Introduction
Of all human brain tumors, >80% are gliomas,
which have a poor prognosis with a 5-year survival rate of <5%
(1). Glioblastoma multiforme (GBM)
accounts for 15% of brain tumors (2). Although multiple methods are available
to treat glioblastoma multiforme (GBM), including radiotherapy,
surgery, chemotherapy and photodynamic therapy (3,4),
survival of patients remains poor. Therefore, it is necessary to
understand the pathogenic processes at the molecular level in order
to identify novel markers and molecular targets that may improve
the diagnosis, predict outcomes and provide novel treatment
approaches.
The forkhead box (FOX) family of proteins are
involved in multiple crucial biological processes and human
diseases (5,6). FOXK1 was first reported in 1994 as a
DNA-binding protein, which specifically binds to the CCAC box motif
(7). FOXK1 contains a forkhead
domain and a forkhead-associated domain, which bear a DNA-binding
region and a phosphopeptide recognition region, respectively
(8,9). FOXK1 has been identified to interact
with four and a half LIM domains 2 in myogenic progenitor cells
(10). In addition, FOXK1 interacts
with Sin3 protein via the Sin3-interacting domain, thereby
regulating myogenic progenitors (10). FOXK1 also takes part in development
and oncogenesis (11). However, the
role of FOXK1 in GBM has remained elusive.
Epithelial to mesenchymal transition (EMT) is a
complex process, during which cells lose their epithelial
properties while gaining mesenchymal characteristics. Multiple
epithelial and mesenchymal markers are known, including E-cadherin
as an epithelial marker and N-cadherin as a mesenchymal marker.
E-cadherin is an important protein that participates in cell
anchoring junctions, so that EMT results in loss of cell-to-cell
contact and therefore facilitates cell motility; therefore, EMT
promotes tumor cell metastasis (12,13).
Several studies indicated that multiple transcription factors are
highly expressed during EMT, including Snail, Slug, ZEB and TWIST,
which repress E-cadherin transcription (14–16).
In order to explore the molecular mechanisms of the
effects of FOXK1 in GBM, the present study detected FOXK1
expression levels in GBM tissues by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
revealed that FOXK1 was not only highly expressed, but positively
associated with tumor size and metastasis. In addition, it was
demonstrated that FOXK1 facilitates EMT through activation of the
transcription of Snail. Furthermore, fluorescence-assisted cell
sorting (FACS) analysis indicated that FOXK1 promotes GBM cell
proliferation through regulating the cell cycle. In brief, FOXK1,
as a crucial transcription factor, has a key function in GBM cell
proliferation and EMT.
Materials and methods
GBM tissue samples and cell lines
A total of 83 pairs of GBM tumor tissues and
adjacent non-tumorous tissues were collected from the neurosurgery
department of Renmin Hospital of Wuhan University between 2013 and
2016. All GBM patients were histologically confirmed. Clinical
data, including patient age, gender, tumor size and metastasis were
collected from the information system of Renmin Hospital of Wuhan
University. All patients have provided informed consent for use of
their data/specimens. All tissue experiments were approved by the
Ethics Committee of Wuhan University (Wuhan, China).
The T98G and LN18 human GBM cell lines, and normal
human astrocytes (NHAs) derived from XCL-1 GFAPp-Nanoluc-Halotag
(ATCC®ACS-5006™) were purchased from the American Type
Tissue Collection (Manassas, VA, USA) and cultured in Dulbecco's
modified Eagle's medium (DMEM; HyClone, Logan, UT, USA) containing
10% fetal bovine serum (FBS; HyClone) at 37°C a humidified
atmosphere containing 5% CO2.
Western blot analysis
To obtain total protein, cells were lysed with
radioimmunoprecipitation assay buffer containing protease inhibitor
cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Following
centrifugation at 13,000 × g for 10 min at 4°C, the protein
concentration in the supernatant was determined using a
bicinchoninic protein assay kit (Pierce; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Total protein (50 µg per lane) was
subjected to 10% SDS-PAGE and transferred protein onto
nitrocellulose membranes (EMD Millipore, Billerica, MA, USA).
Membranes were then blocked in 5% non-fat milk at room temperature
for 1 h, followed by incubation with primary antibodies at 4°C
overnight. Following washing in PBS containing Tween 20, membranes
were then incubated with horseradish peroxidase-secondary
antibodies (1:5,000; Abcam, Cambridge, MA, USA; cat. nos. ab6721
and ab97023) at room temperature for 1 h. Finally, protein bands
were visualized by enhanced chemiluminescence (Thermo Fisher
Scientific, Waltham, MA, USA; cat. no. 32106). The following
antibodies were used: FOXK1 (1:2,000; Abcam; cat. no. ab172730),
EMT antibody kit (1:2,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA; cat. no. 9782) and β-actin (1:5,000;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; cat. no. A2228).
β-actin served as an internal control.
Cell transfection
T98G and LN18 cells were cultured to 60–70%
confluence and transfected with PCMV-Tag2B vector, FOXK1 (Vigene
Biosciences, Inc., Rockville, MD, USA), scramble RNA (SCR) or FOXK1
siRNA (Sigma-Aldrich; Merck KGaA) using the Lipofectamine 2000
reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA; cat. no.
11668019) according to the manufacturer's protocol. Following 48 h
of transfection, cells were used to subsequent experiments.
Anchorage-independent cell growth
assay
Following knockdown or ectopic expression FOXK1 in
T98G and LN18 cells (transfection time, 48 h), ~4×104
cells were re-suspended with 2 ml DMEM supplemented with 0.35%
agarose and layered onto 2 ml of 0.6% agarose/medium in the 6-well
plates. Cells were cultured with 2 ml fresh growth medium every 3
days for 15 days. Colonies were stained with 0.5% crystal violet
and counted. n>50 were defined as a colony. All experiments were
performed at least three times.
