Introduction
Cancer is a prevalent health issue worldwide. The
United States (US) National Cancer Institute reports that ~1/4
mortalities in the US are due to cancer (1). Cancer mortality has declined
continuously over the past two decades; the overall risk of
succumbing to cancer decreased by 20% between 1991 and 2010
(2). However, the World Health
Organization reports that >14 million new cancer cases occur
worldwide every year (3). In
particular, cancer is the leading cause of mortality in China
(3). In addition, the National
Cancer Center of China reported ~4,292,000 new cancer cases and
~2,814,000 cancer-associated mortalities in China in 2015, with
lung cancer being the most common cancer type and the leading cause
of cancer-associated mortality (2).
Stomach, esophageal and liver cancer were also commonly diagnosed,
and were identified as leading causes of cancer-associated
mortality (4).
Although chemotherapy-based treatments have greatly
improved the survival rates of patients with cancer, current
therapeutic strategies also induce significant undesirable side
effects, including inflammation, ulceration and diarrhea (5). Paclitaxel, docetaxel and vinorelbine
have all been widely used as anticancer agents with a good outcome
(6). These drugs work through
promoting tubulin polymerization and inhibiting cell mitosis;
however, they induce significant side effects, including bone
marrow toxicity and neutropenia (7).
Therefore, it is important to develop novel drugs with low
toxicities for the treatment of cancer.
Apoptosis, also called type I programmed cell death,
is the tightly regulated process of cell death. Apoptosis is
essential to the development and maintenance of multicellular
organisms. Compelling functional studies have established the
concept that apoptotic programmed cell death serves as a natural
barrier to cancer development (8,9).
Furthermore, abnormalities in the regulation of cell death are
characteristic of neoplastic disease. Apoptotic signaling pathways
are thus evident drug targets for therapeutic interventions for
cancer, and the promotion of apoptosis may effectively block
neoplastic progression. The B-cell lymphoma 2 (Bcl-2) family of
proteins are significant regulators of apoptosis, and the rate of
apoptosis can be increased by altering the ratio of
Bcl-2-associated X (Bax)/Bcl-2 proteins. With increasing Bax
expression, more cytochrome c is released by the
mitochondria, which activates caspase-9 and −3, leading to
apoptosis.
Triterpenoids exist widely in fungus, ferns and
plants. The triterpenoid lucialdehyde c is separated from
Ganoderma lucidum, while poricoic acid G is derived from
Poriacocos. Triterpenoids consist of several isoprene units
(10). Triterpenoids exert various
biological and pharmacological activities, particularly antitumor
effects (11,12). Numerous triterpenoids, including
ganoderic acid B, have been used to treat various types of
malignancy. Lucialdehyde c has been revealed to exert cytotoxic
effects on Lewis lung carcinoma, T-47D, sarcoma 180 and meth-A
tumor cell lines (13). Furthermore,
3-acetoxylanosta-8,24-dien-21-oic acid (FPOA), which was initially
isolated from Ganoderma tsugae, has been identified to
induce human hepatoma Hep3B cell death by apoptosis (14,15).
With increasing research into triterpenoids, the mechanisms of
their antitumor effects have been demonstrated to include induction
of apoptosis, cell cycle inhibition and regulation of the immune
response (16,17). However, the underlying molecular
mechanism of the antitumor effect of FPOA remains unclear.
Fomitopsis pinicola, a wood-decay fungus, has
long been used in North East China as a traditional medicine to
treat poor leg circulation in the elderly (18). In a previous study by our group, the
triterpenoid FPOA was isolated from the fruiting body of F.
pinicola and was identified to be its principal active
component (19). A previous study
has reported that FPOA induces tumor cell death by apoptosis
(20). Thus, the present study
assessed the antiproliferative effects of FPOA on human hepatoma
HepG2 cells and investigated the underlying molecular mechanisms of
these effects.
