Introduction
Colorectal cancer (CRC), known as either bowel
cancer or colon cancer, develops from the colon or rectum (1). CRC predominantly results from lifestyle
and old age, with very few cases of CRC resulting from genetic
disorders (2). CRC accounts for 13%
of all cancer cases worldwide (3).
In 2012, 1.4 million new cases of CRC were diagnosed and 694,000
mortalities as a result of CRC were recorded (4). CRC is the fourth greatest cause of
cancer-related mortality after lung, stomach and liver cancer
(5).
The purpose of CRC treatment is cure or palliation,
and this depends mainly on factors including the patient's health
status, preference and tumor stage (6). Currently, a combination of surgery,
radiation therapy, chemotherapy and targeted therapy is commonly
adopted for the treatment of CRC (1). A cure may be achieved by surgery if CRC
is detected at an earlier stage, while palliation may be directed
through relieving CRC-related symptoms when CRC is diagnosed later
(7). It is necessary to explore
effective treatments for patients with CRC.
There are types of microRNA (miRNA), which are ~22
nucleotides long, that inhibit protein expression via targeting its
coding gene (8). Generally, miRNA in
animals are complementary to a site in the 3′ untranslated region
(UTR) of their target gene (9). On
account of the involvement of miRNA in eukaryotic cell function,
the dysregulation of miRNA is correlated with disease (10) and cancer (11). Notably, miRNA-based therapies have
been reported to be a potential for cancer treatment (12). miR-766-5p has been demonstrated to be
overexpressed in CRC and promote the cell proliferation of SW480
cells (13). However, the role of
miR-766-5p in cell migration and invasion of CRC has not been
reported. The present study aimed to investigate the aforementioned
question.
Materials and methods
Clinical samples
A total of 31 pairs of tumor tissues and adjacent
tissues, which were at least 2-cm distal to tumor margins, were
collected from 31 patients with CRC who were admitted to the
Affiliated Hospital of Shandong University and underwent surgery
between January 2014 and January 2015. There were 15 male patients
and 16 female patients with the age ranged from 47 to 73 years old.
Following collection, tissues were quickly frozen and stored in
−196°C liquid nitrogen. None of the patients received neoadjuvant
therapy. The present study was approved by the Ethics Committee of
the Affiliated Hospital of Shandong University of Traditional
Chinese Medicine (Jinan, China). Informed consent was obtained from
each patient. Tissues were used for the detection of mRNA
expression levels.
Cell culture
The human CRC cell line, SW480, was purchased from
Nanjing KGI Biotechnology (Nanjing, China) and cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin, and
incubated in a humidified chamber at 37°C with an atmosphere of 5%
CO2 and 95% air.
Plasmid transfection
SW480 cells were seeded into 24-well plates at a
density of 1×105 cells/well. Cells were randomly divided
into two groups: miR-766-5p inhibitor group and miR-negative
control (NC) inhibitor group. Transfection of SW480 cells with 30
µM miR-766-5p inhibitor or miR-NC inhibitor (GeneCopoeia,
Rockville, MD, USA) was conducted by Lipofectamine 2000
transfection reagent (Thermo Fisher Scientific, Inc.) in accordance
with the manufacturer's protocol. SW480 cells were collected 48 h
after transfection for subsequent experiments.
Luciferase activity assay
Suppressor of cancer cell invasion (SCAI) was
predicted to be recognized by miR-766-5p using TargetScan
(http://www.targetscan.org/), and the
recombinant plasmids of pmir-SCAIwt-3′UTR and pmir-SCAImut-3′UTR
(Promega Corporation, Madison, WI, USA) were constructed. SW480
cells were incubated for 24 h at 37°C, followed by co-transfection
of miR-766-5p inhibitor and pmir-SCAIwt-3′UTR or pmir-SCAImut-3′UTR
using Lipofectamine 2000 reagents. After 24 h, luciferase
activities were assessed using the Dual-Luciferase Reporter Assay
System (Promega Corporation). Renilla luciferase was used as an
internal control.
Cell viability analysis
SW480 cells (2×103 cells/well) were
seeded onto 96-well plates. Cell viability was evaluated with a
cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Rockville, MD, USA), according to the manufacturer's protocol, at
24, 48 or 72 h after transfection with miR-766-5p inhibitor or
miR-NC inhibitor. Absorbance was read at 490 nm with an iMARK plate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell apoptosis analysis
Transfected SW480 cells were seeded into 12-well
plates at the density of 2×105/well, cultured for 48 h
at 37°C and collected via centrifugation at 23,200 × g for 5 min at
4°C. Following three washes with PBS, SW480 cells were re-suspended
in 100 µl binding buffer, stained with annexin V-fluorescein
isothiocyanate and propidium iodide (Roche Diagnostics, Basel,
Switzerland) for 15 min at room temperature in the dark. The rate
of cell apoptosis was determined by flow cytometry (Beckman
Coulter, Inc., Miami, FL, USA) in 1 h. The cell number at each
phase was analyzed suing FloJo software (Version 7.6.3, Treestar,
Inc., Ashland, OR, USA).
Cell invasion assay
Cell invasiveness of SW480 cells was evaluated by
Transwell chambers, which were coated with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA). Transfected SW480 cells were
placed in Transwell upper chambers that were coated with gelatin.
Functioning as a chemoattractant, 0.2% BSA containing fibronectin
(Bio-Techne, Shanghai, China) (10 µg/ml) was added to the bottom
chamber. For the invasion assay, 100 µl transfected SW480 cells at
a concentration of 1×105 cells were cultured in the
upper chamber in serum-free Dulbecco's modified Eagle's medium at
37°C in 5% CO2 humidified air. A total of 16 h later,
non-invading cells on the upper chambers were removed with a cotton
swab, and invading cells that reached the bottom chambers were
fixed with 70% ethanol for 15 min at room temperature and stained
by Hemacolor® Rapid staining solution (Merck KGaA,
Darmstadt, Germany) at room temperature for 30 min. The number of
invasive cells was counted under a light microscope (magnification,
×400).
