Introduction
Atherosclerosis, a unique form of arteriosclerosis,
presents as arterial wall thickening due to the
invasion/accumulation of white blood cells and proliferation of
intimal smooth muscle cell (1,2).
Vascular endothelium contributes to the development of
atherosclerosis via abnormal cell proliferation and apoptosis
(3). Endothelial cells maintain the
homeostasis of the vascular system (4). However, the precise molecular
mechanisms which underlie the contributions of the vascular
endothelium to atherosclerosis remain unclear and complex.
After binding to the 3′ untranslated region (UTR) of
target genes, microRNAs (miRNAs, miR) repress protein expression
levels by mRNA destabilization or/and translation inhibition
(5). Recent studies also found that
miRNAs had the ability to reduce mRNA targets by binding to 5′UTR
or protein-coding exons (6,7). Circulating miRNAs serve critical roles
in multiple diseases, including atherosclerosis (8) and cancer (9), thus showing enormous potential as novel
biomarkers for various diseases. Consequently, it has emerged that
miRNAs function as epigenetic regulators in diverse biological
processes, including cell proliferation (10) and tumorigenesis (11). In diseased vascular vessels,
dysregulation of miRNAs, for instance, miR-33 (12) and miR-133 (13), has been detected.
Mitogen activated protein kinase 6 (MAPK6) is an
enzyme which is encoded by the MAPK6 gene in humans (14). MAPK6 has been shown to modulate cell
migration, proliferation and angiogenesis in primary human
umbilical vein endothelial cells (HUVECs), and has been reported to
play a key role in maintaining normal physiological conditions of
vascular smooth muscle cells (VSMCs) (15). In addition, in a previous study, the
knockdown of MAPK6 damaged tube formation by VSMCs (16).
However, it remains unclear whether MAPK6 could be
regulated by miRNA in the functions of the vascular endothelium.
The current study aimed to clarify this.
Patients and methods
Participants
All study participants provided written informed
consent. The current study was approved by the Ethics Committee of
the Huai'an First People's Hospital (Jiangsu, China). Sixty
atherosclerotic patients (mean age, 60.6 years; 36 male and 24
female) and 51 healthy volunteers (mean age, 56.5 years; 31 male
and 20 female) were enrolled in the Huai'an First People's
Hospital. Human vascular endothelial cells (HVECs) were isolated
from the peripheral blood of healthy volunteers and patients with
atherosclerosis using a human vascular endothelial cell separation
medium kit (Sangon Biotech, Shanghai, China). HVECs were used for
detection of the differences of MAPK6/miRNAs between healthy
volunteers and patients with atherosclerosis.
Cell culture and transfection
HUVECs were used for the conducting of the following
in vitro experiments. Briefly, HUVEC cell dissolution was
performed by keeping in 37°C for 5 min, transferred into a
centrifuge tube and centrifuged (125 g, 5–7 min). HUVEC cell
retrieved was balanced by RPMI (Lonza, Allendale, NJ, USA), 10%
fetal bovine serum (Sigma, St. Louis, MO, USA) and inoculated on
60×20 mm Petri dishes (1.3×105 cells/ml). Endothelial
cells were cultivated in 5 ml culture medium, inoculated in flasks
overnight at 37°C <5% CO2 with culture media changing
every other day. When cellular density reached 80%, passaging was
performed at passaging rates of 1:2 or 1:3. After treatment of
trypsin, endothelial cells in suspension were centrifuged, the
obtained pellets were re-suspended in culture medium and inoculated
on new Petri dishes.
HUVECs were seeded in Dulbecco's modified Eagle's
medium containing 10% fetal bovine serum, 1% 100 U/ml penicillin
and 1% 100 mg/ml streptomycin, thereafter, cultured in an incubator
with 5% CO2 at 37°C.
As for cell transfection, hsa-miR-98 mimic or
hsa-miR-negative control (NC) mimic was transfected into HUVECs by
opti-MEM and Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) in
accordance with manufacturer's instructions.