Materials and methods
FPOA extraction and isolation
F. pinicola was collected from Changbai
mountain (Jilin, China). The fruiting bodies were extracted with
petroleum ether and CHCl3, and the CHCl3
extract was separated using a silica gel column. Subsequent elution
with petroleum ether-ethyl acetate yielded FPOA. The methods used
for the extraction, isolation and purification of FPOA have been
described in previous studies by our group (19,20). The
isolated FPOA was characterized using 1H and
13C nuclear magnetic resonance (NMR) spectroscopy with
CDCl3 solvent, as previously described (15,21), and
the data were compared with the previously reported values.
Cell culture
HepG2, MCF-7, HeLa, A549 and MRC-5 cell lines were
obtained from the Norman Bethune Health Science Center of Jilin
University (Changchun, China). All cell lines were maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin,
60 U/ml gentamicin, 2.0 g/l sodium bicarbonate and 2.38 g/l HEPEs.
Cultures were maintained in a humidified incubator at 37°C with 5%
(v/v) CO2.
Cytotoxicity assay
The cytotoxicity assay used was the MTT assay (MTT
Cell Proliferation Assay kit; Beyotime Institute of Biotechnology,
Haimen, China). Cells were plated into 96-well culture plates
(6×103 cells/well) and cultured at 37°C with 5% (v/v)
CO2. The HepG2, MCF-7, HeLa, A549 and MRC-5 cells were
allowed to attach for 24 h prior to the treatment. Mitomycin C
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used as a
positive control, the cells were exposed to various concentrations
of FPOA dissolved in saline and Tween-80 [1 mM; 0 (negative
control), 1.56, 3.13, 6.25, 12.50, 25.00, 50.00, 100.00 and 200.00
µg/ml], and incubation was continued at 37°C with 5% (v/v)
CO2 for 48 h. In total, six replicates of each FPOA
concentration were run and the results were averaged. Following
incubation, stock MTT solution (20 µl; 5 mg/ml) was added to each
well. After 4 h, dimethyl sulfoxide (150 µl) was added to dissolve
the formazan crystals that had formed. The optical densities of
drug-treated wells were measured using a microplate reader at 570
nm. Finally, the FPOA cytotoxicities were expressed as half maximal
inhibitory concentration (IC50) values.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) apoptosis assay
HepG2 cells that were cultured for 24 h in 6-well
plates (1×106 cells/well) were collected, washed and
stained following treatment with different concentrations of FPOA
(0, 12.50, 25.00 and 50.00 µg/ml) for 24 h at 37°C with 5% (v/v)
CO2. Each drug concentration was tested in triplicate.
Cells were then washed twice with PBS and 400 µl 1X binding buffer
was added followed by 5 µl annexin V-FITC conjugate as denoted by
the FITC Annexin V Apoptosis Detection kit (BD Biosciences,
catalogue no. 556547). The cells were then incubated in the dark
for 15 min at 2–8°C, then 5 µl PI was added and incubation was
continued for 5 min. Finally, all the samples were subjected to
flow cytometry analysis (FACSCalibur; BD Biosciences, San Jose, CA,
USA) as previously described (11).
Western blotting
HepG2 cells were seeded into 10-cm-diameter culture
dishes (1×103 cells/dish). After 24 h of incubation, the
cells were treated with different concentrations of FPOA (0, 12.50,
25.00 and 50.00 µg/ml) for 24 h at 37°C with 5% (v/v)
CO2. The cells were then harvested in cell lysis buffer
(cat. no. 9803; Cell Signaling Technology, Inc., Danvers, MA, USA),
incubated for 2 h at 4°C and centrifuged for 15 min at 12,000 × g
at room temperature. The supernatants were collected and protein
concentration was determined using the Bradford assay. Equal
amounts of total protein (50 µg/lane) were subjected to 20%
SDS-PAGE and transferred onto polyvinylidene difluoride membranes.
The membranes were then blocked with 5% (w/v) non-fat dry milk in
Tris-buffered saline-Tween-20 (TBST) for 1 h at room temperature
and then incubated with the primary antibodies directed against
poly(ADP-ribose) polymerase (PARP; cat. no. ENM0145; 1:3,000),
Bcl-2 (cat. no. ENT0470; 1:1,000), Bax (cat. no. ENT0456; 1:1,000),
caspase-9 (cat. no. ESAP14070; 1:750), caspase-3 (cat. no.
