Introduction
Chronic rhinosinusitis (CRS) is one of the most
common otorhinolaryngological diseases, which severely impairs
quality of life and induces a heavy economic burden on patients
(1). However, the etiology and
pathogenesis of CRS remain unknown, making its diagnosis,
classification and treatment challenging (2). A greater understanding of the molecular
pathological mechanisms underlying the onset of CRS may facilitate
the identification of a novel method of diagnosis and the
development of novel therapeutic strategies to treat the
condition.
The majority of patients with CRS also experience
nasal polyps (NP), which has a high rate of recurrence even
following the administration of appropriate drugs and surgical
treatments (3). This has led to the
classification of CRS as either CRS with nasal polyps (CRSwNP) or
CRS without nasal polyps (CRSsNP) (4). It has been demonstrated that the
pro-inflammatory cytokine interleukin-32, is differentially
expressed in the nasal epithelial cells of patients with CRSwNP and
those with CRSsNP (5). Although the
differential expression of inflammatory mediators in CRSwNP and
CRSsNP has been previously demonstrated (6), further studies are required to provide
a basis for the accurate diagnosis and the development of effective
treatment strategies.
Clara cell 10-kDa protein (CC10), also known as
uteroglobin, is a steroid-inducible member of the secretoglobin
family that serves an important role in the regulation of
anti-inflammatory and immunomodulatory activities (7,8). CC10 is
constitutively expressed in the epithelial cells of organs that
directly communicate with the external environment, including the
nose, bronchi and lungs (7–9). Furthermore, CC10 is an important
mediator of inflammatory and allergic responses, as well as
responses to malignant tumors of the respiratory system (10). Pulmonary CC10 protein serves a role
in the T-cell-mediated inflammatory response by modulating
expression of the T helper 2 cytokine (11). Furthermore, the histamine H1 receptor
antagonist fexofenadine hydrochloride significantly elevates the
expression of CC10 protein in nasal epithelial cells, which may
partially account for the therapeutic effect of fexofenadine
hydrochloride in the treatment of allergic disorders (12). Additionally, it has been demonstrated
that increased levels of CC10 are associated with improvements in
bronchial dysplasia and sputum cytometric assessment in patients at
high risk of developing lung cancer (13). However, the expression of CC10 in
patients with CRS and NP and its role in the development of the
disease, remain unknown.
The trefoil factor (TFF) family members TFF1, TFF2
and TFF3 are secreted from a variety of mucous epithelial tissues
and function as important regulators of cell migration, immune
reaction, mucosa repair, angiogenesis and tumorigenesis (14–16). TFF
peptides have also been identified in many other organs, including
the respiratory tract, gallbladder, prostate, uterus, thyroid
gland, salivary glands, mammary glands and the nervous system
(17). TFF1, also known as pS2,
contains 60 amino acid residues and is mainly expressed in the
gastric mucosa as a protective factor against gastric damage
(18). However, it has also been
demonstrated that TFF1 is widely expressed in human and mouse nasal
mucosa, suggesting that it may also regulate the inflammatory
response in the respiratory tract (19–21). The
expression and function of TFF1 in CRS and NP remains unclear.
The present study investigated the differential
expression of CC10 and TFF1 in the nasal mucosa of patients with
CRS and NP to provide further information regarding the pathology
of CRS.
Materials and methods
Patients and samples
Nasal mucosa samples were obtained from different
patient groups as follows: i) 20 samples from patients who were
diagnosed with CRS without NP and had undergone endoscopic sinus
surgery for CRS at Dongying People's Hospital (Donying, China) (13
males, 7 females; age range, 22–70 years); ii) 20 samples from
patients who were diagnosed with NP without CRS and had undergone
endoscopic sinus surgery for NP at Dongying People's Hospital (14
males, 6 females; age range, 20–73 years); and iii) 18 samples from
healthy controls (10 males, 8 females; age range, 18–55 years). All
patients were recruited from September 2014 to October 2015. All
samples were stored at −80°C immediately after collection. CRS and
NP were diagnosed according to the combined criteria developed by
the American Academy of Allergy, Asthma & Immunology and
American Academy of Otolaryngology-Head and Neck Surgery (22,23).
Diagnosis of CRS was based on typical sinusitis symptoms, including
thick mucus, a blocked nose and pain in the face lasting >12
weeks and positive findings from a computer topography (CT) scan of
the unilateral or bilateral nose. NP was determined by the presence
of polyps in the middle meatus or nasal cavity detected by
endoscopy or surgery. Normal nasal mucosa samples collected from
the inferior turbinate or uncinate process of 18 patients with
nasal septum deviation and no sign of anterior ethmoid sinusitis in
CT and nasal endoscopy were used as healthy controls (Table I). These patients were also recruited
from Dongying People's Hospital. The current study was approved by
the Ethics Committee of Dongying People's Hospital and informed
consent was obtained from all participants.
 | Table I.Clinical data of patients with CRS and
NP. |
Table I.