Cell migration assay
Cell migration was evaluated using a wound healing
assay. Transfected SW480 cells were cultured in an incubator at
37°C with 5% CO2. After incubation for 24 h, a wound gap
was created using a 20-µl pipette tip. Transfected SW480 cells were
further cultured for 24 h. Thereafter, wounded monolayers were
photographed with an inverted microscope (magnification, ×400).
Western blotting
Protein lysates were obtained with
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Concentration of protein samples
were determined using the BCA method (Beyotime Institute of
Biotechnology). Protein samples (15 µg/lane) were first separated
by 10% SDS-PAGE and transferred, at 4°C, onto polyvinylidene
difluoride membranes followed by blocking by 5% skim milk at room
temperature for 1 h. Membranes were first incubated overnight with
primary antibodies (SCAI, 12892, 1:1,000, Cell Signaling
Technology, Inc., Danvers, MA, USA; MMP2, ab37150, 1:1,000, Abcam,
Cambridge, UK; p-PI3K, ab151549, 1:1,000, Abcam; p-AKT, 4060,
1:1,000, Cell Signaling Technology, Inc.; GAPDH, 5174, 1:1,000,
Cell Signaling Technology, Inc.) at 4°C, then treated with
horseradish peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 2 h at room temperature.
Blots were visualized with an enhanced chemiluminescent kit
(Beyotime Institute of Biotechnology).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from SW480 cells at 48 h
after transfection and from tissue samples from patients with CRC
using TRIzol (Thermo Fisher Scientific, Inc.), and RT-qPCR for
miRNA was executed using a one-step TaqMan miRNA
reverse-transcription kit (Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used: 95°C for 40 sec at
initial denaturation, followed by 45 cycles for 30 sec at 95°C for
denaturation and 30 sec at 58°C, with the final extension at 72°C
for 35 sec. The relative mRNA expression levels of miR-766
(normalized to U6) and SCAI (normalized to GAPDH), were calculated
using the 2−ΔΔCq method (14). The primer sequences were as followed:
GAPDH, forward 5′-AGCCTCCCGCTTCGCTCTCTGC-3′ and reverse
5′-ACCAGGCGCCCAATACGACCAAA-3′; U6, forward
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse
5′-CGCTTCACGAATTTGCGT-3′; SCAI, forward 5′-CGGGAAACACGAAATTATCC-3′
and reverse 5′-GCTTCTGGAGATGAGGATTCTC-3′; and miR-766, forward
5′-TCGAGTACTTGAGATGGAGTTTT-3′ and reverse
5′-GGCCGCGTTGCAGTGAGCCGAG-3′.
Statistical analysis
All data were presented as the mean ± standard
deviation. The analysis was performed using GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Differences between
two groups were analyzed using a Student's t-test, and differences
between three or more groups were analyzed using one-way analysis
of variance followed by Newman-Keuls analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-766-5p expression is higher in CRC
tissues than normal tissues
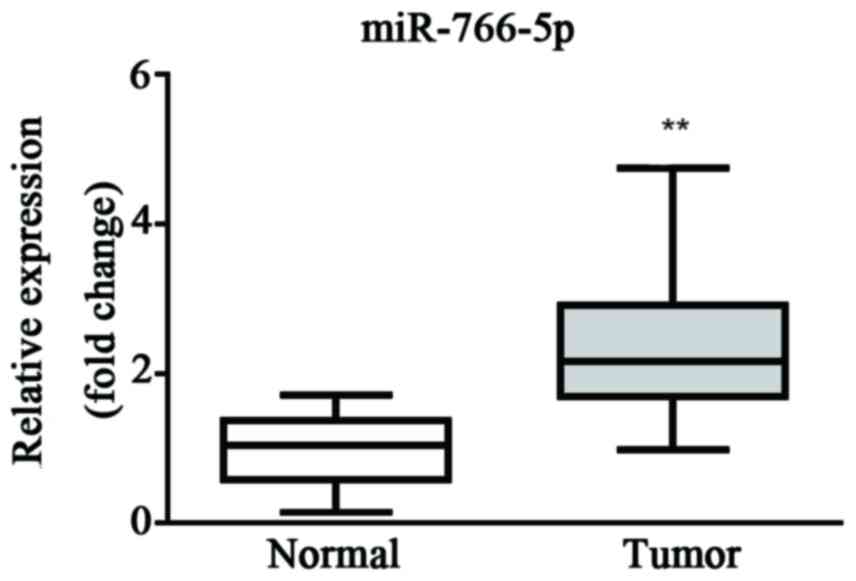
The miR-766-5p expression level in patient tissues
was first evaluated by RT-qPCR. Results demonstrated that the
expression level of miR-766-5p was significantly higher in cancer
tissue than the level in healthy tissue, which suggests that
miR-766-5p may be a promoter during the progression of CRC
(Fig. 1).