Luciferase reporter assays
The binding site between hsa-miR-98 and MAPK6 was
predicted by miRanda. For luciferase reporter assay, HUVECs were
seeded in 24-well plates, afterwards, HUVECs were transfected with
WT or mutant reporter plasmid and hsa-miR-98 mimic or hsa-miR-NC
mimic by Lipofectamine 2000. After 24 h, dual luciferase reporter
assay kit (Promega, Madison, WI, USA) was used for the
determination of relative firefly and renilla luciferase activities
in different groups according to the protocol provided by
manufacturer. Firefly luciferase activity acted as a control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cultured cells using
TRIzol (Invitrogen) according to the manufacturer's instructions,
miScript SYBR-Green PCR kit (Qiagen, Valencia, CA, USA) was used
for the RT-qPCR analysis, which was conducted on an Applied
Biosystems 7900HT fast real-time PCR system (Applied Biosystems,
Foster City, CA, USA). U6 acted as a control for miR-98 and GAPDH
acted as a control for MAPK6. Primers were as: MAPK6 forward,
5′-TAAAGCCATTGACATGTGGG-3′ and reverse, 5′-TCGTGCACAACAGGGATAGA-3′;
GAPDH forward, 5′-ACAAGATGGTGAAGGTCGGTGTGA-3′ and reverse,
5′-AGCTTCCCATTCTCAGCCTTGACT-3′; miR-98,
5′-CCGAGGTAGTAAGTTGTATTGTT-3′; U6, 5′-ACGCAAATTCGTGAAGCGTT-3′. At
last, relative level of hsa-miR-98 and MAPK6 in cells was
quantified with the method of 2−ΔΔCT.
Cell apoptosis assay
At 48 h after cell transfection, HUVECs were
harvested and washed with PBS for three times. HUVEC cells were
added with 5 µl Annexin V-FITC and 5 µl PI, stained in the dark for
15 min with Annexin V-FITC apoptosis detection kit (KeyGEN Biotech,
Nanjing, China). Relative rate of Annexin V-FITC or PI-positive
HUVECs in each group was analyzed by flow cytometry.
Cell proliferation assays
MTT assay (Sigma) was adopted for the analysis of
cell proliferation of HUVECs in each group. HUVECs were first
seeded in 96-well plates and transfected with different plasmids,
at 24 h post-transfection, 25 µl MTT (5 mg/ml) was added into each
well, plates were incubated at 37°C in an incubator for 3 h.
Precipitates in each well were solubilized by 150 µl dimethyl
sulfoxide (DMSO), assessed at 480 nm with versaMax ELISA microplate
reader (Molecular Devices, Sunnyvale, CA, USA).
Western blotting
Proteins were extracted from HUVECs by RIPA lysis
buffer (Invitrogen) and separated by 12% sodium dodecyl sulfate
poly-acrylamide gel electrophoresis before transferring to
polyvinylidene fluoride membranes (Millipore, Danvers, MA, USA).
After blocking with 5% nonfat milk at 37°C for 2 h, membranes were
incubated with the primary antibodies [Bax (dilution, 1:1,000),
Bcl-2 (dilution, 1:1,000), caspase-3 (dilution, 1:1,000), (all from
Cell Signaling Technology), MAPK6 (dilution, 1:500) and GAPDH
(dilution, 1:1,000) (both from Proteintech, Rosemont, IL, USA)]
overnight at 4°C, then HRP-linked secondary antibodies for 2 h at
room temperature. At last, protein bands were visualized by
enhanced chemiluminescence (ECL) (General Electric Healthcare,
Aurora, OH, USA). GAPDH was used as a control.
Statistical analysis
Data were analyzed by SPSS 17.0 (SPSS, Chicago, IL,
USA) with Student's t-test and ANOVA analyses. Results were
expressed as mean ± standard deviation. P<0.05 was considered as
statistically significant.