Clinical data of patients with CRS and
NP.
| Feature | Control | CRS | NP |
|---|
| Number of
patients | 18 | 20 | 20 |
| Sex
(male/female) | 10/8 | 13/7 | 14/6 |
| Age (years) | 18–55 | 22–70 | 20–73 |
| Skin Prick Test | 0 | 4 | 3 |
| History of
asthma | 0 | 0 | 2 |
| History of
smoking | 5 | 9 | 8 |
| Aspirin
intolerance | 0 | 0 | 0 |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA samples were extracted using the
TRIzol® reagent (cat. no. 15596026; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. The ultraviolent absorbance of 2 µl RNA
diluted with 198 µl Milli-Q water was measured at wavelengths of
260 and 280 nm. The concentration and purity of RNA samples was
calculated as follows: RNA concentration (µg/µl)=optical
density(OD)260 ×4 and RNA
purity=OD260/OD280. RNA samples with a purity
of OD260/OD280 >1.8, were used for
RT-qPCR. The cDNA was synthesized using a PrimeScript™ First Strand
cDNA Synthesis kit (cat. no. 6110A; Takara Bio, Inc., Otsu, Japan)
according to the manufacturer's protocol. Reverse transcription was
performed at 42°C for 1 h followed by RNA transcriptase
inactivation at 72°C for 10 min. qPCR reactions were subsequently
performed using a SYBR Fast qPCR mix (cat. no. RR430A; Takara Bio,
Inc.). The primer sequences used in qPCR are presented in Table II. qPCR was performed using the
following settings: Pre-denaturation at 95°C for 10 sec, followed
by 40 cycles of denaturation at 95°C for 5 sec and elongation at
60°C for 30 sec. The threshold cycle of each sample was identified
using LightCycler version 96 software combined with the LightCycler
96 Real-Time PCR system (both Roche Applied Science, Penzberg,
Germany). Relative expression was calculated using the
2−ΔΔCq method and β-actin was used as the internal
standard. Expression of CC10 and TFF1 in patients with CRS and NP
were compared with that in normal nasal mucosa of the inferior
turbinate collected from the control group.
 | Table II.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
|
| Primer sequences
(5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| CC10 |
GATCAAGACATGAGGGAGGCA |
CACAGTGAGCTTTGGGCTATTT |
| TFF1 |
CAATGGCCACCATGGAGAAC |
AACGGTGTCGTCGAAACAGC |
| β-actin |
GAAGGTGAAGGTCGGAGTC |
GGAAGATGGTGATGGGATT |
Immunohistochemical analysis and
hematoxylin and eosin (H&E) staining
The expression of TFF1 and CC10 was determined using
immunohistochemistry, following the streptavidin biotin-peroxidase
complex (SABC) method. Tissues were fixed overnight in 4%
paraformaldehyde at room temperature, embedded in paraffin and
sliced into 4-µm-thick serial sections. Slides were deparafinized
in two treatments of xylene (each 5 min) and transferred to 100%
alcohol for two treatments (each 3 min), then once through 95, 70
and 50% alcohols respectively for 3 min each. The slides were then
incubated in 3% hydrogen peroxide to remove endogenous oxidases for
10 min at room temperature. After rinsing twice in PBS (5 min
each), a citrate buffer were used to performed antigen retrieval
(95–100°C for 10 min). Normal 5% goat serum (cat. no. 5425, Cell
Signaling Technology, Inc., Danvers, MA, USA) was used as a
blocking reagent and the slides were incubated for 1 h at a room
temperature. The remaining steps were performed using an SABC kit
purchased from Wuhan Boster Biological Technology Ltd. (Wuhan,
China) and a diaminobenzidine kit (cat. no. ZLI-9017), which was
purchased from Beijing ZSGB-Bio Co., Ltd. (Beijing, China). Each
kit was used according to the manufacturer's protocol. PBS buffer
was applied to replace primary antibodies in the negative control.
Rabbit anti-human CC10 (cat. no. sc-25555) and mouse anti-human
TFF1 (cat. no. sc-271464) polyclonal antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) and used at
dilutions of 1:800 and 1:100, respectively. Slides were incubated
with CC10 and TFF1 antibodies for 1 h at room temperature. Positive
staining was defined by fine yellow particles in the field of view
using high-magnification optical microscopy. H&E staining was
conducted following a previously published protocol (24).