ESAP10165; 1:400), cytochrome c (cat. no. ENT1186; 1:1,500)
and GAPDH (ESAP10111; 1:1,000; all Elabscience Biotechnology Co.,
Ltd, Wuhan, China), dilution with 5% (w/v) non-fat dry milk in
TBST, overnight at 4°C. GAPDH served as the loading control. Next,
the membranes were incubated with the appropriate horseradish
peroxidase-conjugated secondary antibodies in TBST for 1 h at 4°C.
The protein bands were visualized using enhanced chemiluminescence
(ECL kit; cat. no. P0018; Beyotime Institute of Biotechnology,
Haimen, China). Quantity one version 4.62 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used for analysis.
Statistical analysis
The data are expressed as the mean ± standard
deviation of six experiments. One-way analysis of the variance was
performed to analyze the statistical significance of difference
between groups. All statistical analyses were performed using SPSS
(version 19.0, IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
FPOA extraction and isolation
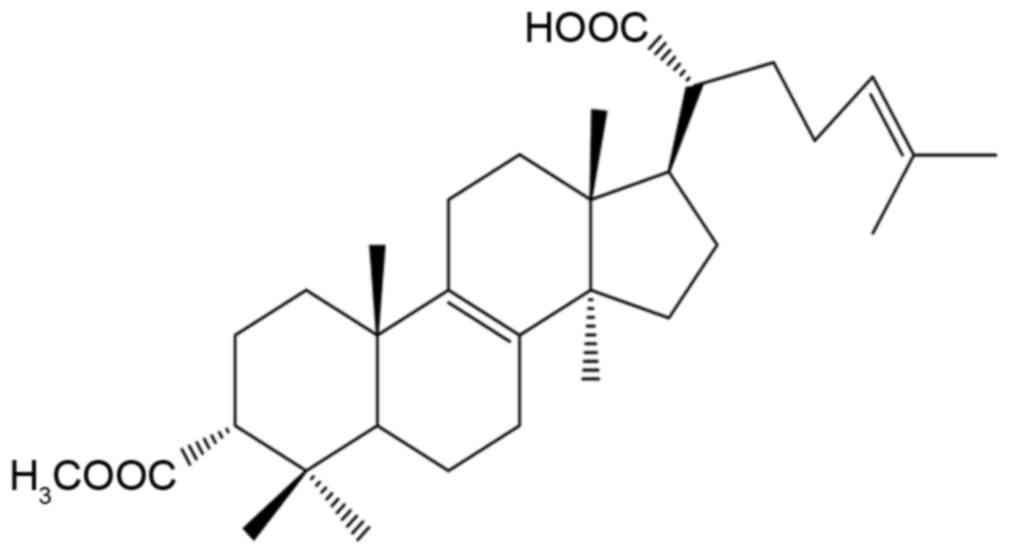
FPOA (Fig. 1) is a
white petroleum ether-ethyl acetate powder with a melting point of
192–194°C and an m/z of 498. The 1H NMR
(CDCl3) and 13C NMR (CDCl3) data
for FPOA are shown in Table I.
 | Table I.Nuclear magnetic resonance data of
3-acetoxylanosta-8,24-dien-21-oic acid (δppm
CDCl3). |
Table I.
Nuclear magnetic resonance data of
3-acetoxylanosta-8,24-dien-21-oic acid (δppm
CDCl3).