RT-qPCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from GBM/adjacent
tissue samples or cells according to the manufacturer's
instructions. Complementary (c)DNA was then generated using
TransScript First-Strand cDNA Synthesis SuperMix (TransGen,
Beijing, China). SYBR MIX (Roche Diagnostics, Basel, Switzerland)
was used to perform PCR in the 7500 Real-Time PCR System to detect
relative mRNA expression. Primers were designed as follows:
E-cadherin forward, 5′-AAACATCATTGATGCAGACC-3′ and reverse,
5′-GATAGATTCTTGGGTTGGGTC-3′; α-catenin forward,
5′-TGTTACACAGGTTACAACCCT-3′ and reverse,
5′-GATCATCTGCGAACTCTCCT-3′; N-cadherin forward,
5′-CAAAGCCTGGAACATATGTG-3′; and reverse,
5′-GTTTGAAAGGCCATATGTGG-3′; fibronectin forward,
5′-AATGTGAACGACACATTCCA-3′ and reverse, 5′-ACCACTTGAGCTTGGATAGG-3′;
Snail forward, 5′-TCTAATCCAGAGTTTACCTTCC-3′; and reverse,
5′-GAAGAGACTGAAGTAGAGGAG-3′; Slug forward,
5′-ACACATACAGTGATTATTTCCC-3′; and reverse,
5′-GAGGAGGTGTCAGATGGA-3′; TWIST forward,
5′-CCAGGTACATCGACTTCCTC-3′; and reverse,
5′-GGAAACAATGACATCTAGGTCTC-3′; FOXK1 forward,
5′-CAGTTACCGCTTTGTGCAG-3′; and reverse, 5′-GAATTCTGCCAGCCTTTGTC-3′;
GAPDH forward, 5′-ATTTCCTGGTATGACAACGA-3′ and reverse,
5′-TTGATGGTACATGACAAGGTG-3′. GAPDH was used as an internal control.
The following thermocycling conditions were used: 5 min at 98°C,
denaturation at 98°C for 30 sec, annealing at 57°C for 30 sec and
extension at 72°C for 40 sec, performed for 30 cycles. The relative
expression of gene was analyzed using the 2−ΔΔCq method
(17). All experiments were
performed at least three times.
Transwell migration assay. LN18 cells with knockdown
or ectopic expression of FOXK1 (transfection time, 48 h) were
seeded onto Transwell membrane inserts (Corning, Inc., Corning, NY,
USA) at 5×103 cells per well in DMEM without serum. DMEM
containing 10% FBS was added to the lower chamber. Following
incubation for 8 h at 37°C, cells were fixed in 4%
paraformaldehyde, followed by staining with 0.5% crystal violet at
room temperature for 15 min. For each membrane, migrated cells in 6
random fields at ×20 magnification were counted. All experiments
were performed in triplicate.
Cell Counting kit (CCK)-8 assay. Cell proliferation
was detected using a CCK-8 assay as previously described (18). In brief, T98G and LN18 cells were
transfected with empty vector or FLAG-FOXK1, or with control small
interfering RNA (siRNA) or FOXK1 siRNA for 48 h and then seeded in
96-well plates at 4×103 cells/well in 200 µl DMEM
containing 10% FBS. Following incubation for 24 h, 20 µl CCK-8
stain in 200 µl DMEM was added to each well, followed by incubation
for 2 h at 37°C. Finally, the absorbance of each well was measured
at 450 nm.
Apoptosis and cell cycle analysis. For apoptosis and
cell cycle analysis, T98G and LN18 cells with knockdown or ectopic
expression of FOXK1 (transfection time, 48 h) were collected and
washed three times with PBS. Following fixation in 70% cold
ethanol, cells were stained with Annexin V-propidium iodide (PI)
solution or PI solution for apoptosis or cell cycle analysis,
respectively. Finally, FACS analysis was performed to detect
apoptosis and cell cycle distribution. All experiments were
performed at least three times.
Chromatin immunoprecipitation (ChIP) assay. The ChIP
assay was performed using an EZ ChIP Kit (EMD Millipore). In brief,
cells were lysed and sonicated to produce chromatin fragments of
200-1×103 bp. Immunoprecipitated chromatin was detected
using 3 µg anti-FOXK1 antibody (ab18196; Abcam, Cambridge, MA, USA)
and specific primers were used to amplify the target gene promotor
region. The same quantity of Immunoglobulin G (IgG; ab172730;
Abcam) served as a negative control. The PCR assay was performed
using 2X EasyTaq PCR SuperMix (TransGene) according to the
manufacturer's instructions. The PCR conditions were as follows: 5
min at 98°C, denaturation at 98°C for 30 sec, annealing at 57°C for
30 sec and extension at 72°C for 40 sec, performed for 30 cycles.
The primers used were as follows: TWIST forward,
5′-AGGCGCTATCAAATTCCC-3′ and reverse, 5′-AAGGCAGCAGAGCCAGAG-3′;
Snail forward, 5′-ATGGCAGCTCACTGTGGC-3′ and reverse,
5′-CGCTGGCTTCCTTTCATT-3′; Slug forward, 5′-CCACCTCACCCTCCAAAC-3′
and reverse, 5′-CACATGAAGATCACCCTA-3′. The products were detected
by agarose gel electrophoresis with ethidium bromide staining.
Input groups served as a positive control and IgG groups served as
a negative control.
Luciferase reporter assay
Cells were seeded in 6-well plates at
6×104 cells/well and transfected with the vector, FOXK1,
pGL3-Snail, Renilla (Vigene Biosciences, Inc.) as the cell density
reached 70–80% confluence. After 24 h of transfection, an
illuminometer was used to quantify the luciferase activity. All
experiments were performed at least three times.