Western blot analysis
Total protein from human nasal mucosa tissues was
extracted using radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China), following the
manufacturer's protocol. Following the measurement of protein
concentration using a Pierce™ BCA Protein assay kit (no. 23227;
Pierce; Thermo Fisher Scientific, Inc.), total protein from the
control, CRS and NP groups (15 µg) was subjected to 15% SDS-PAGE
and blotted onto PVDF membranes. PVDF membranes were then blocked
with TBS buffer containing 5% milk powder for 2 h at room
temperature, washed 3 times for 5 min using TBS buffer, incubated
with anti-CC10, anti-TFF1 or anti-GAPDH (1:1,000; cat no. AF0006;
Beyotime Institute of Biotechnology) antibodies for 1 h at room
temperature, washed three times for 5 min using TBS buffer and
incubated with horseradish peroxidase-conjugated secondary
antibodies (1:10,000; cat nos. A4416 and A6154; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 1 h at room temperature. Rabbit
anti-human CC10 (1:500; cat. no. sc-25555) polyclonal antibodies
and mouse anti-human TFF1 (1:500, cat. no. sc-271464) polyclonal
antibodies were purchased from Santa Cruz Biotechnology, Inc. The
membranes were subsequently rinsed with TBS three times, the blots
were visualized using an enhanced chemiluminescence western blot
detection reagent (cat. no. 321096; Thermo Fisher Scientific, Inc.)
and exposed to X-ray film.
Statistical analysis
Data analysis was performed using SPSS version 16.0
(SPSS, Inc., Chicago, IL, USA) and P<0.05 was determined to
indicate a statistically significant difference. One-way analysis
of variance was used followed by Tukey's post-hoc test. The
correlation between TFF1 and CC10 expression was analyzed using
Spearman's correlation analysis.
Results
Expression of CC10 in human nasal
mucosa from patients with CRS and NP
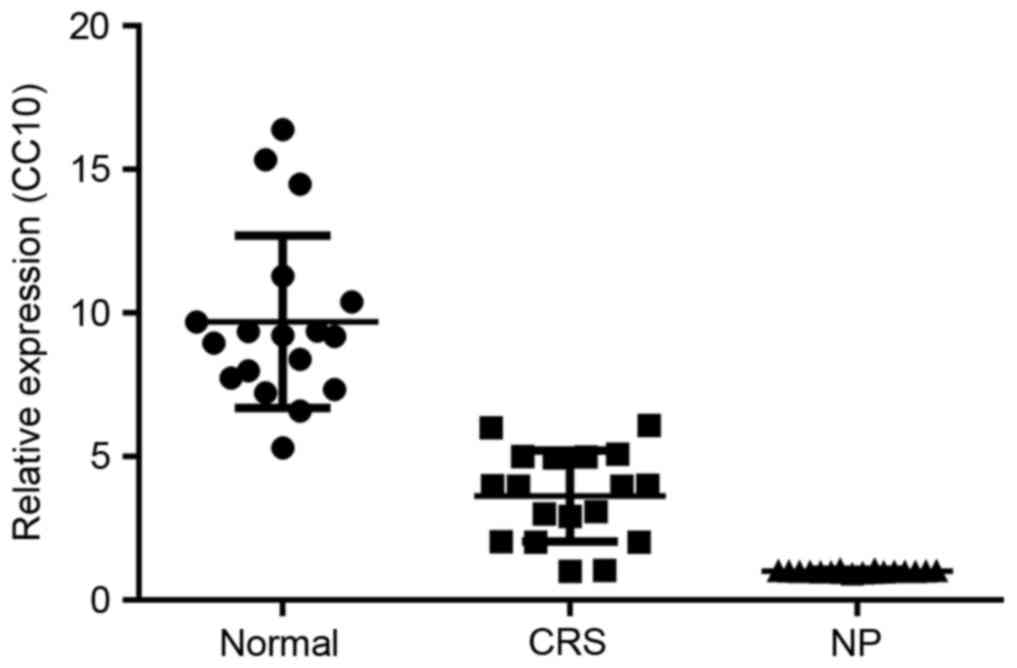
The expression of CC10 mRNA in the control, CRS and
NP groups was initially analyzed using RT-qPCR. The expression of
CC10 mRNA in the CRS group was significantly decreased compared
with that of the control group (P<0.05; Fig. 1). Furthermore, the expression of CC10
mRNA was significantly decreased in the NP group compared with that
of the CRS group (P<0.05; Fig.
1).