| Position | δ (13C),
ppm | δ (1H),
ppm |
|---|
| C-1 |
30.36 |
|
| C-2 |
23.38 |
|
| C-3 |
77.89 |
|
| C-4 |
36.76 |
|
| C-5 |
45.35 |
|
| C-6 |
17.95 |
|
| C-7 |
25.96 |
|
| C-8 | 133.88 |
|
| C-9 | 134.51 |
|
| C-10 |
36.89 |
|
| C-11 |
20.83 |
|
| C-12 |
28.95 |
|
| C-13 |
44.25 |
|
| C-14 |
49.56 |
|
| C-15 |
27.03 |
|
| C-16 |
30.87 |
|
| C-17 |
47.16 |
|
| C-18 |
15.98 | 0.759
(3,s) |
| C-19 |
18.84 | 0.973 (3,
s) |
| C-20 |
47.59 |
|
| C-21 | 182.52 |
|
| C-22 |
32.49 |
|
| C-23 |
25.91 |
|
| C-24 | 123.57 | 5.093 (1,
t) |
| C-25 | 132.24 |
|
| C-26 |
17.64 | 1.588 (3,
s) |
| C-27 |
25.67 | 1.676 (3,
s) |
| C-28 |
27.55 | 0.858 (3,
s) |
| C-29 |
21.85 | 0.909 (3,
s) |
| C-30 |
24.34 | 0.934 (3,
s) |
| OCOMe (C-32) |
21.32 | 2.06 (3,
s) |
| OCOMe (C-31) | 170.79 |
|
Cytotoxicity of FPOA on cancer cell
lines
As demonstrated in Table
II, FPOA evidently inhibited the growth of HepG2, MCF-7, and
HeLa cells that had IC50 values of 42.10, 52.25 and
53.19 µM, respectively. However, it had no effect on MRC-5 and A549
cells that had IC50 values of 365.09 and 279.14 µM,
respectively. Furthermore, when mitomycin C was used as a positive
control the IC50 values on the MCF-7 and A549 tumor
cells were 63.76 and 94.75 µM, respectively, confirming that FPOA
had no effect on these cell lines. Furthermore, the mitomycin C
results confirmed that FPOA was particularly toxic to the HepG2
cells. Therefore, the HepG2 cell line was selected to further
evaluate the antitumor actions of FPOA.
 | Table II.FPOA and mitomycin C cytotoxicity
against HepG2, MCF-7, HeLa, A549 and MRC-5 cells. |
Table II.
FPOA and mitomycin C cytotoxicity
against HepG2, MCF-7, HeLa, A549 and MRC-5 cells.
|
|
|
Inhibition
