Introduction
Bronchial asthma is a type of disease with symptoms
of bronchial hyper-responsiveness and airway obstruction. It is
characterized by chronic airway inflammation and bronchial asthma.
A relatively more unified view reveals that asthma is a development
process, in which epithelial cells, fibroblasts, dendritic cells,
eosinophils, mastocytes, T lymphocytes and other inflammatory cells
interact with constituent cells of the airway, and the secreted
inflammatory mediators participate in the development of asthma
(1–3).
According to available data, the prevalence,
mortality and treatment costs of asthma clearly show the rising
trends globally. The prevalence of asthma is increased by
approximately 50% every 10 years (4). Findings have shown that, the T-helper
type 2 (Th2) subgroup in the cluster of differentiation 4
(CD4)+T cells plays a key role in the occurrence of
asthma, and it mediates the occurrence of II inflammatory responses
by secreting a large number of cytokines, thus greatly promoting
the occurrence of asthma (5,6). Interleukin-9 (IL-9) has long been
considered a Th2 cytokine, that plays an important role in the
pathogenesis of asthma, parasitic infection in the body and the
occurrence process of tuberculosis (7,8). Newly
identified Th9 cells are CD4+ T effector cells and are
different from Th2, which are differentiated by the combined
stimulation of transforming growth factor β (TGF-β) and IL-4. They
can secrete IL-9 and IL-10, albeit the former is the main type, and
participate in asthma and parasitic infection-mediated immune
responses such as Th2 cells (9–11).
In the present study, we established a mouse model
of bronchial asthma to observe the roles of Th9 cells and their
cytokine IL-9 in the pathogenesis of bronchial asthma.
Materials and methods
Reagents and instruments
Thirty female-specific pathogen-free (SPF) Bagg'
albino (BALB)/c mice weighing 20.65±2.35 g on average were included
in the present study. Ovalbumin (OVA) was purchased from Sigma (St.
Louis, MO, USA); aluminum hydroxide gel was purchased from Pierce
(Rockford, IL, USA); IL-9 enzyme-linked immunosorbent assay (ELISA)
kits were purchased from Boster Biological Technology Co., Ltd.
(Wuhan, China); Roswell Park Memorial Institute (RPMI)-1640 medium
was purchased from Gibco (Grand Island, NY, USA); fluorescein
isothiocyanate (FITC)-labeled anti-human CD4 monoclonal antibodies,
phycoerythrin (PE)-labeled anti-human IL-9 monoclonal antibodies
and 2-SYBR® Green Real-time Polymerase Chain Reaction
(PCR) Master Mixes were purchased from Vazyme Biotech Co., Ltd.
(Nanjing, China). β-actin mouse monoclonal antibodies and goat
anti-mouse horseradish peroxidase (HRP)-labeled secondary
antibodies were purchased from Zhongshan Golden Bridge Biological
Technology Co., Ltd. (Beijing, China); phorbol 12-myristate
13-acetate (PMA), ionomycin and monensin were purchased from Sigma.
The microplate reader was purchased from Thermo Fisher Scientific,
Inc. (Waltham, MA, USA) and the flow cytometer was purchased from
Becton-Dickinson (Franklin Lakes, NJ, USA).
The present study was approved by the Ethics
Committee of Children's Hospital of Zhengzhou (Dongsan Street
Hospital).
Establishment of a mouse model of
bronchial asthma
OVA was used for sensitization and induction of a
mouse model of bronchial asthma. Fifteen mice in the observation
group were intraperitoneally injected with 0.1 ml saline solution
containing 50 µg OVA and 2 mg aluminum hydroxide (at the
concentration of 10%) on days 0 and 14. From day 21, the mice were
placed in a closed box and atomized with 2.5% OVA solution for 7
days. In the control group, 15 mice were treated with sterile
saline instead of OVA solution in the sensitization and induction
phases. The usage and dosage were consistent with those in the
observation group. The mice were sacrificed at the last 24 h after
the atomization.
Detection of non-invasive pulmonary
functions of mice
A Buxco non-invasive pulmonary function instrument
(Buxco Respiratory Products, Data Sciences International) was
connected, and the standard value was set. The airway
responsiveness of mice in the two groups was detected. Then the two
groups of mice were placed into a non-invasive pulmonary function
instrument box. After the mice adapted to the environment for 5
min, 20 µl mean corpuscular hemoglobin (MCH) was added at
concentrations of 0, 6.25, 12.50 and 25.00 mg/ml for induction
atomization, respectively. MCH at each concentration was used for
atomization for 1 min, and the results were recorded for 3 min. At
the end of the experiment, data and statistical results were output
automatically. The ratio of specific airway resistance (sRaw)
represented the level of airway responsiveness.