Expression of TFF1 in the nasal mucosa
of patients with CRS and NP
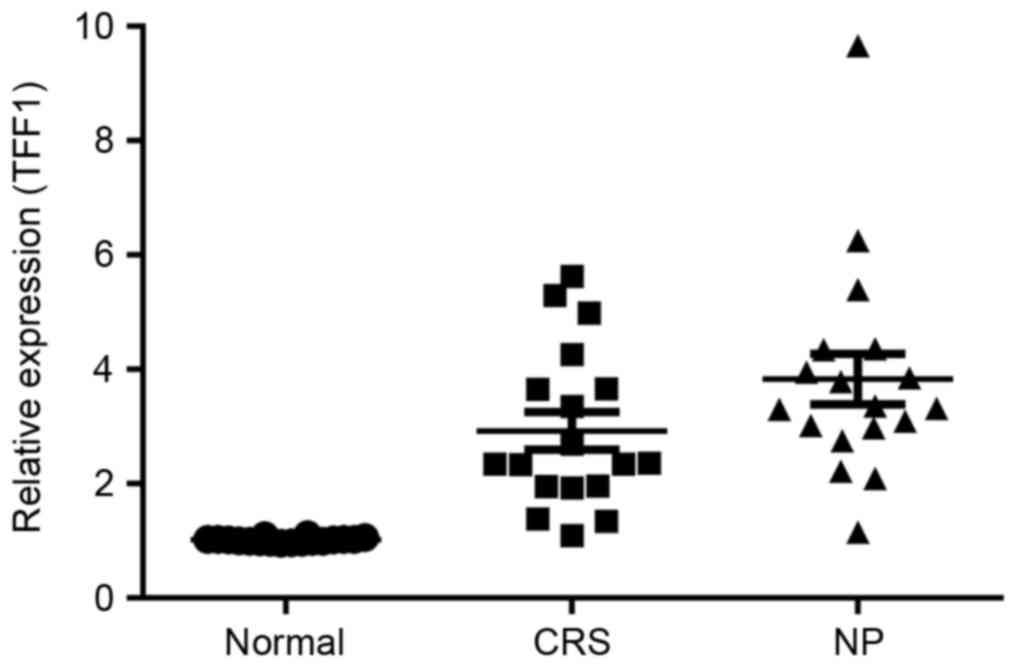
The potential role served by TFF1 in the
pathogenesis of CRS and NP was investigated by determining the
expression of TFF1 in the control, CRS and NP groups using RT-qPCR.
The results demonstrated that the expression of TFF1 in the CRS
group was significantly increased compared with that in the control
group (P<0.05; Fig. 2). TFF1
expression in the NP group exhibited a further significant increase
compared with the CRS group (P<0.05; Fig. 2).
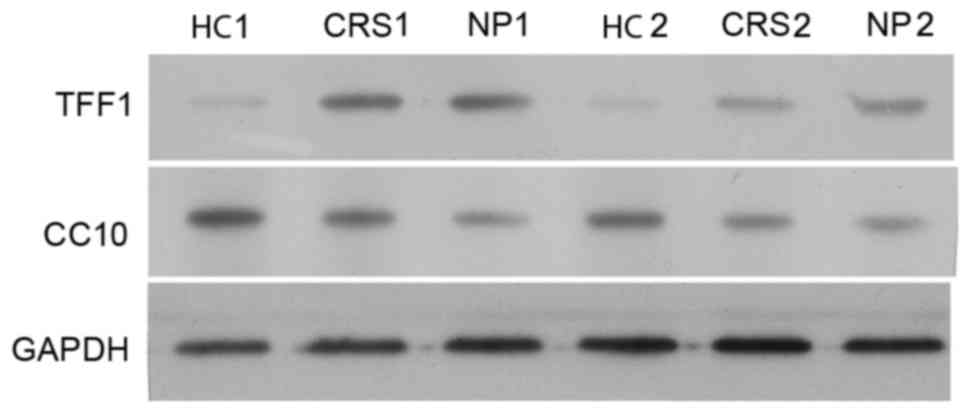
CC10 and TFF1 protein expression in
human nasal mucosa of patients with CRS and NP
The expression of CC10 and TFF1 proteins in nasal
mucosa tissues from the control, CRS and NP groups was confirmed
using western blot analysis. Two samples of HC, CRS and NPS were
randomly selected to perform western blot analysis on. The
expression of CC10 protein in the CRS and NP groups was lower
compared with those of the control group and a larger decrease in
the expression of CC10 protein was observed in the NP group
compared with the CRS group (Fig.
3). This is consistent with the results of the RT-qPCR analysis
(Fig. 2). By contrast, the
expression of TFF1 protein in the CRS and NP groups was higher
compared with the control (Fig. 3).
There was no difference in expression of TFF1 protein between the
CRS and NP groups (Fig. 3).