ratio (%) of different concentrations (µg/ml) |
|---|
|
|
|
|
|---|
| Compound | Cell line | 0 | 1.56 | 3.13 | 6.25 | 12.50 | 25.00 | 50.00 | 100.00 | 200.00 | IC50
(µM) |
|---|
| FPOA | MRC-5 |
0.00±1.88 |
3.99±0.78 |
6.56±1.41a |
5.62±1.76 |
2.14±0.65 |
4.61±0.97 |
14.13±1.48b |
31.00±2.96b |
53.03±3.45b | 365.09 |
|
| HepG2 |
0.00±3.43 |
−4.47±1.90 |
−16.94±2.83b |
21.73±4.36b |
24.01±0.68b |
58.84±3.02b |
85.47±0.84b |
81.89±0.47b |
83.62±0.73b | 42.10 |
|
| MCF-7 |
0.00±2.48 |
7.03±4.42 |
9.97±2.84a |
15.78±4.57a |
27.40±3.01b |
47.69±2.32b |
87.79±0.42b |
88.36±1.03b |
87.70±0.48b | 52.25 |
|
| HeLa |
0.00±3.03 |
−0.13±2.02 |
17.83±2.71b |
19.33±3.09b |
27.20±1.94b |
47.96±0.51b |
72.33±2.09b |
89.36±0.74b |
90.17±0.62b | 53.19 |
|
| A549 |
0.00±2.17 |
9.95±3.21b |
11.61±1.92b |
14.67±1.76b |
14.39±2.10b |
11.13±1.09b |
34.41±2.64b |
37.19±2.66b |
64.15±1.34b | 279.14 |
| Mitomycin C | MRC-5 |
0.00±1.35 |
3.32±0.77 |
2.70±0.43 |
4.84±1.23a |
5.89±1.42a |
6.29±1.59a |
18.09±1.78b |
40.74±5.49b |
52.68±4.74b | 397.13 |
|
| HepG2 |
0.00±3.35 |
7.15±1.50 |
18.63±4.05b |
8.26±2.59 |
34.25±3.41b |
48.95±3.75b |
63.66±3.57b |
77.99±3.02b |
79.23±4.01b | 78.58 |
|
| MCF-7 |
0.00±3.15 |
9.94±3.35 |
19.62±3.34b |
20.19±5.08a |
38.86±0.89b |
53.33±2.29b |
66.72±3.91b |
84.24±3.75b |
82.25±2.39b | 63.76 |
|
| HeLa |
0.00±4.03 |
6.51±2.04 |
15.34±3.08a |
26.96±4.75b |
39.86±3.99b |
50.13±3.96b |
62.72±4.35b |
76.82±2.71b |
79.19±4.17b | 74.13 |
|
| A549 |
0.00±3.03 |
7.92±1.16a |
6.79±1.42 |
19.99±2.68b |
22.53±2.17b |
43.09±2.69b |
61.31±3.79b |
73.56±2.70b |
69.53±4.47b | 94.75 |
FPOA induces HepG2 cell apoptosis
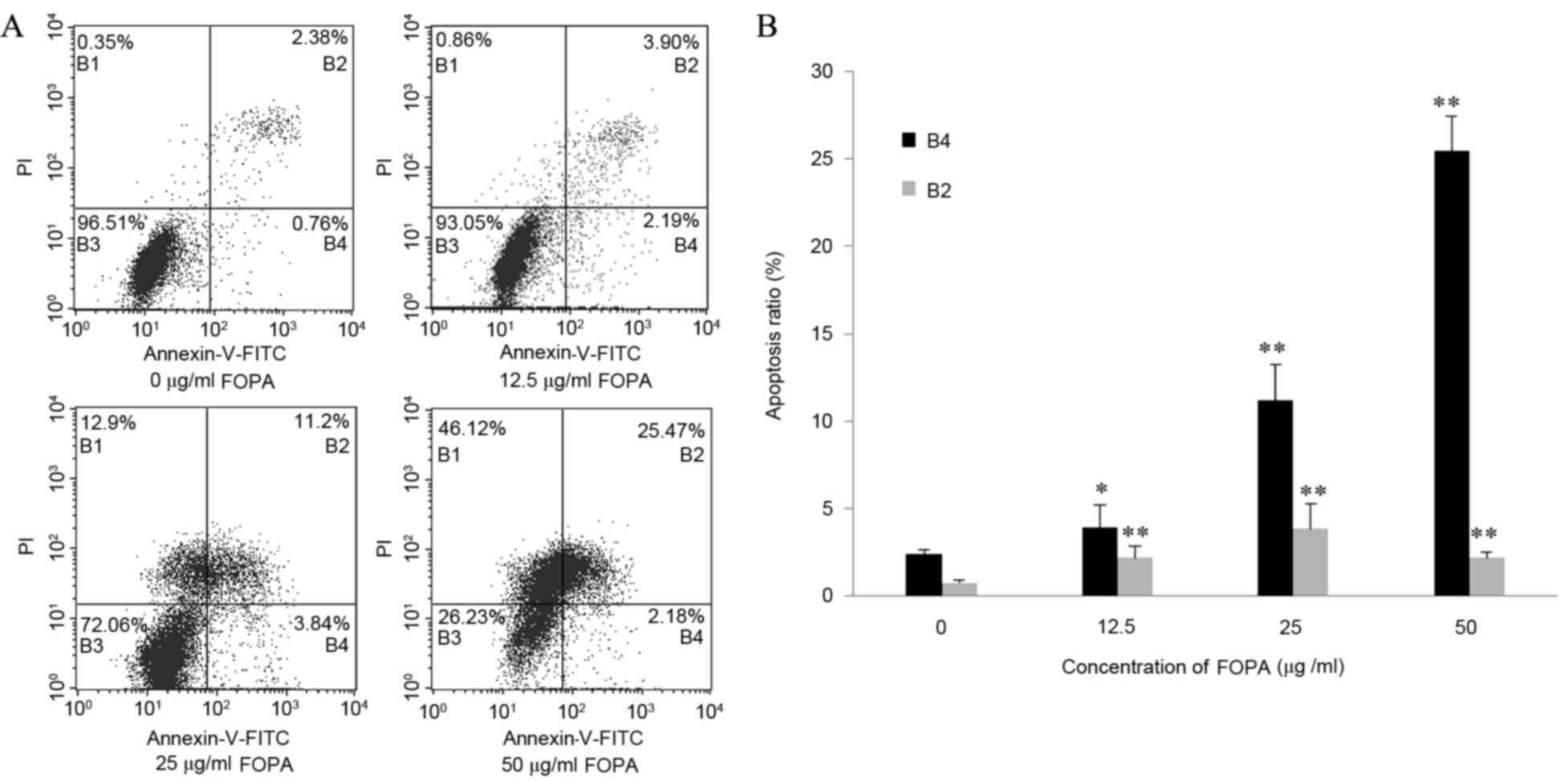
As demonstrated in Fig.