Detection of Th9 lymphocytes in
peripheral blood of mice
Blood from abdominal aorta of mice in the two groups
was extracted and mononuclear cells were isolated by lymphocyte
separation medium. RMPI-1640 complete culture medium was used for
cell suspension, and the cell concentration was adjusted to
2×106/ml. The cells were then inoculated into 6-well
plates with 1 ml per well. The medium was added mixed with PMA (25
ng/ml), ionomycin (1 µg/ml) and monensicillin (1.7 µg/ml), and
cultured in an incubator with 5% CO2 at 37°C for 6 h.
Cells were collected, placed in the flow tube (testing tube and
control tube), and centrifuged for 5 min at rate of 2,600 × g. The
supernatant was then discarded, and 100 µl phosphate-buffered
saline (PBS) was added for resuspension. FITC-CD4 antibodies (10
µl) were added in the testing and control tubes and incubated in
the dark at room temperature for 15 min. The tissues were washed
twice with PBS, 100 µl rupture agent was added, placed at room
temperature in the dark for 10 min, and centrifuged at the rate of
2,600 × g for 5 min. The supernatant was then discarded, 2 µl
PE-IL-9 antibodies were added to the testing tube, and 2 µl of the
same antibodies were added in the control tube for control. The
antibodies were incubated at room temperature for 30 min. After
being washed twice, the cells were resuspended in 0.5 ml PBS. The
percentage of CD4+IL-9+ T cells in
CD4+ T cells was detected.
Detection of the level of IL-9
messenger ribonucleic acid (mRNA) in lung tissues of mice
Lung tissues of mice in the two groups were placed
in diethyl pyrocarbonate (DEPC)-treated 1.5 ml Eppendorf (EP)
tubes, respectively. TRIzol (1 ml) (Invitrogen) was added, and an
ultrasonic cell crusher was used for tissue homogenate. Chloroform
(200 µl) was added, thoroughly mixed with tissues and left to stand
at room temperature for 3 min. Tissues were then centrifuged at the
rate of 9,100 × g at 4°C for 15 min. The supernatant was
transferred to another 1.5 ml EP tube, and tissues were added and
mixed thoroughly with 500 µl isopropyl alcohol and left to stand at
room temperature for 10 min. Subsequently the tissues were
centrifuged at the rate of 5,800 × g at 4°C for 5 min. The
supernatant was discarded, and 50 µl ribonuclease (RNase)-free
water-soluble liquid was added to obtain the total RNA. The
concentration and optical densitity (OD)260/OD80 ratio were
measured. The total RNA with (OD) 260/OD80 ratio between 1.8 and
2.0 were used for reverse transcription. A 20 µl reverse
transcription reaction system was established to obtain
complementary deoxyribonucleic acid (cDNA), RNA was reversely
transcribed into single-stranded cDNA according to the protocol of
the reverse transcription kits (Takara Biomedical Technology Co.,
Ltd., Dalian, China). IL-9 primers used were: F:
5′-GTGACATACATCCTTGCCTC-3′ and R: 5′-GTGGTACAACAGTTGGG-3′.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were:
5′-CTCTGCTCCTCCTGTTCGAC-3′, and 5′-GCGCCCAATACGACCAAATC-3′. A 20 µl
reverse transcription quantitative PCR system (Vazyme Biotech Co.,
Ltd.) was established. The reaction conditions for the PCR were:
denaturation at 95°C for 90 sec annealing; at 95°C for 15 sec
extension; at 57°C for 20 sec and further elongation; at 66°C for
30 sec; with a total of 35 cycles. Data were measured, and
quantitative analysis was conducted (∆∆Cq method).
Determination of the level of IL-9
proteins in peripheral blood and lung tissues of mice
The level of IL-9 in peripheral blood was detected
using ELISA kits, in strict accordance with the protocol of the
kit. IL-9 in lung tissues was analyzed by western blot analysis.