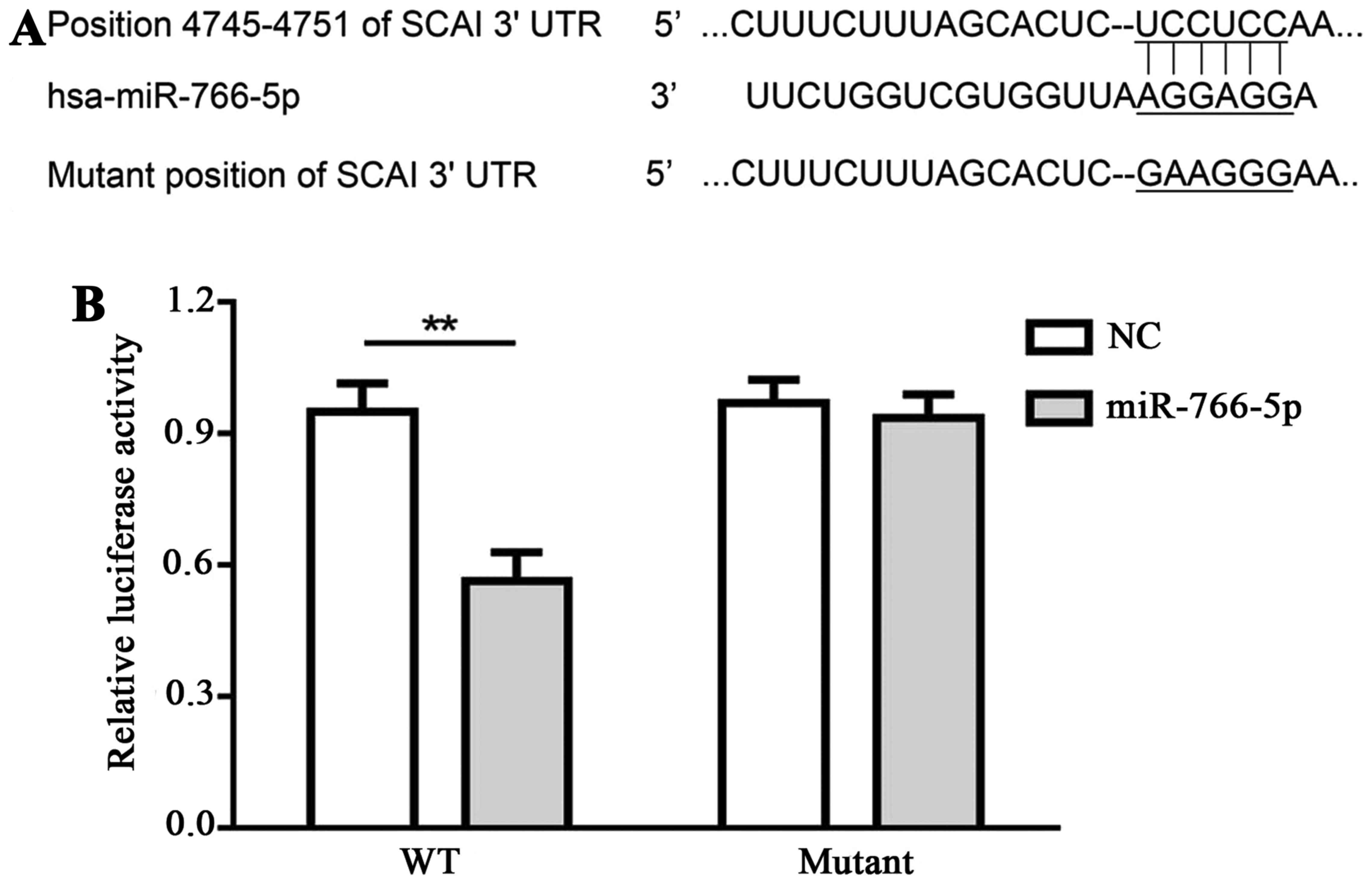
Interaction between miR-766-5p and its
target, SCAI, in SW480 cells
TargetScan was applied for the prediction of
interaction between miR-766-5p and its potential targets. SCAI
demonstrated a relative higher score when compared with other
potential targets, thus, SCAI was selected in the present study.
Binding sequences between miR-766-5p and SCAI 3′UTR, as well as the
mutant sequences, are presented in Fig.
2A. Luciferase activity in SW480 cells that were transfected
with pmir-SCAIwt-3′UTR and miR-766-5p inhibitor was decreased
compared with cells transfected with pmir-SCAIwt-3′UTR and miR-NC
inhibitor, while there was no significant difference in cells
transfected with pmir-SCAImut-3′UTR and miR-766-5p inhibitor in
comparison with cells transfected with pmir-SCAImut-3′UTR and
miR-NC inhibitor (Fig. 2B). These
results demonstrate that miR-766-5p targets and binds with the
3′UTR of SCAI.
Characteristics of patients with
CRC
Patients' age, sex, tumor size, tumor
differentiation degree and metastasis are presented in Table I. There were no significant
differences in patient age or gender; however, there were
significant differences in tumor size, differentiation degree and
metastasis.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Factor | n | P-value |
|---|
| Gender |
|
|
| Male | 15 |
|
|
Female | 16 | >0.05 |
| Age, years |
|
|
|
<60 | 17 |
|
| ≥60 | 14 | >0.05 |
| Tumor size, cm |
|
|
|
<5 | 11 |
|
| ≥5 | 20 | <0.05 |
| Tumor differentiation
degree |
|
|
| Low
degree | 9 |
|
|
Intermediate degree | 22 | <0.05 |
| Metastasis |
|
|
| No | 10 |
|
| Yes | 21 | <0.05 |
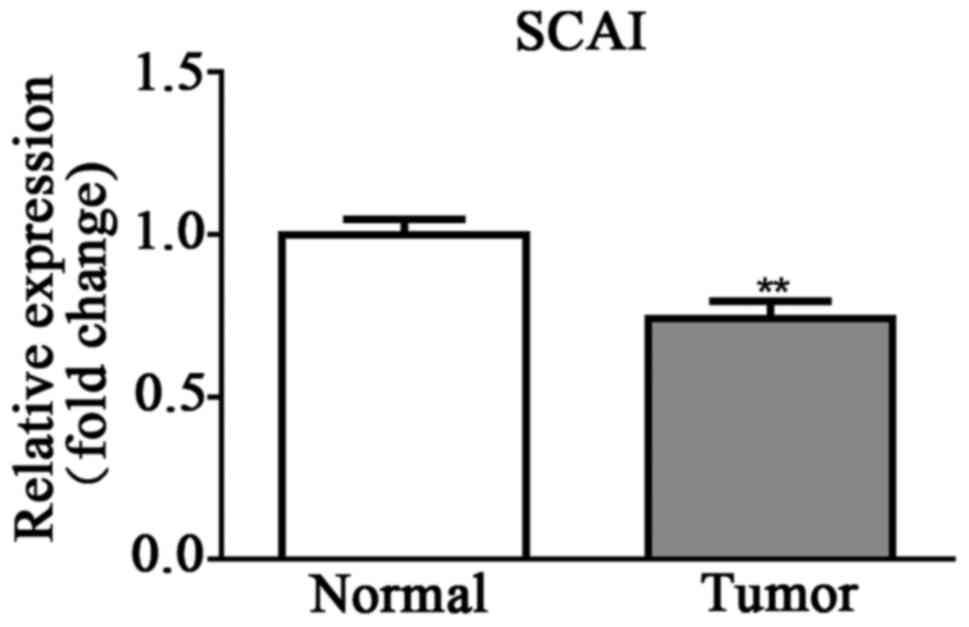
SCAI expression is lower in CRC
tissues than in normal tissues
SCAI expression level in patient tissues was
determined by RT-qPCR. Results demonstrated that the SCAI
expression level was significantly lower in cancer tissue than in
healthy tissue (Fig. 3).