Statistical analysis
All results were analyzed with SPSS 21.0 statistical
software (IBM Corp., Armonk, NY, USA). The association between
FOXK1 expression and clinicopathological parameters was evaluated
by Pearson's Chi-square test. All values are expressed as the mean
± standard error of the mean. The expression of FOXK1 in GBM cell
lines and NHAs was analyzed with the Chi-squared test. The
two-tailed Student's t-test was used to assess differences between
two groups. The survival analysis was performed using Kaplan-Meir
analysis. Analysis of variance followed by Tukey's post hoc test
was used to assess the differences between multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
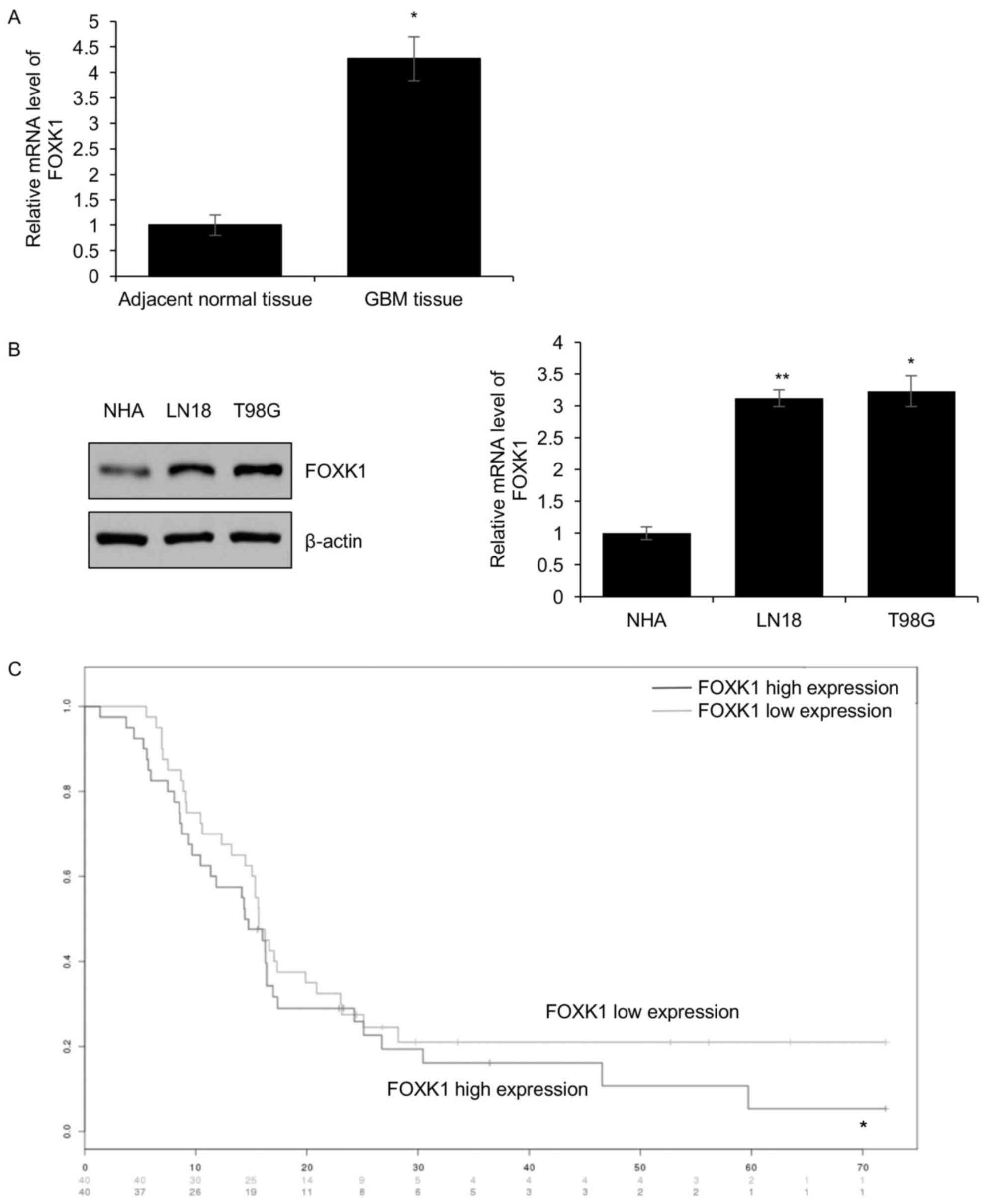
FOXK1 is overexpressed in GBM tissues
and cell lines
FOXK1 is a crucial transcription factor, and several
studies regarded it as a tumor suppressor, while multiple studies
also indicated that FOXK1 promotes EMT and cell metastasis. The
role of FOXK1 in GBM has remained to be elucidated. The present
study first examined the expression of FOXK1 in human GBM tissues
and adjacent non-tumorous tissues using RT-qPCR. In a total of 83
pairs of human GBM and adjacent non-tumorous tissue samples, FOXK1
was overexpressed in the GBM tissues compared with that in adjacent
non-tumorous tissues (Fig. 1A).
In addition, the expression of FOXK1 was identified
to be positively associated with metastasis, tumor size and tumor
stage. However, the age and gender of the patients were not
associated with FOXK1 (Table I).
Furthermore, the expression of FOXK1 was detected in the T98G and
LN18 GBM cell lines as well as in NHAs. The results indicated that,
compared with that in NHAs, FOXK1 was obviously overexpressed in
the GBM cell lines (Fig. 1B).
Consequently, the influence of FOXK1 expression on the survival of
GBM patients was analyzed. The results demonstrated that the 5-year
survival in the group with low FOXK1 expression was significantly
higher than that in the group with high expression of FOXK1
(Fig. 1C).
 | Table I.Clinicopathological parameters of
patients with glioblastoma multiforme (n=83). |
Table I.