Lung tissues (5 mg) of mice in the two groups were taken,
respectively, and placed in a homogenizer for full grinding. The
cell suspension was transferred into a 1.5 ml EP tube, and tissues
were centrifuged at the rate of 2,600 × g at 4°C for 10 min. The
supernatant was discarded, and the suspension was added with
radioimmunoprecipitation assay (RIPA) lysate (Beyotime
Biotechnology, Shanghai, China). The mixed liquor was vibrated for
full mixing and then left to stand on ice for 30 min and tissues
were centrifuged at the rate of 9,100 × g at 4°C for 10 min. The
bicinchoninic acid assay (BCA) kit (Beyotime Biotechnology) was
used to detect the content of the proteins. After the sodium
dodecyl sulfate polyacrylamide gel (10%) electrophoresis (SDS-PAGE)
was performed, the gel was transferred to polyvinylidene difluoride
(PVDF) membranes. After specific blocking in bovine serum albumin
(BSA) for 1 h, the tissues were washed with phosphate-buffered
saline supplemented with Tween-20 (PBST) 3 times for 5 min each
time. Mouse anti-human IL-9 monoclonal antibodies (diluted at
1:500; cat. no. 564255) were incubated at 4°C for 12 h, and tissues
were washed with PBST 3 times for 5 min each time. Rabbit
anti-mouse IgG monoclonal antibodies (1:1,000; cat. no. ZB-2305)
were then incubated at room temperature for 1 h, and the tissues
were washed with PBST 3 times for 5 min each time. Membranes were
coated with luminescent liquid for development with β-actin as the
internal reference.
Statistical analysis
The experimental results were expressed as mean ±
standard deviation, and SPSS 19.0 software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Comparisons between
groups were performed using LSD test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Analysis of mouse behavioral
manifestations and pulmonary functions
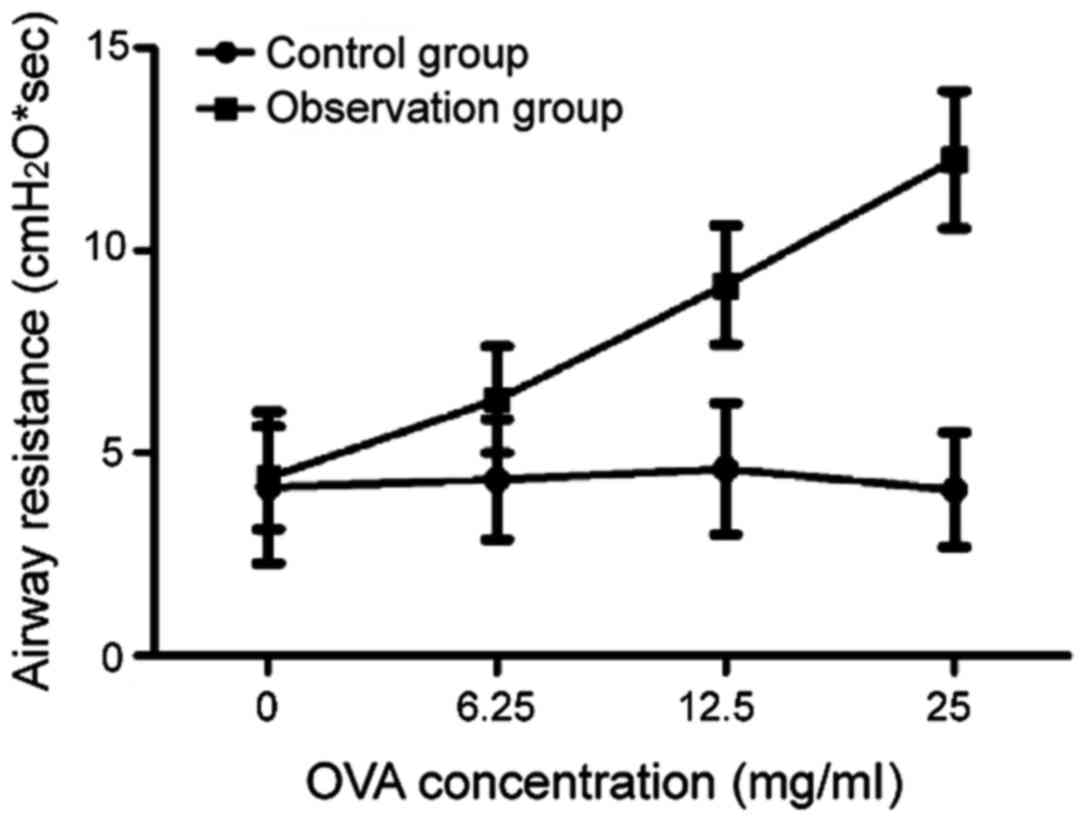
After the induction of OVA, varying degrees of
symptoms emerged, such as forelimbs scratching the nose, dysphoria,
lying prostrately without motion, depression, cyanosis, tachypnea,
abdominal convulsions, urinary and fecal incontinence. After
induction of MCH, the sRaw of mice in the observation group was
significantly higher than that in the control group, and the
difference was statistically significant (P<0.05). With the
increase of MCH concentration, the sRaw of mice in the observation
group was increased gradually, indicating that mice in the
observation group had bronchial hyper-responsiveness. The results
of the analysis of mouse behavioral manifestations and pulmonary
function verified that the mouse model was established successfully
in the observation group (Fig.