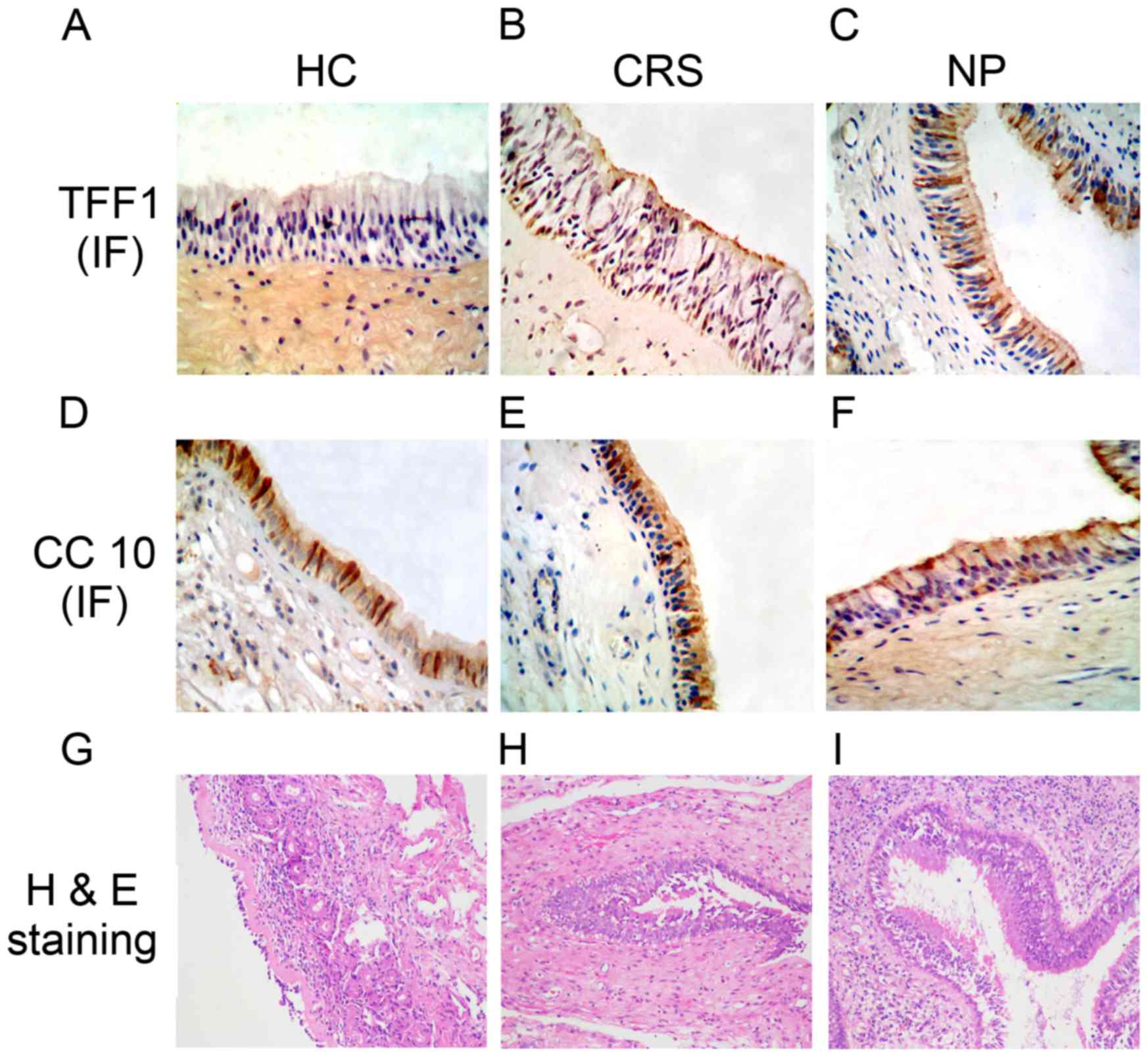
Immunohistochemical analysis
Immunohistochemical staining was performed using the
SABC method to measure the expression of TFF1 and CC10. The results
demonstrated that TFF1 was primarily expressed in goblet and
ciliated cells and distributed in the cytoplasmic regions
surrounding the nucleus (Fig. 4A-C).
The expression of TFF1 in the mucosa tissues of patients in the CRS
(Fig. 4B) and NP (Fig. 4C) groups was increased compared with
that of the control group (Fig. 4A).
Additionally, the results determined that CC10 was mainly expressed
in the epithelial cells of the nasal mucosa from all groups
(Fig. 4D-F). The staining densities
in the control group (Fig. 4D) were
lower compared with the CRS (Fig.
4E) and NP (Fig. 4F) groups,
which clearly demonstrated that expression of CC10 is decreased in
patients with CRS and NP.
The results of H&E staining clearly identified a
lesion in the CRS and NP groups, which was not present in the
control group (Fig. 4G-I). A
thickened mucous layer, hyperplastic goblet cells and deciduous
cilia were also detected in the CRS (Fig. 4H) and NP (Fig. 4I) groups however, these were more
marked in the NP group compared with the CRS group.