2A, HepG2 cells were distributed into four quadrants by flow
cytometry after Annexin/PI staining: Viable
(Annexin−/PI−); early apoptotic
(Annexin+/PI−); late apoptotic
(Annexin+/PI+) and necrotic
(Annexin−/PI+). The rate of apoptosis in the
control group (early and late apoptotic) was 3.14±0.47%. The
apoptosis rate of the HepG2 cells significantly increased following
FPOA treatment at all doses (P<0.05 vs. the control group;
Fig. 2B). This effect was observed
in a dose-dependent manner, with the apoptotic rate of the 50 µg/ml
FPOA group reaching 27.65±0.79% (Fig.
2B). These results indicate that FPOA induces significant
apoptosis in HepG2 cells in a dose-dependent manner.
Effect of FPOA on apoptosis-associated
protein expression
As demonstrated in Fig.
3, the protein expression of Bcl-2 and PARP were downregulated
in HepG2 cells exposed to FPOA for 24 h, while the protein
expression levels of Bax, cytochrome c, caspase-9,
caspase-cleaved PARP and caspase-3 increased. These results
demonstrated that expression of the antiapoptotic protein Bcl-2 was
inhibited by FPOA in a dose-dependent manner, whereas the levels of
proapoptotic Bax were increased. Furthermore, expression of
caspases-3, a key apoptotic protein, was activated, indicating that
apoptosis occurred.
 | Figure 3.Western blotting analysis of protein
extracts obtained from HepG2 cells treated with FPOA (0, 12.5, 25
and 50 µg/ml) for 24 h. Western blot and quantification of (A) PARP
and cleaved PARP proteins, (B) Bax/Bcl-2, (C) cyt c, (D)
caspase-9 and (E) caspase-3. *P<0.05, **P<0.01 vs. the
control group. FPOA, 3-acetoxylanosta-8,24-dien-21-oic acid; PARP,
poly (ADP) ribose polymerase; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; cyt c, cytochrome c. |
Discussion
Apoptosis is a fundamental process that takes place
throughout an organism's life. A regulated amount of apoptosis
allows for the development and maintenance of normal organs
(22). The majority of anticancer
drugs in clinical use induce tumor cell apoptosis, via activating
several apoptotic signaling pathways, including those of the
mitochondria and endoplasmic reticulum.
FPOA has previously been revealed to cause Hep 3B
cell death by apoptosis (14).
However, the apoptotic pathway induced by FPOA remains unclear. The
structure of the triterpenoid ganoderic acid from fungi is similar
to FPOA, the primary difference being the carboxyl position.
Previous studies have revealed that ganoderic acid induces
apoptosis in numerous tumor cells, including Bel-7402, HepG2 and
HeLa cells (23,24). Additionally, various molecular
mechanisms for this effect have been reported, including
apoptosis-associated proteins, death receptors, oxidative stress
and immunomodulation (23,24).