1).
Comparison of Th9 cell subgroups of
mice between the two groups
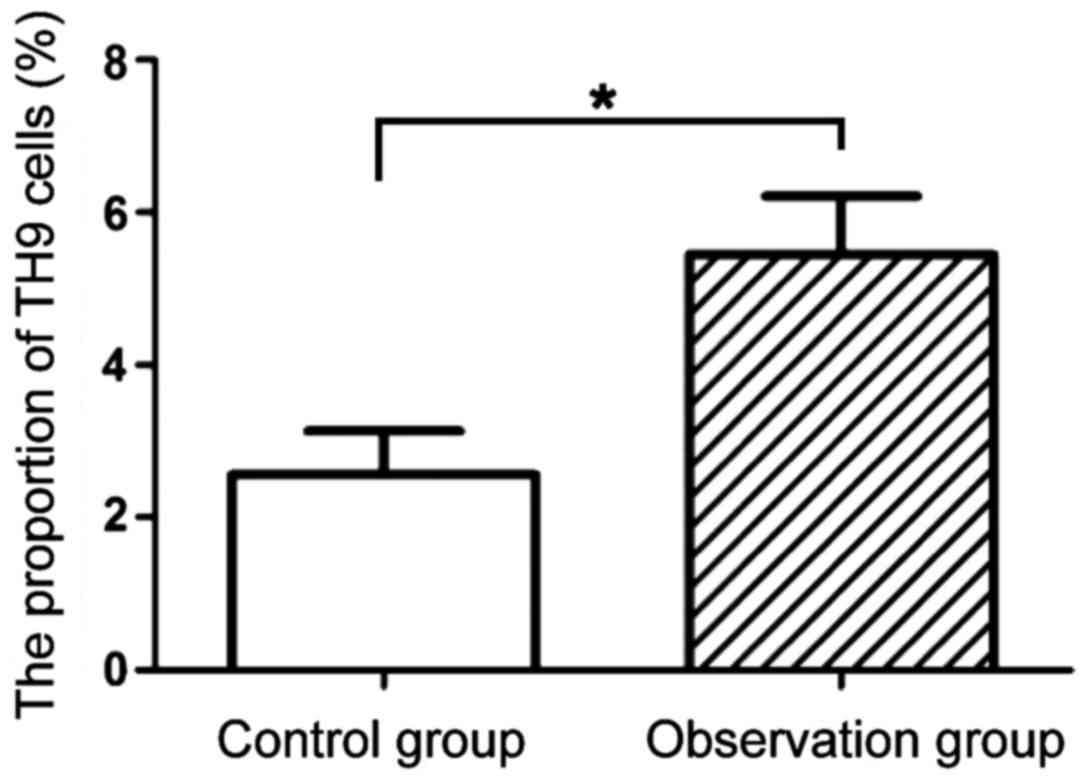
The results of flow cytometry are shown in Figs. 2 and 3. Compared with the proportion of Th9 cells
in peripheral blood of mice in the control group (2.56±0.57), that
in the observation group (5.45±0.76) was significantly increased
(P<0.05).