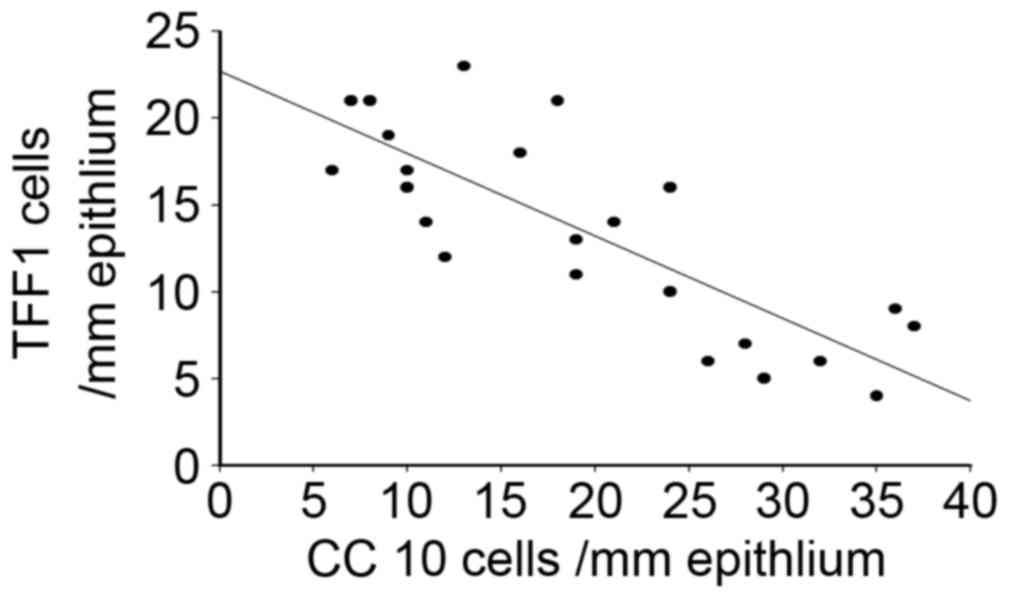
Correlation of CC10 and TFF1
expression in human nasal mucosa of patients with CRS and NP
Considering the differential expression of CC10 and
TFF1 in human nasal mucosa from patients with CRS and NP, a
Spearman's correlation analysis was performed. The results
demonstrated that there was a significant negative correlation
between the expression of CC10 and TFF1 in the tissues of patients
with CRS and NP (r=−0.89, P<0.05; Fig. 5), suggesting that CC10 and TFF1 may
interact during the development of CRS and NP.
Discussion
The pathogenesis of CRS and NP is not fully
understood; however, previous studies have focused on several
hypotheses, including bacterial infections, fungal colonization,
local anatomical structure variation, trauma, allergic reactions
and environmental pollution. CRS and NP may be caused by these
factors and mediated by mucosal immune dysfunction and excessive
inflammatory reactions. These may therefore serve a critical role
in the early development of CRS and NP and indicates that treating
local mucosal immune disorders and excessive inflammatory reaction
may attenuate the development of CRS and NP (25,26).
The role served by CC10 in the progression and
development of CRS and NP remains controversial. It has been
demonstrated that CC10 is expressed in the goblet, non-mucinous and
non-ciliated cells of the nasal sinus mucosa epithelium and that
the number of CC10-positive cells is negatively correlated with
inflammatory cell infiltration and the number of goblet cells. This
indicates that downregulation of CC10 expression may lead to
anti-inflammatory network dysfunction in the epithelial cells of
the upper respiratory tract and a persistent severe inflammatory
response eventually leads to the formation of NP (9). CC10 expression is negatively correlated
with preoperative CT scores, as well as postoperative nasal
endoscopy and symptom scores (8). By
contrast, fexofenadine hydrochloride elevates the expression of
CC10 protein in nasal epithelial cells, which may partially account
for its therapeutic effect on allergic disorders (12). Furthermore, increased CC10 expression
is associated with improvements in bronchial dysplasia and sputum
cytometric assessment in patients at high risk of developing lung
cancer (13). A previous study
focusing on the effect of inflammatory cytokines on CC10 expression
using nasal mucosa tissue culture identified that tumor necrosis
factor-α, interleukin (IL)-1β and IL-4 inhibited the expression of
CC10, whereas IL-10 and interferon-γ promoted the expression of
CC10 (8). These contradictory
results suggest that CC10 expression is very sensitive to
pathological conditions and its differential expression may be
context-dependent. In the present study, CC10 expression was
significantly increased in the nasal mucosa tissues of patients
with CRS and NP compared with the control group, which further
demonstrates that CC10 serves a role in the pathology of CRS and
NP.
Previous studies have demonstrated that TFF peptides
are highly expressed in the goblet cells, submucosal glands and
ciliary epithelial cells of the respiratory tract; however, TFF has
not been identified in the alveolar epithelium. Among the three TFF
peptides, TFF3 exhibited the highest expression in the human
respiratory tract, followed by TFF1, whereas TFF2 was undetectable
(19,20,27). The
current study analyzed the expression of TFF1 in human nasal mucosa
using RT-qPCR and identified a significant decrease in the
expression of TFF1 in the nasal mucosa of patients with CRS and NP.