Results
Different expression levels of MAPK6
in the HVECs of healthy volunteers and atherosclerotic
patients
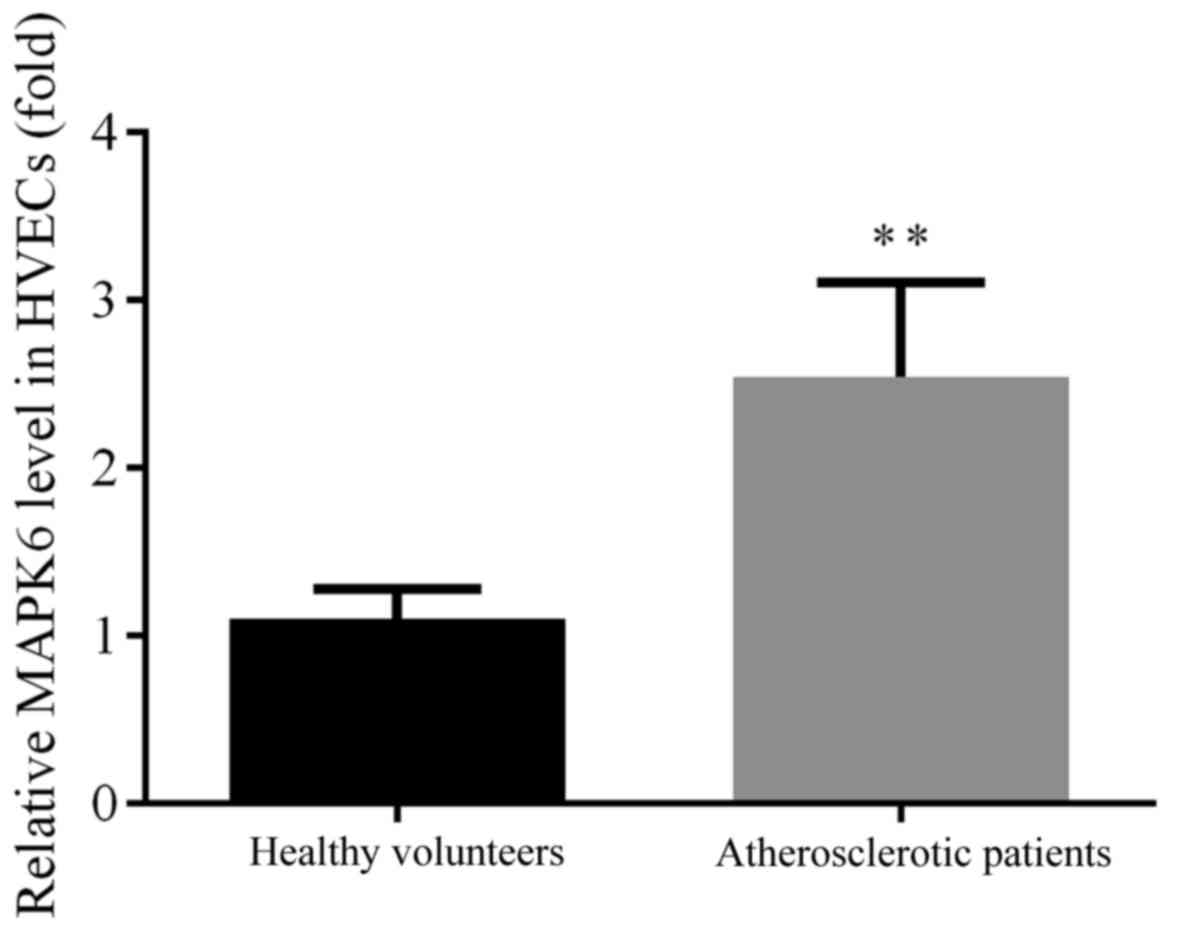
MAPK6 modulates the migration, proliferation and
angiogenesis of primary HUVECs (15). Therefore, the expression levels of
MAPK6 in HVECs from healthy individuals and subjects with
atherosclerosis were detected by RT-qPCR. The expression level of
MAPK6 was found to be significantly upregulated in the HVECs of
patients with atherosclerosis when compared with HVECs from healthy
volunteers (P<0.01; Fig. 1).
Prediction of the targets of
hsa-miR-98
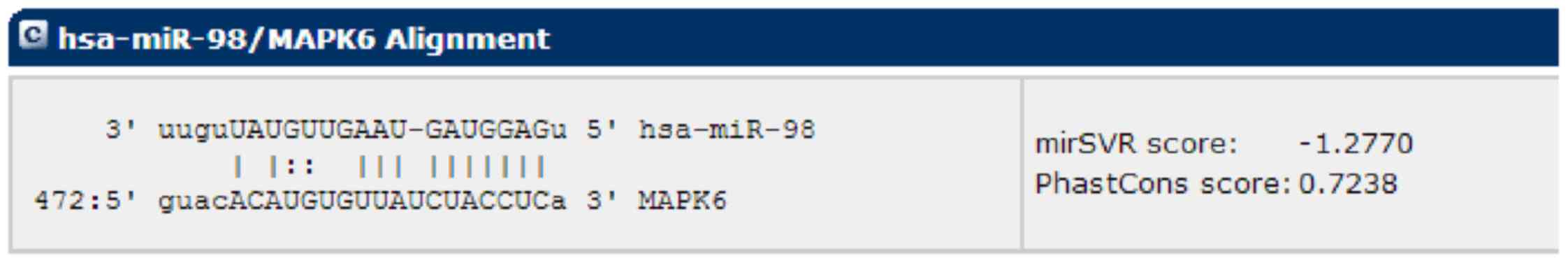
Mounting evidence suggests that, in cardiovascular
biology, miRNAs have the ability to regulate gene transcriptional
decay and repression by binding target miRNAs (17). A bioinformatic analysis of miRanda
was carried out to identify potential miRNAs that target MAPK6,
which exhibited a potential seed sequence of hsa-miR-98 in
MAPK6-3′-UTR (Fig. 2).