Clinicopathological parameters of
patients with glioblastoma multiforme (n=83).
|
|
| FOXK1 protein
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Parameter | n (%) | Low (n=30)% | High (n=53)% | P-value | Chi-square
values |
|---|
| Gender |
|
|
|
|
|
|
Male | 51 (61.4) | 18 (21.6) | 33 (39.8) | 0.839 | 0.041 |
|
Female | 32 (38.6) | 12 (14.5) | 20 (24.1) |
|
|
| Age (years) |
|
|
|
|
|
|
≥40 | 49 (59.0) | 21 (25.4) | 28 (33.7) | 0.126 | 2.335 |
|
<40 | 34 (41.0) | 9 (10.8) | 25 (30.1) |
|
|
| Tumor size
(cm) |
|
|
|
|
|
| ≥2 | 43 (51.8) | 5 (6) | 38 (45.8) | <0.001 | 23.236 |
|
<2 | 40 (48.2) | 25 (30.1) | 15 (18.1) |
|
|
| Pathological
grade |
|
|
|
|
|
|
I–II | 39 (47.0) | 19 (22.9) | 20 (24.1) | 0.025 | 5.039 |
|
III–IV | 44 (53.0) | 11 (13.2) | 33 (39.8) |
|
|
| Metastasis |
|
|
|
|
|
|
Yes | 44 (53.0) | 7 (8.4) | 37 (44.6) | <0.001 | 16.613 |
| No | 39 (47.0) | 23 (27.7) | 16 (19.3) |
|
|
FOXK1 promotes EMT of GBM cells
through activation of SNAIL transcription
EMT promotes the cancer cell metastasis ability of
most carcinomas. High expression of FOXK1 is closely associated
with metastasis, which suggested that FOXK1 may participate in the
EMT. To test this hypothesis, FOXK1 was knocked down by FOXK1 siRNA
in T98G cells and the knockdown efficiency was assessed. As
presented in Fig. 2A, FOXK1 was
depleted by 70–80%. Furthermore, EMT markers were also
significantly changed. At the mRNA as well as at the protein level,
the expression of the epithelial markers E-cadherin and α-catenin
was increased, while that of the mesenchymal markers N-cadherin and
fibronectin was obviously decreased (Fig. 2B). Furthermore, the expression of
Snail, a transcription factor that promotes EMT, was decreased when
FOXK1 was silenced, whereas Slug and TWIST were not affected
(Fig. 2B). As Snail is an important
transcription factor which promotes the EMT process, it was then
further explored whether FOXK1 promotes EMT through transcriptional
regulation of SNAIL. For this purpose, a luciferase reporter assay
was utilized to determine the effect of FOXK1 on SNAIL promoter
activity. As presented in Fig. 2C,
FOXK1 transcriptionally activated the SNAIL gene promoter in T98G
and LN18 cells. In addition, the ChIP assay demonstrated that