Immunohistochemistry and western blot analysis also clearly
demonstrated that the expression of TFF1 protein was significantly
increased in patients with CRS and NP, indicating that TFF1 may
serve an important role in the progression of the disease. It has
been demonstrated that TFF1 is mainly expressed in the goblet
cells, cilia epithelial cells and mucosal glands of the respiratory
tract from the nasal cavity to the bronchioles and its expression
pattern overlaps with the secretion of airway mucin, which is
distributed in the cytoplasmic regions surrounding the nucleus
(28). In combination with the
results of the current study, the expression of TFF in the human
nasal mucosa tissues suggests that they serve a potential role in
the pathology of CRS and NP.
Furthermore, it was previously demonstrated in that
there was a negative correlation between the expression of CC10 and
TFF1 during the progression of inflammation and alteration of the
cell phenotype (29). However, the
association between CC10 and TFF1 expression in human nasal mucosa
tissues during the development CRS and NP remains unknown. The
current study demonstrated that the expression of CC10 was
significantly increased in the nasal mucosa tissues of patients
with CRS and NP, whereas the expression of TFF1 was significantly
decreased. Spearman's correlation analysis identified a negative
correlation between the expression of TFF1 and CC10.
TFF1 is an important regulator of epithelial cell
migration and the inflammatory process in the airway, which may
serve an important regulatory role in the process of airway defense
and injury repair (15,20). TFF1 delays the transition from G1 to
the S-phase in the cell cycle, thus regulating tumor inhibition,
mucosal protection, cell apoptosis and differentiation (30). The expression of TFF1 in inflammation
and malignant tumors indicates the status of prognosis and is
significantly negatively correlated with the therapeutic effect of
hormone therapy (29,31). The decreased expression of TFF1 and
its negative correlation with CC10 in human nasal mucosa in the
current study suggests that TFF1 deficiency may contribute to the
onset and progression of CRS and NP, and regulation of the
expression of TFF1 and CC10 may be a potential treatment strategy
for patients with CRS and NP.
In conclusion, the current study identified the
differential expression of CC10 and TFF1 in human nasal mucosa
tissues from patients with CRS and NP. The negative correlation
between CC10 and TFF1 suggests that they may serve a role the
pathogenesis of CRS and NP. Therefore, the expression of these two
markers may be a potential target for the diagnosis and treatment
of CRS and NP in the future.
References
|
1
|
Fokkens W, Lund V and Mullol J; European
Position Paper on Rhinosinusitis and Nasal Polyps group, : European
position paper on rhinosinusitis and nasal polyps 2007. Rhinol
Suppl. 20:1–136. 2007.PubMed/NCBI
|
|
2
|
Rosenfeld RM, Andes D, Bhattacharyya N,
Cheung D, Eisenberg S, Ganiats TG, Gelzer A, Hamilos D and Hudgins
PA III: Clinical practice guideline: Adult sinusitis. Otolaryngol
Head Neck Surg. 137(3 Suppl): S1–S31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaliner MA, Osguthorpe JD, Fireman P, Anon
J, Georgitis J, Davis ML, Naclerio R and Kennedy D: Sinusitis:
Bench to bedside. Current findings, future directions. J Allergy
Clin Immunol. 99 Suppl:S829–S848. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Polzehl D, Moeller P, Riechelmann H and
Perner S: Distinct features of chronic rhinosinusitis with and
without nasal polyps. Allergy. 61:1275–1279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keswani A, Chustz RT, Suh L, Carter R,
Peters AT, Tan BK, Chra R, Azam T and Dinarello CA: Differential
expression of interleukin-32 in chronic rhinosinusitis with and
without nasal polyps. Allergy. 67:25–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Zele T, Claeys S, Gevaert P, Van Maele
G, Holtappels G, Van Cauwenberge P and Bachert C: Differentiation
of chronic sinus diseases by measurement of inflammatory mediators.
Allergy. 61:1280–1289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh G and Katyal SL: Clara cell
proteins. Ann N Y Acad Sci. 923:43–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Z, Lu X, Zhang XH, Bochner BS, Long
XB, Zhang F, Wang H and Cui YH: Clara cell 10-kDa protein
expression in chronic rhinosinusitis and its cytokine-driven
regulation in sinonasal mucosa. Allergy. 64:149–157. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Kim J, Sypek JP, Wang IM, Horton H,
Oppenheim FG and Bochner BS: Gene expression profiles in human
nasal polyp tissues studied by means of DNA microarray. J Allergy
Clin Immunol. 114:783–790. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Yu HJ, Wang N, Zhang YN, Huang SK,
Cui YH and Liu Z: Clara cell 10-kDa protein inhibits T(H)17
responses through modulating dendritic cells in the setting of
allergic rhinitis. J Allergy Clin Immunol. 131:387–394.e1-12. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hung CH, Chen LC, Zhang Z, Chowdhury B,
Lee WL, Plunkett B, Chen CH, Myers AC and Huang SK: Regulation of
TH2 responses by the pulmonary Clara cell secretory 10-kd protein.