Comparison of the level of IL-9 mRNA
of mice between the two groups
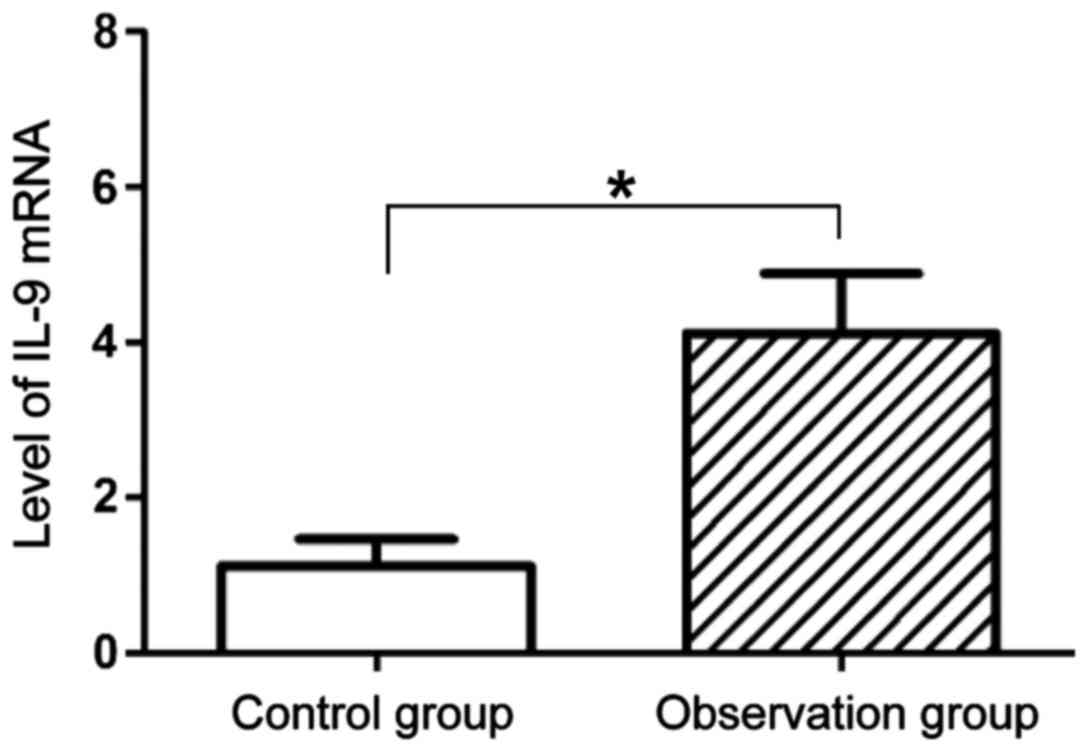
The expression level of IL-9 mRNA in lung tissues
was analyzed by qPCR. As shown in Fig.
4, the expression level of IL-9 mRNA in lung tissues of mice in
the observation group (4.12±0.76) was significantly higher than
that in the control group (1.12±0.34), and the difference was
statistically significant (P<0.05).
Comparison of the level of IL-9
proteins in peripheral blood and lung tissues of mice between the
two groups
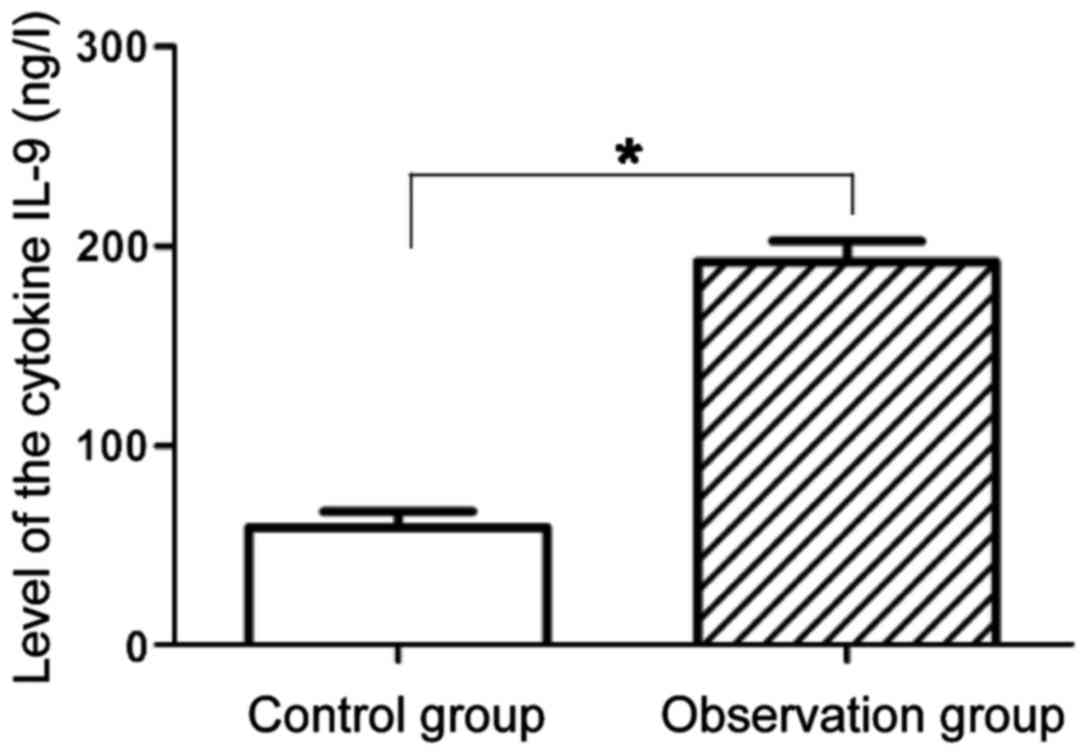
Results of ELISA are shown in Fig. 5. Compared with the expression level
of IL-9 proteins in peripheral blood of mice in the control group
59.43±7.91 ng/l, that in the observation group 192.33±10.23 ng/l
was significantly increased, and the difference was statistically
significant (P<0.05). Results of western blot analysis of the
level of IL-9 proteins in lung tissues indicated that the
expression level of IL-9 proteins in lung tissues of mice in the
observation group was significantly higher than that in the control
group, and the difference was statistically significant (P<0.05)
(Fig. 6).
Discussion
Bronchial asthma is one of the common chronic
diseases of the respiratory system. There are approximately 230
million individuals with chronic asthma worldwide (12). The morbidity and mortality rates of
asthma worldwide have gradually increased in recent years, which
poses a great threat to human health (13). Virus infection, allergens and air
pollution can induce the occurrence of acute asthma. Chronic
non-specific airway inflammation and increased airway
responsiveness are the most important features of asthma (14,15). At
present, the imbalance of the number and function of Th1/Th2 in
CD4+ T lymphocyte subgroups is closely related to the
pathogenesis of asthma. The incidence of asthma is mainly affected
by Th2 cell-mediated immune responses (16). Previous findings showed that IL-9
plays an important role in asthma, parasitic infections and other
Th2-related diseases (17). However,
it has also been found that the function of IL-9 is not completely
identical to that of other Th2-related cytokines. Therefore,