endogenous FOXK1 bound to the promoter region of SNAIL, but not
Slug and TWIST (Fig. 2D). In brief,
the present results revealed that FOXK1 activated the transcription
of SNAIL.
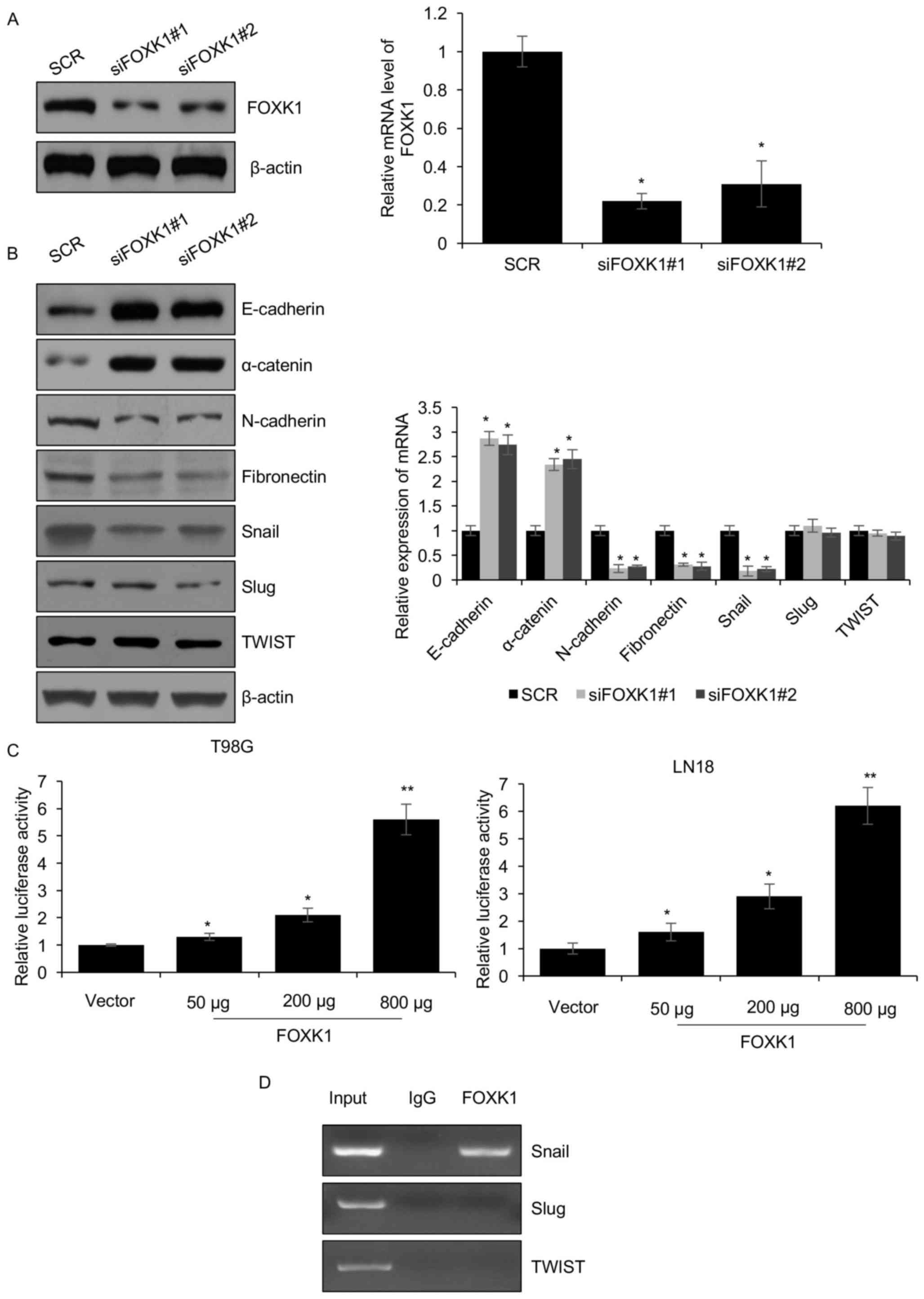 | Figure 2.FOXK1 promotes EMT through
transcriptional activation of SNAIL in glioblastoma multiforme
cells. (A) Two different siRNAs were utilized to knockdown FOXK1 in
T98G cells. After transfection for 48 h, FOXK1 knockdown efficiency
was assessed by western blotting and RT-qPCR. (B) Knockdown of
FOXK1 regulates EMT-associated protein expression. FOXK1 depletion
led to suppression of the mesenchymal marker N-cadherin and
fibronectin, but promoted the expression of the epithelial markers
E-cadherin and α-catenin. The protein and mRNA levels were detected
by western blotting and RT-qPCR, respectively. (C) Co-transfection
with SNAIL-Luciferase reporter vector and Renilla construct, and
different amounts of FOXK1 expression vector (50, 200 or 800
ng/well) in T98G and LN18 cells. After transfection for 36 h, cells
were collected and subjected to the luciferase reporter assay. All
experiments were performed at least three times. *P<0.05,
**P<0.01 vs. SCR/control group. (D) A chromatin
immunoprecipitation assay was performed in T98G cells with rabbit
normal IgG or anti-FOXK1, followed by PCR with specific primers for
SNAIL. PCR products were indicated. EMT, epithelial to mesenchymal
transition; PCR, polymerase chain reaction; FOXK1, forkhead box K1;
IgG, immunoglobulin G; SCR, scrambled control; siFOXK1, small
interfering RNA targeting FOXK1. |
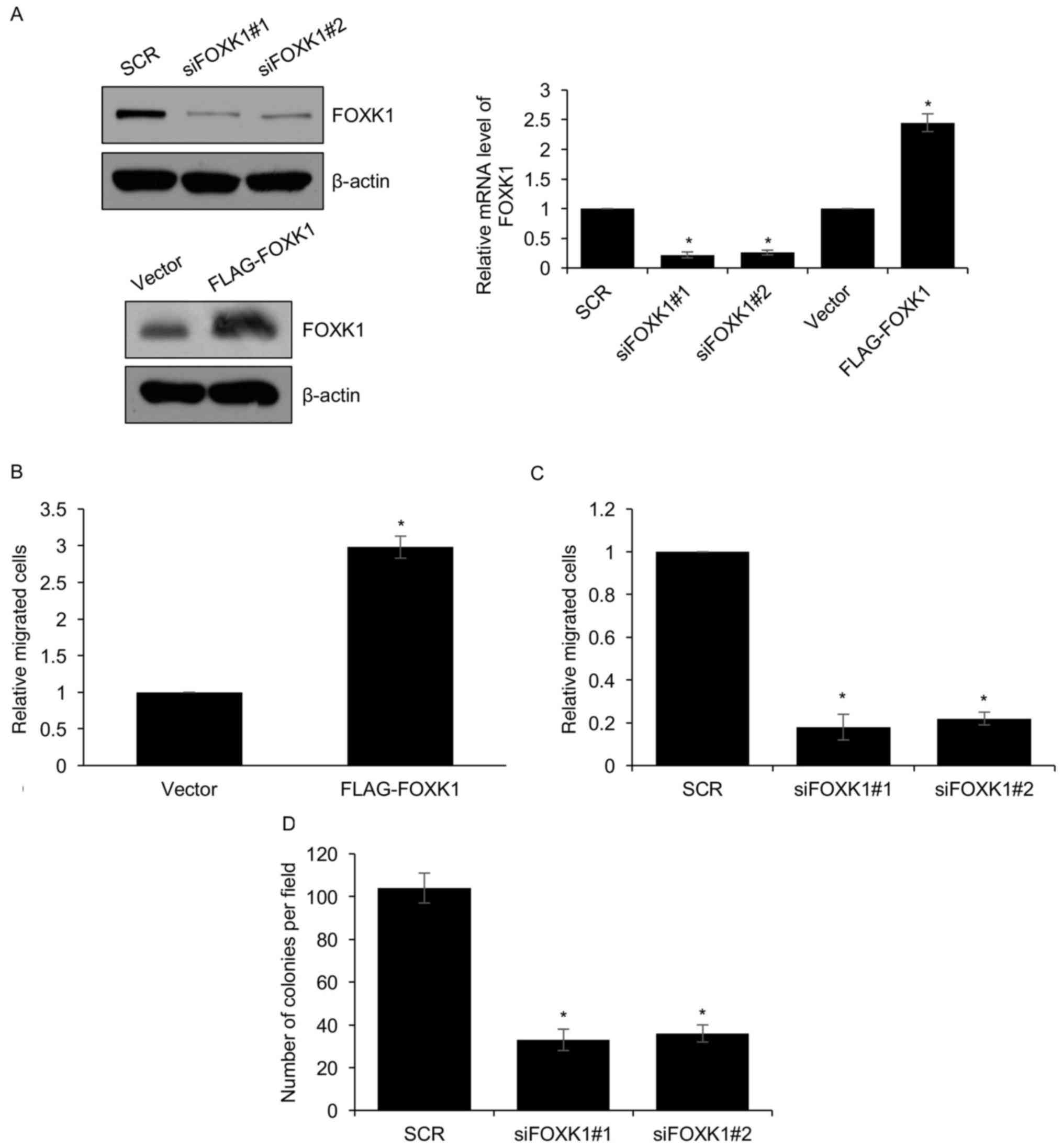
FOXK1 facilitates GBM cell
metastasis
An enhanced capacity to form metastasis is a
characteristic of cancer that is associated with the EMT, and
metastasis is the reason for poor prognosis. As the above mentioned
results suggested that FOXK1 promotes EMT through transcriptional
activation of SNAIL, it was hypothesized that FOXK1 regulates GBM
cell metastasis. In order to verify this hypothesis, a Transwell
migration assay was performed using LN18 cells in which FOXK1 was
ectopically overexpressed or knocked down as verified by western
blot analysis and RT-qPCR (Fig. 3A).