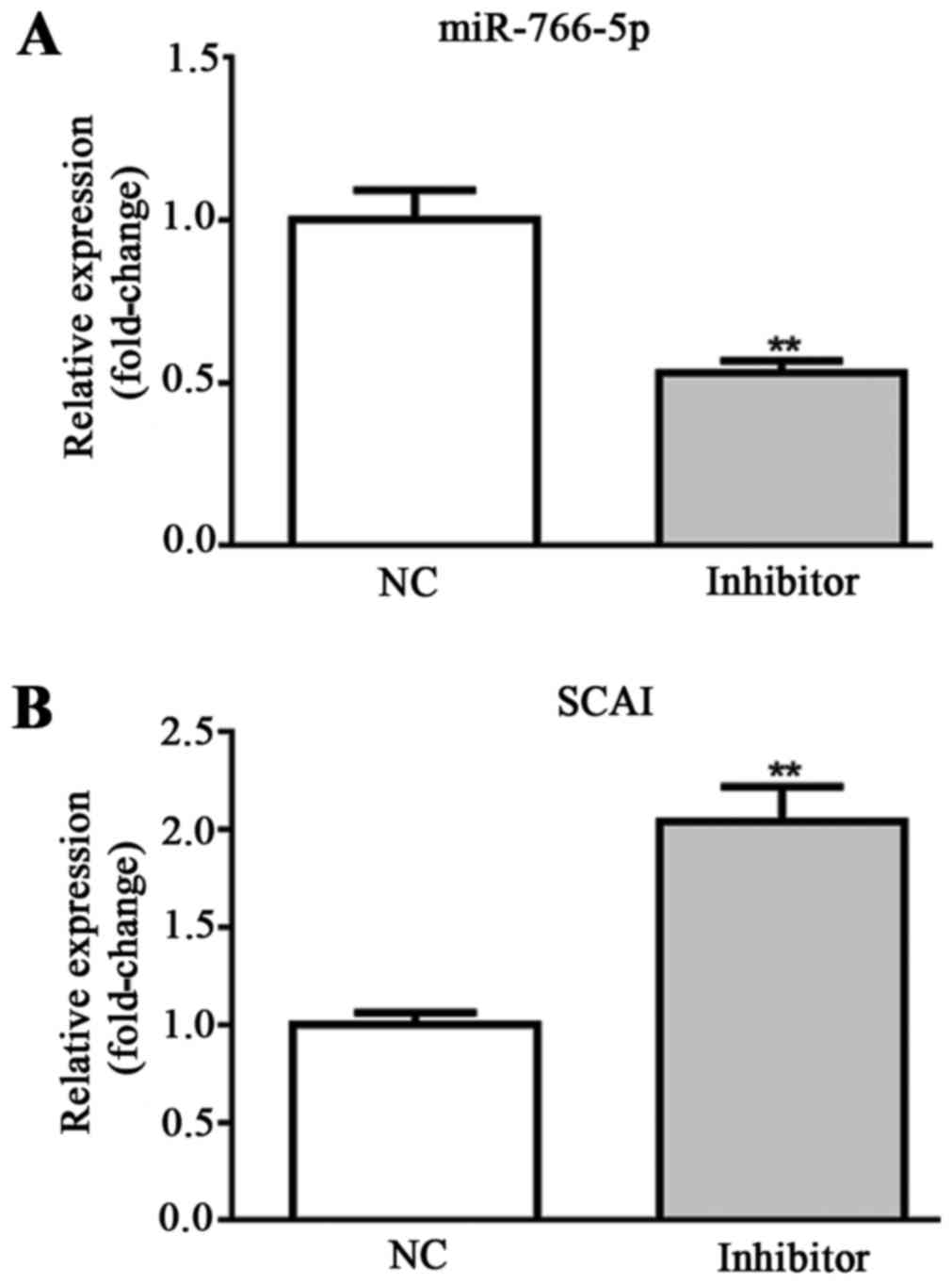
Effects of miR-766-5p inhibitor on
miR-766-5p/SCAI expression in SW480 cells
For in vitro experiments, SW480 cells were
transfected with miR-NC inhibitor and miR-766-5p inhibitor. The
cells in the two different groups were used for the detection of
miR-766-5p and SCAI expression levels by RT-qPCR. Results
demonstrated that, compared with the NC group, miR-766-5p
expression was significantly decreased and SCAI expression was
significantly increased in the miR-766-5p inhibitor group (Fig. 4A and B). These results suggest that
SW480 cells were successfully transfected with miR-766-5p
inhibitor. The effects of miR-766-5p on cell behaviors and the
corresponding molecules were subsequently determined.