Bcl-2 family members serve an important role in
regulating apoptosis, particularly at the mitochondrial level
(25). Bcl-2 family proteins can be
divided into proapoptotic, including Bax, Bcl-2 homologous
antagonist/killer and Bcl-2-interacting killer, and antiapoptotic,
including Bcl-2, B-cell lymphoma-extra large and Bcl-2-like protein
2. Bax and Bcl-2 are important proteins in the process of
apoptosis. The ratio of proapoptotic Bax to antiapoptotic Bcl-2 is
a critical determinant of the extent of cellular susceptibility to
apoptosis (26). The present study
revealed that FPOA downregulated the expression of the Bcl-2
protein and upregulated the expression of Bax protein in HepG2
cells, resulting in a significant increase in the Bax/Bcl-2 ratio
and inducing apoptosis. An increased Bax/Bcl-2 ratio increases
mitochondrial membrane permeability, allowing cytochrome c
to combine with other factors and activate caspase family proteins,
triggering apoptosis (27). The
present study also identified an increase in cytochrome c
release from the mitochondria as the Bax/Bcl-2 ratio increased.
The caspase family of proteins serve an important
role in the process of apoptosis. Caspase-9 is an upstream protease
in the apoptotic signal transduction pathway and is a key enzyme of
apoptosis, while caspase-3 functions further downstream. Cleaved
PARP is the protein substrate of caspases-3. The present study used
western blot analysis to detect cleaved PARP, caspase-9 and
caspase-3 levels. Cleaved PARP, caspase-9 and caspase-3 levels were
determined to be increased significantly in FPOA-treated cells. In
conclusion, the results of the present study demonstrated that the
FPOA-induced apoptosis of HepG2 cells was associated with activated
caspase family proteins. Furthermore, the mitochondrial apoptotic
pathway was determined to be the underlying molecular mechanism for
this effect. These results indicate that FPOA is a potential
candidate for the development of anticancer drugs.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31270088), and the
Program for Yangtze River Scholars and Innovative Research Teams
(grant no. IRT1134).
References
|
1
|
National Cancer Institute (NCI), .
Profiles of six cancers: introductionCancer: Changing the
Conversation: The Nation's Investment in Cancer Research. U.S.
Department of Health and Human Services; pp. 34–51. 2012
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
3
|
Stewart BW and Wild CP: World Cancer
Report 2014. IARC Press; Lyon: 2014
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar
|
|
5
|
Krishnan V and Rajasekaran AK: Clinical
nanomedicine: A solution to the chemotherapy conundrum in pediatric
leukemia therapy. Clin Pharmacol Ther. 95:168–178. 2014. View Article : Google Scholar
|
|
6
|
Huang H, Liu T, Guo J, Yu L, Wu X, He Y,
Li D, Liu J, Zhang K, Zheng X and Goodin S: Brefeldin A enhances
docetaxel-induced growth inhibition and apoptosis in prostate
cancer cells in monolayer and 3D cultures. Bioorg Med Chem Lett.
27:2286–2291. 2017. View Article : Google Scholar
|
|
7
|
Diao Y, Ma X, Min W, Lin S, Kang H, Dai Z,
Wang X and Zhao Y: Dasatinib promotes paclitaxel-induced
necroptosis in lung adenocarcinoma with phosphorylated caspase-8 by
c-Src. Cancer Lett. 379:12–23. 2016. View Article : Google Scholar
|
|
8
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar
|
|
9
|
Lowe SW, Cepero E and Evan G: Intrinsic
tumour suppression. Nature. 432:307–315. 2004. View Article : Google Scholar
|
|
10
|
Boh B, Berovic M, Zhang J and Zhi-Bin L:
Ganoderma lucidum and its pharmaceutically active compounds.
Biotechnol Annu Rev. 13:265–301. 2007. View Article : Google Scholar
|
|
11
|
Chen G, Qian W, Li J, Xu Y and Chen K:
Exopolysaccharide of Antarctic bacterium Pseudoaltermonas sp. S-5
induces apoptosis in K562 cells. Carbohydr Polym. 121:107–114.
2015. View Article : Google Scholar
|
|
12
|
Ren Y, Yuan C, Deng Y, Kanagasabai R, Ninh
TN, Tu VT, Chai HB, Soejarto DD, Fuchs JR, Yalowich JC, et al:
Cytotoxic and natural killer cell stimulatory constituents of
Phyllanthus songboiensis. Phytochemistry. 111:132–140. 2015.