The Transwell assay revealed that overexpression of FOXK1 in LN18
cells increased the number of cells that transgressed through the
membrane compared with that in the control group (Fig. 3B). The opposite result was seen when
FOXK1 was silenced in LN18 cells, as the number of cells
transgressed to the lower side of the membrane was significantly
decreased (Fig. 3C). In addition,
the anchorage-independent cell growth assay demonstrated that
silencing of FOXK1 expression suppressed colony formation (Fig. 3D). All of these results supported
that FOXK1 stimulated cell migration.
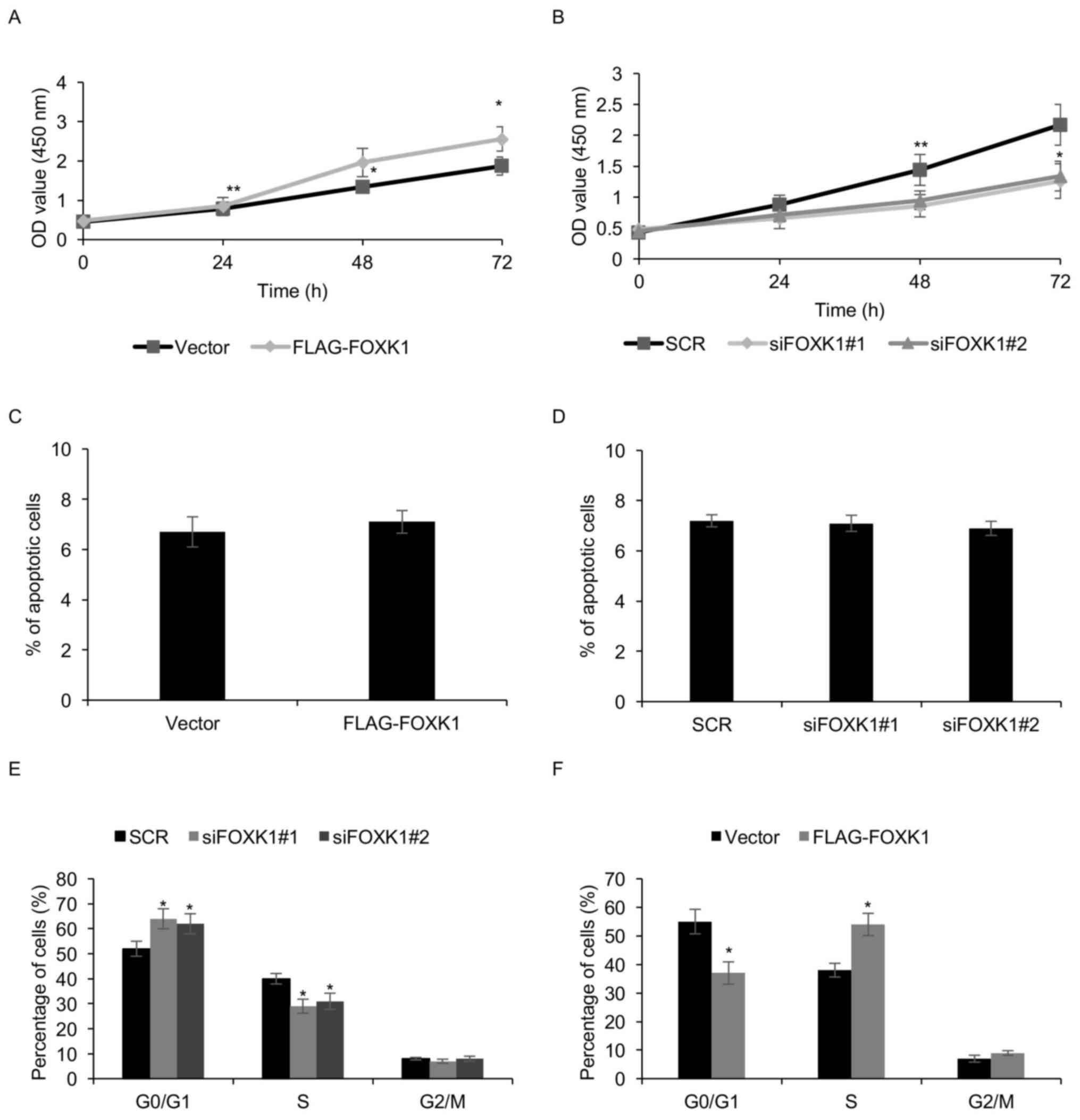
FOXK1 levels affect GBM cell
proliferation through regulating the cell cycle
Considering the higher expression of FOXK1 in GBM
tumor tissues than in adjacent non-tumorous tissues and high
expression of FOXK1 being positively associated with the tumor
size, it was speculated that FOXK1 may regulate GBM cell
proliferation. In order to determine the role of FOXK1 in cell
proliferation, T98G cells with ectopic expression of FOXK1 or
knockdown were subjected to a CCK8 assay. The results demonstrated
that FOXK1 overexpression obviously increased T98G cell growth,
whereas FOXK1 knockdown inhibited T98G cell growth (Fig. 4A and B).