CD4+ Th effector cells secreting IL-9 are a class of
independent cell subgroups, namely Th9 cells (9,18). Due
to the important role of IL-9 in asthma, the action mechanism of
Th9 cells, the main cells secreting IL-9, in bronchial asthma has
drawn increasing attention.
In the present study, the mechanism of Th9 cells and
the cytokine IL-9 in the study of pathogenesis of asthma was
studied by establishing a mouse model of bronchial asthma. The flow
cytometry analysis results of Th9 lymphocytes mouse in the model of
bronchial asthma revealed that the level of Th9 cells in mice with
asthma was significantly increased compared with that in normal
mice, suggesting that Th9 cells play significant roles in the
occurrence and development of asthma. IL-9 can act on a variety of
inflammatory and tissue cells and play an important role in
inflammation and allergic reactions, constituting a pathological
factor of asthma (19). Researchers
suggested that the level of IL-9 in patients with asthma can be
used as one of the indicators for determining disease severity
(20). In this study, both RT-PCR
and western blot results indicated that the levels of IL-9 mRNA and
proteins in lung tissues of mice with asthma were significantly
higher than those in normal mice, which were consistent with those
of Th9 cells.
In summary, Th9 cells, as a kind of important
effector T cells, promote the occurrence and development of
bronchial asthma together with their main cytokine IL-9, and may
become an important indicator for determining the condition of
asthma, thus providing a certain guidance for the clinical
treatment of asthma.
References
|
1
|
Bui TT, Piao CH, Song CH, Shin HS and Chai
OH: Bupleurum chinense extract ameliorates an OVA-induced murine
allergic asthma through the reduction of the Th2 and Th17 cytokines
production by inactivation of NFκB pathway. Biomed Pharmacother.
91:1085–1095. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Junghans-Rutelonis AN, Tackett AP, Suorsa
KI, Chaney JM and Mullins LL: Asthma-specific cognitions,
self-focused attention, and fear of negative evaluation in
adolescents and young adults diagnosed with childhood-onset asthma.