View Article : Google Scholar
|
|
13
|
Gao JJ, Min BS, Ahn EM, Nakamura N, Lee HK
and Hattori M: New triterpene aldehydes, lucialdehydes A-C, from
Ganoderma lucidum and their cytotoxicity against murine and human
tumor cells. Chem Pharm Bull (Tokyo). 50:837–840. 2002. View Article : Google Scholar
|
|
14
|
Gan KH, Fann YF, Hsu SH, Kuo KW and Lin
CN: Mediation of the cytotoxicity of lanostanoids and steroids of
Ganoderma tsugae through apoptosis and cell cycle. J Nat Prod.
61:485–487. 1998. View Article : Google Scholar
|
|
15
|
Lin CN, Fann YF and Chung MI: Steroids of
formosan Ganoderma tsugae. Photochemistry. 46:1143–1146. 1997.
View Article : Google Scholar
|
|
16
|
Muhammad D, Hubert J, Lalun N, Renault JH,
Bobichon H, Nour M and Voutquenne-Nazabadioko L: Isolation of
flavonoids and triterpenoids from the fruits of Alphitonia
neocaledonica and evaluation of their anti-oxidant, anti-tyrosinase
and cytotoxic activities. Phytochem Anal. 26:137–144. 2015.
View Article : Google Scholar
|
|
17
|
Pan JM, Zhou L, Wang GB, Xia GW, Xue K,
Cui XG, Shi HZ, Liu JH and Hu J: Fatsioside A inhibits the growth
of glioma cells via the induction of endoplasmic reticulum
stress-mediated apoptosis. Mol Med Rep. 11:3493–3498. 2015.
View Article : Google Scholar
|
|
18
|
Zhao XH, Bao HY and Cui BK: Progress of
researches on chemical constituents and pharmacogical actions of
Fomitopsis pinicola. J Fungal Research. 8:119–124. 2010.
|
|
19
|
Liu HB, Bao HY and Cui BK: Chemical
constituents of Fomitiporia ellipsoidea fruiting bodies.
Mycosystema. 30:459–463. 2011.
|
|
20
|
Song MJ, Bao HY, Bau T and Li Y: Antitumor
activity and structure-activity relationship of four steroids from
Fomitiporia ellipsoidea. Mycosystema. 34:293–300. 2015.
|
|
21
|
Lin CN, Tome WP and Won SJ: A lanostanoid
of Formosan Ganoderma lucidum. Photochemistry. 29:673–675. 1990.
View Article : Google Scholar
|
|
22
|
Kliche KO and Höffken K: The role of
apoptosis in hematologic malignancies and modulation of apoptosis
as a new therapeutic approach. J Cancer Res Clin Oncol.
125:226–231. 1999. View Article : Google Scholar
|
|
23
|
Liu RM, Li YB, Liang XF, Liu HZ, Xiao JH
and Zhong JJ: Structurally related ganoderic acids induce apoptosis
in human cervical cancer HeLa cells: Involvement of oxidative
stress and antioxidant protective system. Chem Biol Interact.
240:134–144. 2015. View Article : Google Scholar
|
|
24
|
Tang W, Liu JW, Zhao WM, Wei DZ and Zhong
JJ: Ganoderic acid T from Ganoderma lucidum mycelia induces
mitochondria mediated apoptosis in lung cancer cells. Life Sci.
80:205–211. 2006. View Article : Google Scholar
|
|
25
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar
|
|
26
|
Wang WD and Chen ZT: Bcl-2/Bax ratio and
the ‘life or death fate’ of cells. Chin J Cancer Biother.
4:393–396. 2007.
|
|
27
|
Whiteman M, Chu SH, Siau JL, Rose P,
Sabapathy K, Schantz JT, Cheung NS, Spencer JP and Armstrong JS:
The pro-inflammatory oxidant hypochlorous acid induces
Bax-dependent mitochondrial permeabilisation and cell death through
AIF-/EndoG-dependent pathways. Cell Signal. 19:705–714. 2007.
View Article : Google Scholar : PubMed/NCBI
|