Examination of SW480 cell
viability
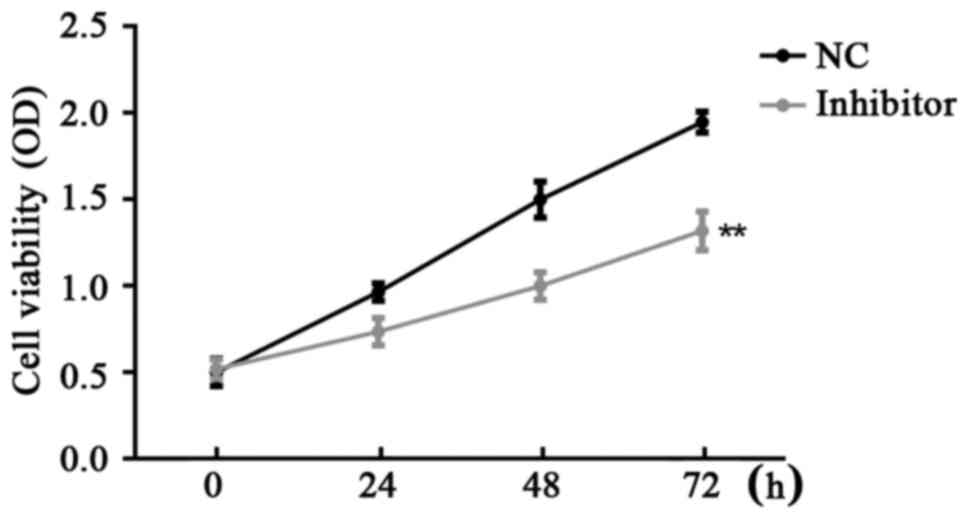
CCK-8 assay was used for the examination of SW480
cell viability in the different groups. Results demonstrated that
miR-766-5p inhibitor significantly inhibited SW480 cell
proliferation compared with the NC group at 24, 48 and 72 h after
transfection (Fig. 5).
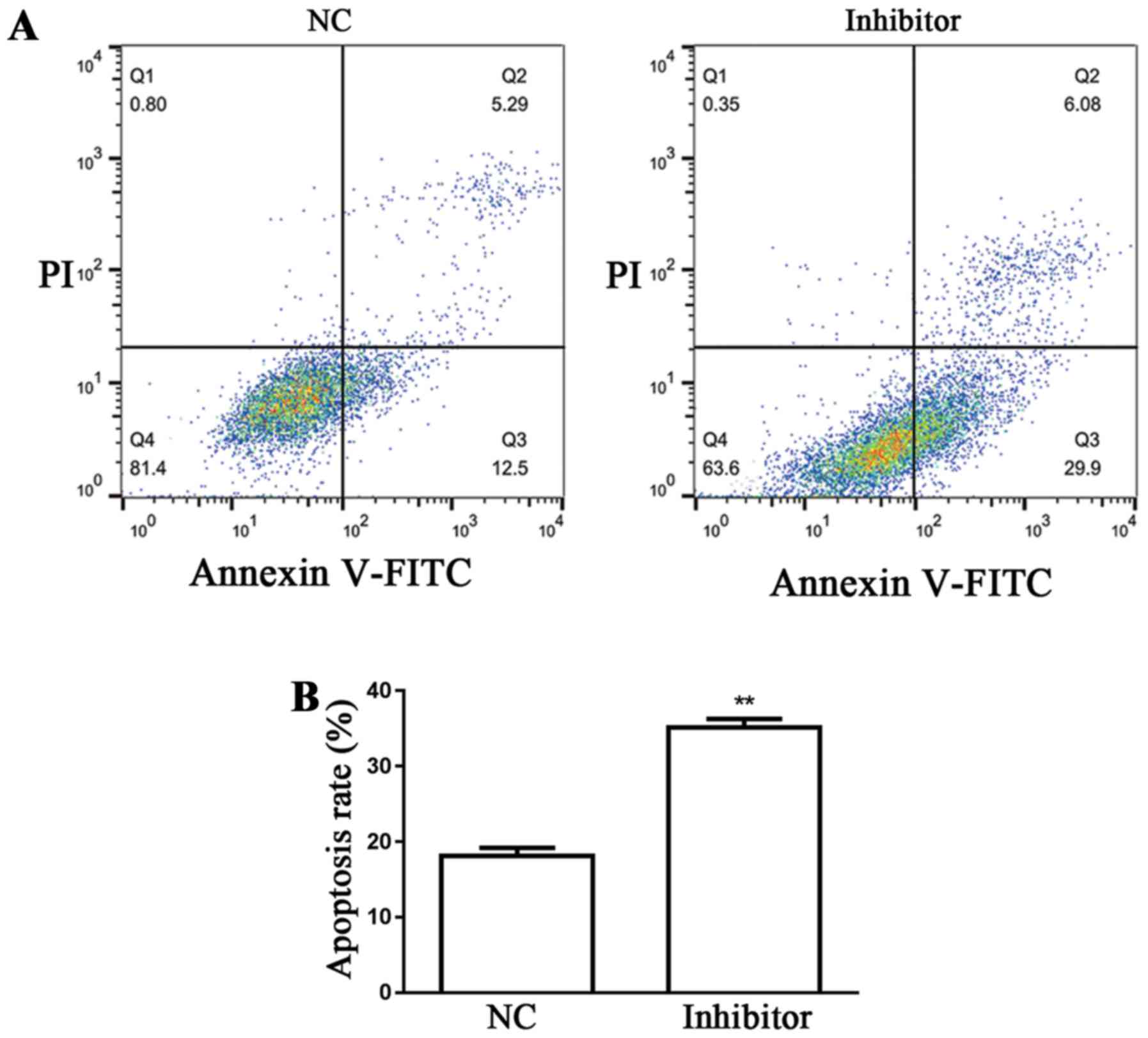
Detection of SW480 cell apoptosis
Flow cytometry was used for the examination of SW480
cell apoptosis in the different groups. Results demonstrated that
miR-766-5p inhibitor significantly promoted SW480 cell apoptosis
(35.98%) compared with the NC group (17.79%) (Fig. 6A and B).