J Allergy Clin Immunol. 114:664–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nogaki T, Asano K, Furuta A, Kanai K,
Suzaki I, Kanei A and Suzaki H: Enhancement of clara cell 10-kD
protein (CC10) production from nasal epithelial cells by
fexofenadine hydrochloride. Asian Pac J Allergy Immunol.
30:139–145. 2012.PubMed/NCBI
|
|
13
|
Chen J, Lam S, Pilon A, McWilliams A,
Macaulay C and Szabo E: Higher levels of the anti-inflammatory
protein CC10 are associated with improvement in bronchial dysplasia
and sputum cytometric assessment in individuals at high risk for
lung cancer. Clin Cancer Res. 14:1590–1597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oertel M, Graness A, Thim L, Bühling F,
Kalbacher H and Hoffmann W: Trefoil factor family-peptides promote
migration of human bronchial epithelial cells: Synergistic effect
with epidermal growth factor. Am J Respir Cell Mol Biol.
25:418–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoffmann W: Trefoil factors TFF (trefoil
factor family) peptide-triggered signals promoting mucosal
restitution. Cell Mol Life Sci. 62:2932–2938. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kjellev S: The trefoil factor family-small
peptides with multiple functionalities. Cell Mol Life Sci.
66:1350–1369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Belovari T, Bijelić N, Tolušić Levak M and
Baus Lončar M: Trefoil factor family peptides TFF1 and TFF3 in the
nervous tissues of developing mouse embryo. Bosn J Basic Med Sci.
15:33–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thim L and May FE: Structure of mammalian
trefoil factors and functional insights. Cell Mol Life Sci.
62:2956–2973. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee SH, Lee SH, Oh BH, Lee HM, Choi JO and
Jung KY: Expression of mRNA of trefoil factor peptides in human
nasal mucosa. Acta Otolaryngol. 121:849–853. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
dos Santos Silva E, Ulrich M, Döring G,
Botzenhart K and Gött P: Trefoil factor family domain peptides in
the human respiratory tract. J Pathol. 190:133–142. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kouznetsova I, Chwieralski CE, Bälder R,
Hinz M, Braun A, Krug N and Hoffmann W: Induced trefoil factor
family 1 expression by trans-differentiating Clara cells in a
murine asthma model. Am J Respir Cell Mol Biol. 36:286–295. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slavin RG, Spector SL, Bernstein IL,
Kaliner MA, Kennedy DW, Virant FS, Wald ER, Khan DA, Blessing-Moore
J, Lang DM, et al: The diagnosis and management of sinusitis: A
practice parameter update. J Allergy Clin Immunol. 116(6 Suppl):
S13–S47. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scadding GK, Durham SR, Mirakian R, Jones
NS, Drake-Lee AB, Ryan D, Dixon TA, Huber PA and Nasser SM; British
society for allergy and clinical immunology, : BSACI guidelines for
the management of rhinosinusitis and nasal polyposis. Clin Exp
Allergy. 38:260–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou Y, Wang Y, Wang SB, Kong YG, Xu YU,
Tao ZZ and Chen SM: Characteristic expression and significance of
CCL19 in different tissue types in chronic rhinosinusitis. Exp Ther
Med. 11:140–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Norlander T, Westrin KM and Stierna P: The
inflammatory response of the sinus and nasal mucosa during
sinusitis: Implications for research and therapy. Acta Otolaryngol
Suppl. 515:38–44. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bernstein JM: The molecular biology of
nasal polyposis. Curr Allergy Asthma Rep. 1:262–267. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wiede A, Jagla W, Welte T, Köhnlein T,
Busk H and Hoffmann W: Localization of TFF3, a new mucus-associated
peptide of the human respiratory tract. Am J Respir Crit Care Med.
159:1330–1335. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomasetto C and Rio MC: Pleiotropic
effects of Trefoil Factor 1 deficiency. Cell Mol Life Sci.
62:2916–2920. 2006. View Article : Google Scholar
|
|
29
|
Cui YH, Wang YY and Liu Z:
Transdifferentiation of Clara cell 10-kDa protein secreting cells
in experimental allergic rhinitis. Am J Rhinol Allergy. 25:145–151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bossenmeyer-Pourié C, Kannan R, Ribieras
S, Wendling C, Stoll I, Thim L, Tomasetto C and Rio MC: The trefoil
factor 1 participates in gastrointestinal cell differentiation by
delaying G1-S phase transition and reducing apoptosis. J Cell Biol.
157:761–770. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perry JK, Kannan N, Grandison PM, Mitchell
MD and Lobie PE: Are trefoil factors oncogenic? Trends Endocrinol
Metab. 19:74–81. 2008. View Article : Google Scholar : PubMed/NCBI
|