To further explore the mechanism by which FOXK1
enhances cell proliferation, it was first assessed whether FOXK1
suppressed cell apoptosis, but no marked effect of FOXK1
overexpression or knockdown on the apoptotic rates of T98G cells
was observed (Fig. 4C and D). Next,
the effect of FOXK1 on the cell cycle was assessed using FACS. The
results indicated that following knockdown of FOXK1 in T98G cells,
the percentage of cells in the G0/G1 phase
was obviously increased, while the percentage of cells in the
S-phase was significantly decreased (Fig. 4E). By contrast, overexpression of
FOXK1 in T98G cells decreased the percentage of cells in the
G0/G1 phase and obviously increased the
percentage of cells in the S-phase (Fig.
4F). A similar result was seen in LN18 cells (data not shown).
The above results revealed that FOXK1 expression promoted GBM cell
proliferation through regulating the cell cycle.
Discussion
Gliomas account for almost 80% of brain tumors and
GBM has become the most prevalent subtype of gliomas (19,20). It
is difficult to treat malignant gliomas, and the survival of GBM
patients under treatment is only 12–15 months (21,22).
The members of the forkhead family have multiple
functional roles during embryogenesis (23–25).
Several studies indicated that FOX proteins have crucial roles in
various cancer types (26,27). For instance, FOXA1 has been reported
to be highly expressed in numerous cancer types, including bladder
(28), breast (29), prostate (30) and pancreatic cancers (31). The roles of FOXK1, as a member of the
FOX family, have remained to be fully elucidated in cancer, and it
has been reported to have tumor suppressor (32) as well as oncogenic funcions (33).
The present study reported that FOXK1 is highly
expressed in GBM tumor tissues compared with that in adjacent
normal tissues. Analysis of GBM cell lines compared with NHAs
provided similar results. As expected, high expression of FOXK1 was
positively associated with several clinicopathological
characteristics, namely tumor size and metastasis. These results
indicated that FOXK1 has a crucial function in GBM development.
Metastasis is the major cause of cancer-associated
mortality, causing >90% of fatalities of carcinoma patients, and
EMT promotes the metastasis ability of cancer cells for most
carcinomas. Multiple reasons have been suggested for the low
incidence of extracranial metastasis of GBM, including inhibition
of extracranial growth of glioblastoma cells through the immune
system (34), the blood-brain
barrier (35), short survival
periods (36) or the absence of
lymphatic channels in the central nervous system (37), and a previous report indicated
extracranial metastases after organ transplantation from GBM donors
(38). In the present study, it was
demonstrated that the transcription factor FOXK1 takes part in
regulating the EMT. Knockdown of endogenous FOXK1 was sufficient to
suppress EMT. This observation was consisted with previous study
(33). The present study indicated
that FOXK1 regulated Snail, a key transcription factor which
promotes EMT. The luciferase reporter assay and the ChIP assay
confirmed that FOXK1 activates Snail transcription. In addition,
the Transwell assay and the anchorage-independent cell growth assay
indicated that FOXK1 promotes GBM cell metastasis.
The CCK-8 and colony formation assay revealed that
ectopic expression of FOXK1 significantly promoted GBM cell
proliferation, whereas depletion of FOXK1 suppressed cell
proliferation. As previous reports have demonstrated, FOXK1
promotes cell proliferation in prostate cancer (39), colorectal cancer (40) and gastric cancer (41). In order to investigate whether the
enhancing effect of FOXK1 on cell proliferation is caused by
inhibition of apoptosis or promotion of the cell cycle, cell
apoptosis was assessed after FOXK1 was silenced; however, no
significant effect was observed. Next, the cell cycle assay
demonstrated that ectopic expression of FOXK1 caused an obvious
increase in the percentage of cells in the S-phase and decreased
the percentage of cells in the G0/G1-phase.
These results inferred that FOXK1 promotes cell proliferation
through facilitating cell cycle progression, but the detailed
molecular mechanisms remain to be elucidated by further
studies.
References
|
1
|
Dunbar E and Yachnis AT: Glioma diagnosis:
Immunohistochemistry and beyond. Adv Anat Pathol. 17:187–201. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young RM, Jamshidi A, Davis G and Sherman
JH: Current trends in the surgical management and treatment of
adult glioblastoma. Ann Transl Med. 3:1212015.PubMed/NCBI
|
|
3
|
Stylli SS, Howes M, MacGregor L, Rajendra
P and Kaye AH: Photodynamic therapy of brain tumours: Evaluation of
porphyrin uptake versus clinical outcome. J Clin Neurosci.
11:584–596. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stylli SS, Kaye AH, MacGregor L, Howes M
and Rajendra P: Photodynamic therapy of high grade glioma-long term
survival. J Clin Neurosci. 12:389–398. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hannenhalli S and Kaestner KH: The
evolution of Fox genes and their role in development and disease.
Nat Rev Genet. 10:233–240. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jackson BC, Carpenter C, Nebert DW and
Vasiliou V: Update of human and mouse forkhead box (FOX) gene
families. Hum Genomics. 4:345–352. 2010.PubMed/NCBI
|
|
7
|
Bassel-Duby R, Hernandez MD, Yang Q,
Rochelle JM, Seldin MF and Williams RS: Myocyte nuclear factor, a
novel winged-helix transcription factor under both developmental
and neural regulation in striated myocytes. Mol Cell Biol.
14:4596–4605. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clark KL, Halay ED, Lai E and Burley SK:
Co-crystal structure of the HNF-3/fork head DNA-recognition motif
resembles histone H5. Nature. 364:412–420. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Durocher D and Jackson SP: The FHA domain.
FEBS Lett. 513:58–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi X, Bowlin KM and Garry DJ: Fhl2
interacts with Foxk1 and corepresses Foxo4 activity in myogenic
progenitors. Stem Cells. 28:462–469. 2010.PubMed/NCBI
|
|
11
|
Carlsson P and Mahlapuu M: Forkhead
transcription factors: Key players in development and metabolism.