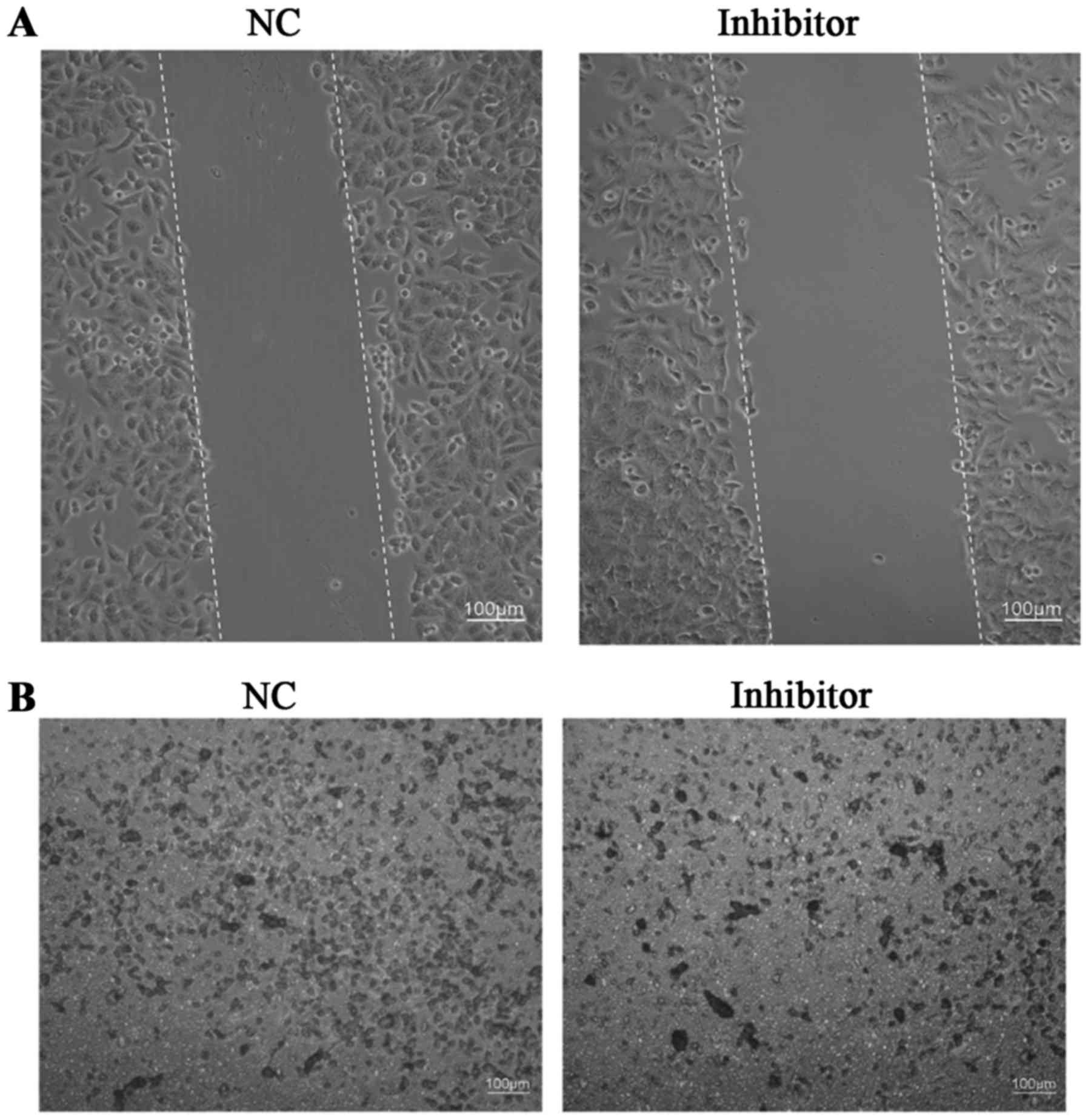
Evaluation of SW480 cell
migration/invasion
A wound healing assay was used for the examination
of SW480 cell migration, while Transwell assays were used for the
examination of SW480 cell invasion in different groups. Results
demonstrated that the miR-766-5p inhibitor markedly inhibited SW480
cell migration/invasion compared with that observed in the NC group
(Fig. 7A and B).
