, Maehara T, Yamamoto T and
Masuda M: Assessment of spontaneous hemopneumothorax: Indications
for surgery. Asian Cardiovasc Thorac Ann. 23:435–438. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haciibrahimoglu G, Cansever L, Kocaturk
CI, Aydogmus U and Bedirhan MA: Spontaneous hemopneumothorax: Is
conservative treatment enough? Thorac Cardiovasc Surg. 53:240–242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de Perrot M, Deléaval J, Robert J and
Spiliopoulos A: Spontaneous hemopneumothorax-results of
conservative treatment. Swiss Surg. 6:62–4. 2000. View Article : Google Scholar : PubMed/NCBI
|