Psychol Health Med. 23:69–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nguyen DT, Kit BK, Brody D and Akinbami
LJ: Prevalence of high fractional exhaled nitric oxide among US
youth with asthma. Pediatr Pulmonol. 52:737–745. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai MK, Lin YC, Huang MY, Lee MS, Kuo CH,
Kuo PL, Lin CH and Hung CH: The effects of asthma medications on
reactive oxygen species production in human monocytes. J Asthma.
Jul 11–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaiser SV, Rodean J, Bekmezian A, Hall M,
Shah SS, Mahant S, Parikh K, Morse R, Puls H and Cabana MD:
Pediatric Research in Inpatient Settings (PRIS) Network: Rising
utilization of inpatient pediatric asthma pathways. J Asthma. May
19–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galli SJ: Mast cells and KIT as potential
therapeutic targets in severe asthma. N Engl J Med. 376:1983–1984.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freĭdin MB, Puzyrev VP, Ogorodova LM,
Saliukova OA, Kamaltynova EM, Kulmanakova IM and Petrovskaia IuA:
Analysis of the association between the T113M polymorphism of the
human Il-9 gene and bronchial asthma. Genetika. 36:559–561.
2000.(In Russian). PubMed/NCBI
|
|
8
|
Shao L, Cong Z, Li X, Zou H, Cao L and Guo
Y: Changes in levels of IL-9, IL-17, IFN-γ, dendritic cell numbers
and TLR expression in peripheral blood in asthmatic children with
Mycoplasma pneumoniae infection. Int J Clin Exp Pathol.
8:5263–5272. 2015.PubMed/NCBI
|
|
9
|
Li C, Jiang X, Luo M, Feng G, Sun Q and
Chen Y: Mycobacterium vaccae nebulization can protect against
asthma in Balb/c mice by regulating Th9 expression. PLoS One.
11:e01611642016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koch S, Sopel N and Finotto S: Th9 and
other IL-9-producing cells in allergic asthma. Semin Immunopathol.
39:55–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang JY, Zheng J, Xing HY and Jia XH:
Determination of Th9 cells and IL-9 in children with Mycoplasma
pneumoniae infection. Zhongguo Dang Dai Er Ke Za Zhi. 17:308–311.
2015.(In Chinese). PubMed/NCBI
|
|
12
|
Poole JA: Asthma is a major
noncommunicable disease affecting over 230 million people worldwide
and represents the most common chronic disease among children. Int
Immunopharmacol. 23:3152014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sposato B, Scalese M, Moschini G and
Migliorini MG: Can we modulate asthma maintenance treatment level
with disease seasonal variations? Eur Rev Med Pharmacol Sci.
19:942–949. 2015.PubMed/NCBI
|
|
14
|
Chiu CD, Chen HJ, Saw HP, Yao NW, Yen HR
and Kao CH: Asthma and early herniated intervertebral disc disease.
Curr Med Res Opin. 33:2019–2025. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang X, Chen Y, Feng G, Luo M, Sun Q and
Li C: Th9 cells and related cytokines increase in the lung of mice
with bronchial asthma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
31:1067–1070. 2015.(In Chinese). PubMed/NCBI
|
|
16
|
Song LJ, Wang ZX, Chi BR and Yang GZ:
Regulatory effect of diammonium glycyrrhizinate on Th1/Th2
deviation in bronchial asthma: Experiment with rats. Zhonghua Yi
Xue Za Zhi. 87:2865–2867. 2007.(In Chinese). PubMed/NCBI
|
|
17
|
Gong F, Pan YH, Huang X, Zhu HY and Jiang
DL: From bench to bedside: Therapeutic potential of interleukin-9
in the treatment of asthma. Exp Ther Med. 13:389–394. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ying X, Su Z, Bie Q, Zhang P, Yang H, Wu
Y, Xu Y, Wu J, Zhang M, Wang S, et al: Synergistically increased
ILC2 and Th9 cells in lung tissue jointly promote the pathological
process of asthma in mice. Mol Med Rep. 13:5230–5240. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoppenot D, Malakauskas K, Lavinskienė S,
Bajoriūnienė I, Kalinauskaitė V and Sakalauskas R: Peripheral blood
Th9 cells and eosinophil apoptosis in asthma patients. Medicina
(Kaunas). 51:10–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng Y, Wang Z, Chang C, Lu L, Lau CS and
Lu Q: Th9 cells and IL-9 in autoimmune disorders: Pathogenesis and
therapeutic potentials. Hum Immunol. 78:120–128. 2017. View Article : Google Scholar : PubMed/NCBI
|