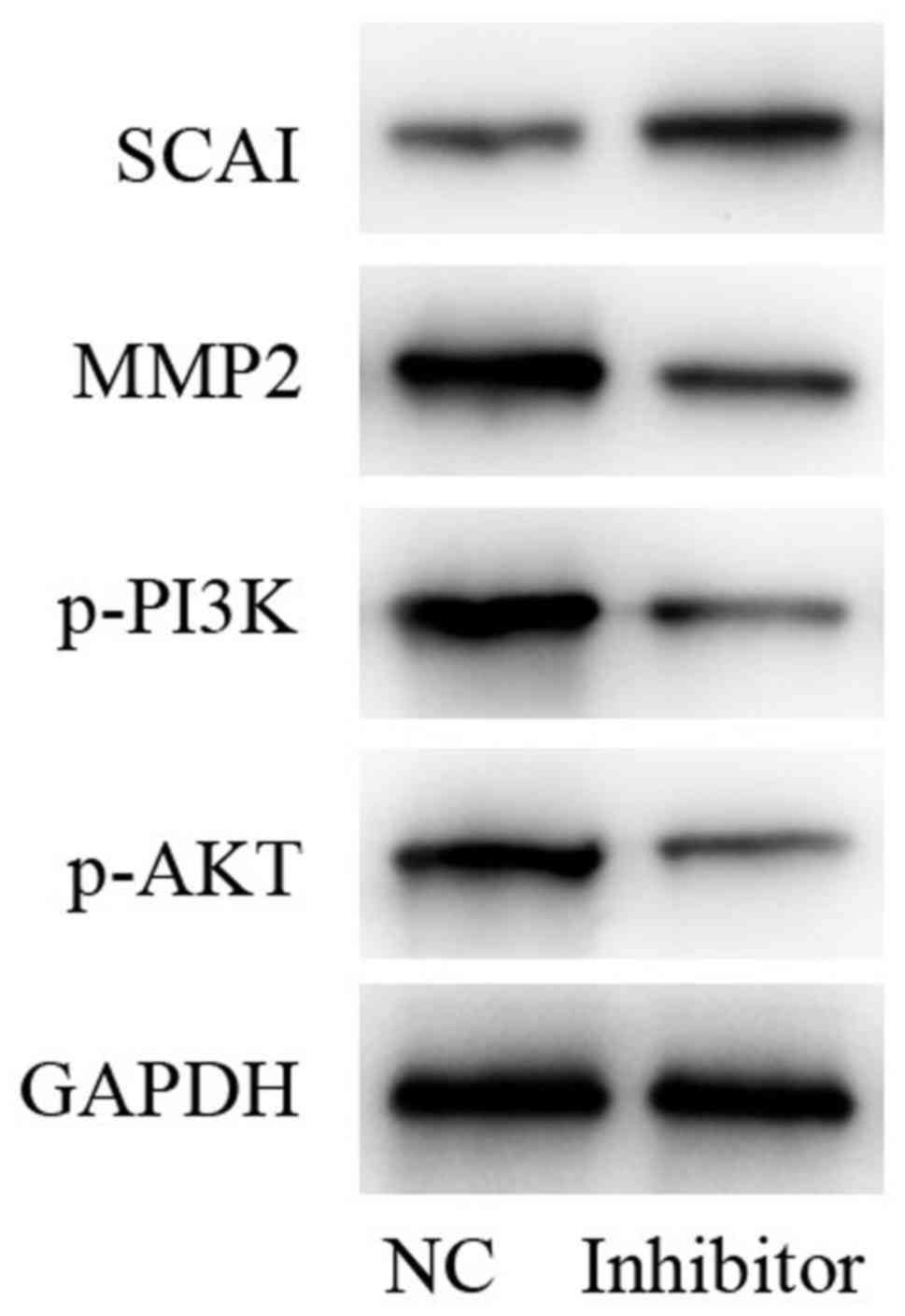
Determination of protein levels of
SCAI, phosphoinositide 3-kinase (PI3K)/AKT and matrix
metalloproteinase-2 (MMP2)
In comparison with the NC group, protein expression
levels of MMP2 and phosphorylated (p)-PI3K/AKT were decreased,
while SCAI was increased following treatment with miR-766-5p
inhibitor. GAPDH was used as an internal control (Fig. 8). Taken together, these results
indicated that, miR-766-5p inhibitor repressed the activation of
p-PI3K/p-AKT signaling by targeting SCAI.
Discussion
miRNA function as oncogenes or tumor suppressors
(15,16). Mounting evidence suggests that miRNA
serve fundamental roles in cell proliferation, migration and
invasion (17–19). Aberrant expression of miRNA has been
increasingly explored (17–19). Furthermore, miR-766-5p was reported
to be overexpressed in CRC and promote the cell proliferation of
SW480 cells (13). The present study
aimed to investigate the role of miR-766-5p in SW480 cells.
SCAI 3′UTR was verified to be recognized by
miR-766-5p. The present study demonstrated that SCAI was
deregulated in tumor tissues compared with normal tissues, which
was consistent with a previous study that also reported the
decrease of SCAI in human cancer, implying a tumor suppressive role
(20,21). In the present study, western blotting
revealed that, following miR-766-5p inhibitor administration, SCAI
protein level was upregulated in SW480 cells in comparison with the
level in the NC group, demonstrating the interaction between
miR-766-5p and SCAI. RNA-mediated interference-induced knockdown of
SCAI has been demonstrated to increase cell migration ability
(22). However, the effects of SCAI
on SW480 cell behaviors were yet to be investigated.
Metastasis, the major cause of cancer mortality, is
a complex process caused by changes in proto-oncogenes and tumor
suppressor genes, which are implicated in cancer cell growth,
migration, invasion and angiogenesis (23,24). As
acknowledged, cell migration and invasion serve pivotal roles in
angiogenesis, tissue repair and immune response, which are all
deregulated in cancer cells (25,26). In
the present study, the influence of miR-766-5p inhibitor on SW480
cell proliferation, apoptosis, migration and invasion was
investigated. Results demonstrated that, miR-766-5p inhibitor
treatment reduced cell proliferation, migration and invasion
ability, and induced cell apoptosis of SW480 cells. Taken together,
the miR-766-5p inhibitor slowed down the process of CRC. The
present study also explored whether there are other molecules that
are influenced by miR-766-5p.
MMP family proteolytic enzymes are indispensable for
the remodeling of extracellular matrix, of which the degradation is
a prerequisite for the invasion and metastasis of cancer (27,28). In
the present study, it was demonstrated that the protein expression
level of MMP2 was notably decreased following miR-766-5p inhibitor
treatment compared to that following treatment with the NC.
Additionally, the PI3K/AKT/mammalian target of rapamycin pathway
was reported to be a potential target for prevention of CRC
(29,30). In the present study, protein
expression levels of p-PI3K and p-AKT were also found to be
markedly decreased following miR-766-5p inhibitor treatment
compared to the levels in the NC group.
In conclusion, treatment with a miR-766-5p inhibitor
reduces cell proliferation, migration, invasion, and MMP2/PI3K/AKT
protein expression in the SW480 CRC cell line, and induces cell
apoptosis via targeting SCAI. The findings of the present study
propose a possible treatment target for CRC.