Dev Biol. 250:1–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bailey JM, Singh PK and Hollingsworth MA:
Cancer metastasis facilitated by developmental pathways: Sonic
hedgehog, Notch, and bone morphogenic proteins. J Cell Biochem.
102:829–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nieto MA, Sargent MG, Wilkinson DG and
Cooke J: Control of cell behavior during vertebrate development by
Slug, a zinc finger gene. Science. 264:835–839. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cohen MS, Hussain HB and Moley JF:
Inhibition of medullary thyroid carcinoma cell proliferation and
RET phosphorylation by tyrosine kinase inhibitors. Surgery.
132:960–967. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu Y and Parada LF: The molecular and
genetic basis of neurological tumours. Nat Rev Cancer. 2:616–626.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwartzbaum JA, Fisher JL, Aldape KD and
Wrensch M: Epidemiology and molecular pathology of glioma. Nat Clin
Pract Neurol. 2:494–503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cloughesy TF, Cavenee WK and Mischel PS:
Glioblastoma: From molecular pathology to targeted treatment. Annu
Rev Pathol. 9:1–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katoh M and Katoh M: Human FOX gene family
(Review). Int J Oncol. 25:1495–1500. 2004.PubMed/NCBI
|
|
24
|
Yang Y, Hou H, Haller EM, Nicosia SV and
Bai W: Suppression of FOXO1 activity by FHL2 through SIRT1-mediated
deacetylation. EMBO J. 24:1021–1032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murakami H, Aiba H, Nakanishi M and
Murakami-Tonami Y: Regulation of yeast forkhead transcription
factors and FoxM1 by cyclin-dependent and polo-like kinases. Cell
Cycle. 9:3233–3242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Teh MT, Wong ST, Neill GW, Ghali LR,
Philpott MP and Quinn AG: FOXM1 is a downstream target of Gli1 in
basal cell carcinomas. Cancer Res. 62:4773–4780. 2002.PubMed/NCBI
|
|
27
|
Müller SM, Terszowski G, Blum C, Haller C,
Anquez V, Kuschert S, Carmeliet P, Augustin HG and Rodewald HR:
Gene targeting of VEGF-A in thymus epithelium disrupts thymus blood
vessel architecture. Proc Natl Acad Sci USA. 102:pp. 10587–10592.
2005; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reddy OL, Cates JM, Gellert LL, Crist HS,
Yang Z, Yamashita H, Taylor JA III, Smith JA Jr, Chang SS, Cookson
MS, et al: Loss of FOXA1 drives sexually dimorphic changes in
urothelial differentiation and is an independent predictor of poor
prognosis in bladder cancer. Am J Pathol. 185:1385–1395. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Badve S, Turbin D, Thorat MA, Morimiya A,
Nielsen TO, Perou CM, Dunn S, Huntsman DG and Nakshatri H: FOXA1
expression in breast cancer-correlation with luminal subtype A and
survival. Clin Cancer Res. 13:4415–4421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mirosevich J, Gao N, Gupta A, Shappell SB,
Jove R and Matusik RJ: Expression and role of Foxa proteins in
prostate cancer. Prostate. 66:1013–1028. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jungert K, Buck A, von Wichert G, Adler G,
König A, Buchholz M, Gress TM and Ellenrieder V: Sp1 is required
for transforming growth factor-beta-induced mesenchymal transition
and migration in pancreatic cancer cells. Cancer Res. 67:1563–1570.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun T, Wang H, Li Q, Qian Z and Shen C:
Forkhead box protein k1 recruits TET1 to act as a tumor suppressor
and is associated with MRI detection. Jpn J Clin Oncol. 46:209–221.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu Y, Peng Y, Wu M, Zhang W, Zhang M, Xie
R, Zhang P, Bai Y, Zhao J, Li A, et al: Oncogene FOXK1 enhances
invasion of colorectal carcinoma by inducing epithelial-mesenchymal
transition. Oncotarget. 7:51150–51162. 2016.PubMed/NCBI
|
|
34
|
Fonkem E, Lun M and Wong ET: Rare
phenomenon of extracranial metastasis of glioblastoma. J Clin
Oncol. 29:4594–4595. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liwnicz BH and Rubinstein LJ: The pathways
of extraneural spread in metastasizing gliomas: A report of three
cases and critical review of the literature. Hum Pathol.
10:453–467. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pasquier B, Pasquier D, Lachard A, N'Golet
A, Panh MH and Couderc P: Extraneural metastasis of central nervous
system tumours (author's transl). Bull Cancer. 66:25–28. 1979.(In
French). PubMed/NCBI
|
|
38
|
Müller C, Holtschmidt J, Auer M, Heitzer
E, Lamszus K, Schulte A, Matschke J, Langer-Freitag S, Gasch C,
Stoupiec M, et al: Hematogenous dissemination of glioblastoma
multiforme. Sci Transl Med. 6:247ra1012014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen F, Xiong W, Dou K and Ran Q:
Knockdown of FOXK1 suppresses proliferation, migration and invasion
in prostate cancer cells. Oncol Res. 25:1261–1267. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu M, Wang J, Tang W, Zhan X, Li Y, Peng
Y, Huang X, Bai Y, Zhao J, Li A, et al: FOXK1 interaction with FHL2
promotes proliferation, invasion and metastasis in colorectal
cancer. Oncogenesis. 5:e2712016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peng Y, Zhang P, Huang X, Yan Q, Wu M, Xie
R, Wu Y, Zhang M, Nan Q, Zhao J, et al: Direct regulation of FOXK1
by C-jun promotes proliferation, invasion and metastasis in gastric
cancer cells. Cell Death Dis. 7:e24802016. View Article : Google Scholar : PubMed/NCBI
|