References
|
1
|
National Cancer Institute (NCI), . Colon
Cancer Treatment (PDQ®)-Patient Version. NCI; Bethesda,
MD: 2017, https://www.cancer.gov/types/colorectal/patient/colon-treatment-pdqFebruary
27–2017
|
|
2
|
IARC, . World Cancer Report 2014: World
Health Organization. Chapter. 5:52014.
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
4
|
IARC, . World Cancer Report 2014: World
Health Organization. Chapter. 1:12014.
|
|
5
|
Brody H: Colorectal cancer. Nature.
521:S12015. View
Article : Google Scholar
|
|
6
|
Stein A, Atanackovic D and Bokemeyer C:
Current standards and new trends in the primary treatment of
colorectal cancer. Eur J Cancer. 47 Suppl 3:S312–S314. 2011.
View Article : Google Scholar
|
|
7
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar
|
|
9
|
Wang XJ, Reyes JL, Chua NH and Gaasterland
T: Prediction and identification of Arabidopsis thaliana microRNAs
and their mRNA targets. Genome Biol. 5:R652004. View Article : Google Scholar
|
|
10
|
Mraz M and Pospisilova S: MicroRNAs in
chronic lymphocytic leukemia: From causality to associations and
back. Expert Rev Hematol. 5:579–581. 2012. View Article : Google Scholar
|
|
11
|
Musilova K and Mraz M: MicroRNAs in B cell
lymphomas: How a complex biology gets more complex. Leukemia.
29:1004–1017. 2015. View Article : Google Scholar
|
|
12
|
Trang P, Weidhaas JB and Slack FJ:
MicroRNAs as potential cancer therapeutics. Oncogene. 27 Suppl
2:S52–S57. 2008. View Article : Google Scholar
|
|
13
|
Li YC, Li CF, Chen LB, Li DD, Yang L, Jin
JP and Zhang B: MicroRNA-766 targeting regulation of SOX6
expression promoted cell proliferation of human colorectal cancer.
Onco Targets Ther. 8:2981–2988. 2015. View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar
|
|
16
|
Osada H and Takahashi T: MicroRNAs in
biological processes and carcinogenesis. Carcinogenesis. 28:2–12.
2007. View Article : Google Scholar
|
|
17
|
Shen F, Cai WS, Feng Z, Li JL, Chen JW,
Cao J and Xu B: miR-492 contributes to cell proliferation and cell
cycle of human breast cancer cells by suppressing SOX7 expression.
Tumour Biol. 36:1913–1921. 2015. View Article : Google Scholar
|
|
18
|
Zhang Z, Sun J, Bai Z, Li H, He S, Chen R
and Che X: MicroRNA-153 acts as a prognostic marker in gastric
cancer and its role in cell migration and invasion. Onco Targets
Ther. 8:357–364. 2015.
|
|
19
|
Li X, Wang J, Jia Z, Cui Q, Zhang C, Wang
W, Chen P, Ma K and Zhou C: miR-499 regulates cell proliferation
and apoptosis during late-stage cardiac differentiation via Sox6
and cyclin D1. PLoS One. 8:e745042013. View Article : Google Scholar
|
|
20
|
Brandt DT, Baarlink C, Kitzing TM, Kremmer
E, Ivaska J, Nollau P and Grosse R: SCAI acts as a suppressor of
cancer cell invasion through the transcriptional control of
beta1-integrin. Nat Cell Biol. 11:557–568. 2009. View Article : Google Scholar
|
|
21
|
Chen X, Hu W, Xie B, Gao H, Xu C and Chen
J: Downregulation of SCAI enhances glioma cell invasion and stem
cell like phenotype by acti-vating Wnt/β-catenin signaling. Biochem
Biophys Res Commun. 448:206–211. 2014. View Article : Google Scholar
|
|
22
|
Juliano R: SCAI blocks MAL-evolent effects
on cancer cell invasion. Nat Cell Biol. 11:540–542. 2009.
View Article : Google Scholar
|
|
23
|
Turajlic S and Swanton C: Metastasis as an
evolutionary process. Science. 352:169–175. 2016. View Article : Google Scholar
|
|
24
|
Brábek J, Mierke CT, Rösel D, Veselý P and
Fabry B: The role of the tissue microenvironment in the regulation
of cancer cell motility and invasion. Cell Commun Signa. 8:222010.
View Article : Google Scholar
|
|
25
|
Haeger A, Wolf K, Zegers MM and Friedl P:
Collective cell migration: Guidance principles and hierarchies.
Trends Cell Biol. 25:556–566. 2015. View Article : Google Scholar
|
|
26
|
Clark AG and Vignjevic DM: Modes of cancer
cell invasion and the role of the microenvironment. Curr Opin Cell
Biol. 36:13–22. 2015. View Article : Google Scholar
|
|
27
|
Galliera E, Tacchini L and Corsi Romanelli
MM: Matrix metalloproteinases as biomarkers of disease: Updates and
new insights. Clin Chem Lab Med. 53:349–355. 2015. View Article : Google Scholar
|
|
28
|
Cathcart J, Pulkoski-Gross A and Cao J:
Targeting matrix metalloproteinases in cancer: Bringing new life to
old ideas. Genes Dis. 2:26–34. 2015. View Article : Google Scholar
|
|
29
|
Ballard-Barbash R, Friedenreich CM,
Courneya KS, Siddiqi SM, McTiernan A and Alfano CM: Physical
activity, biomarkers, and disease outcomes in cancer survivors: A
systematic review. J Natl Cancer Inst. 104:815–840. 2012.
View Article : Google Scholar
|
|
30
|
Pandurangan AK: Potential targets for
prevention of colorectal cancer: A focus on PI3K/Akt/mTOR and Wnt
pathways. Asian Pac J Cancer Prev. 14:2201–2205. 2013. View Article : Google Scholar
|