Different expression levels of miR-98
in the HVECs of healthy volunteers and atherosclerotic
patients
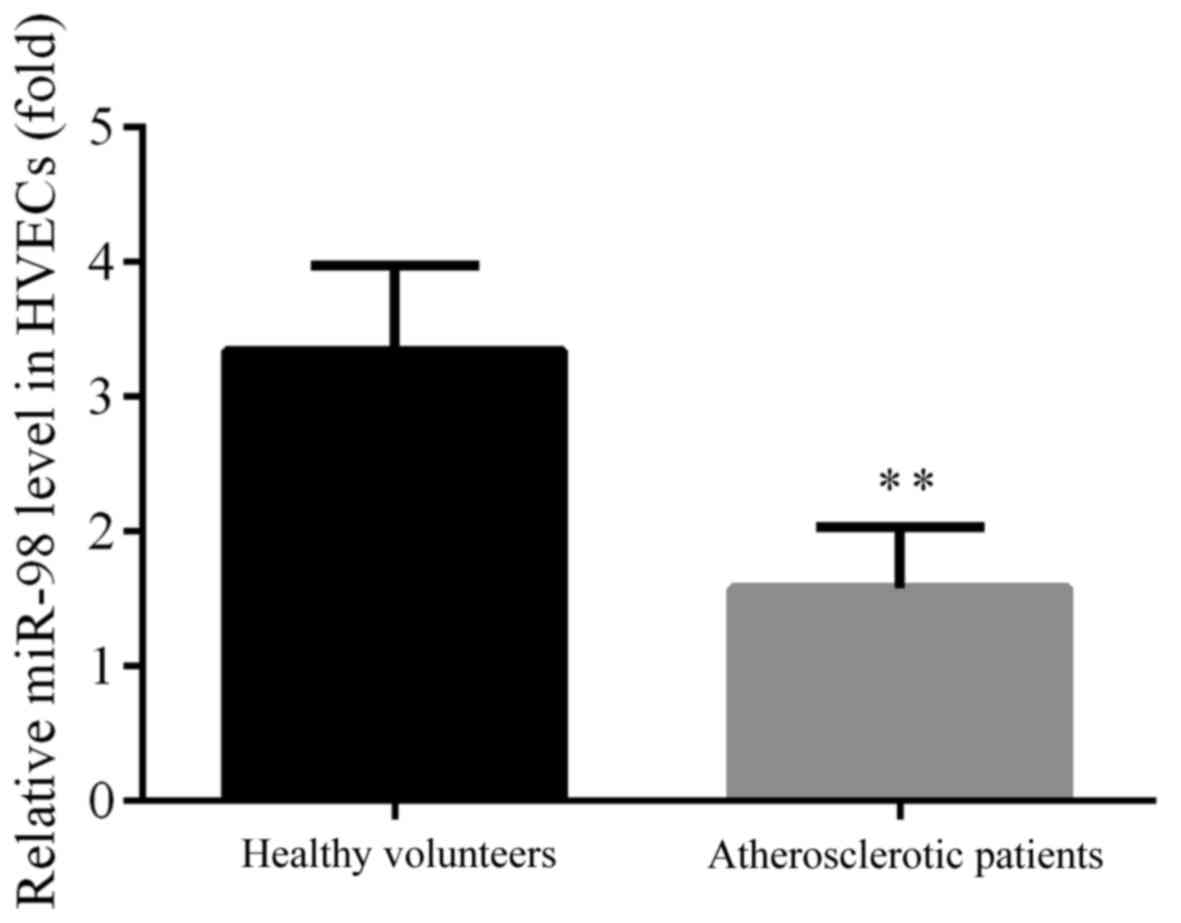
As acknowledged, miRNAs serve pivotal roles in
atherosclerosis, and hsa-miR-98 which was reported to be aberrantly
expressed in the serum of atherosclerotic patients was selected for
the current research according to the results in a microarray
analysis (18). The expression
levels of hsa-miR-98 in the HVECs from healthy and atherosclerotic
subjects were investigated by analysis using RT-qPCR. The
expression of hsa-miR-98 was found to be significantly
downregulated in the HVECs from atherosclerotic patients compared
with the healthy volunteers (P<0.01; Fig. 3).
Effects of overexpression of miR-98 on
cell proliferation
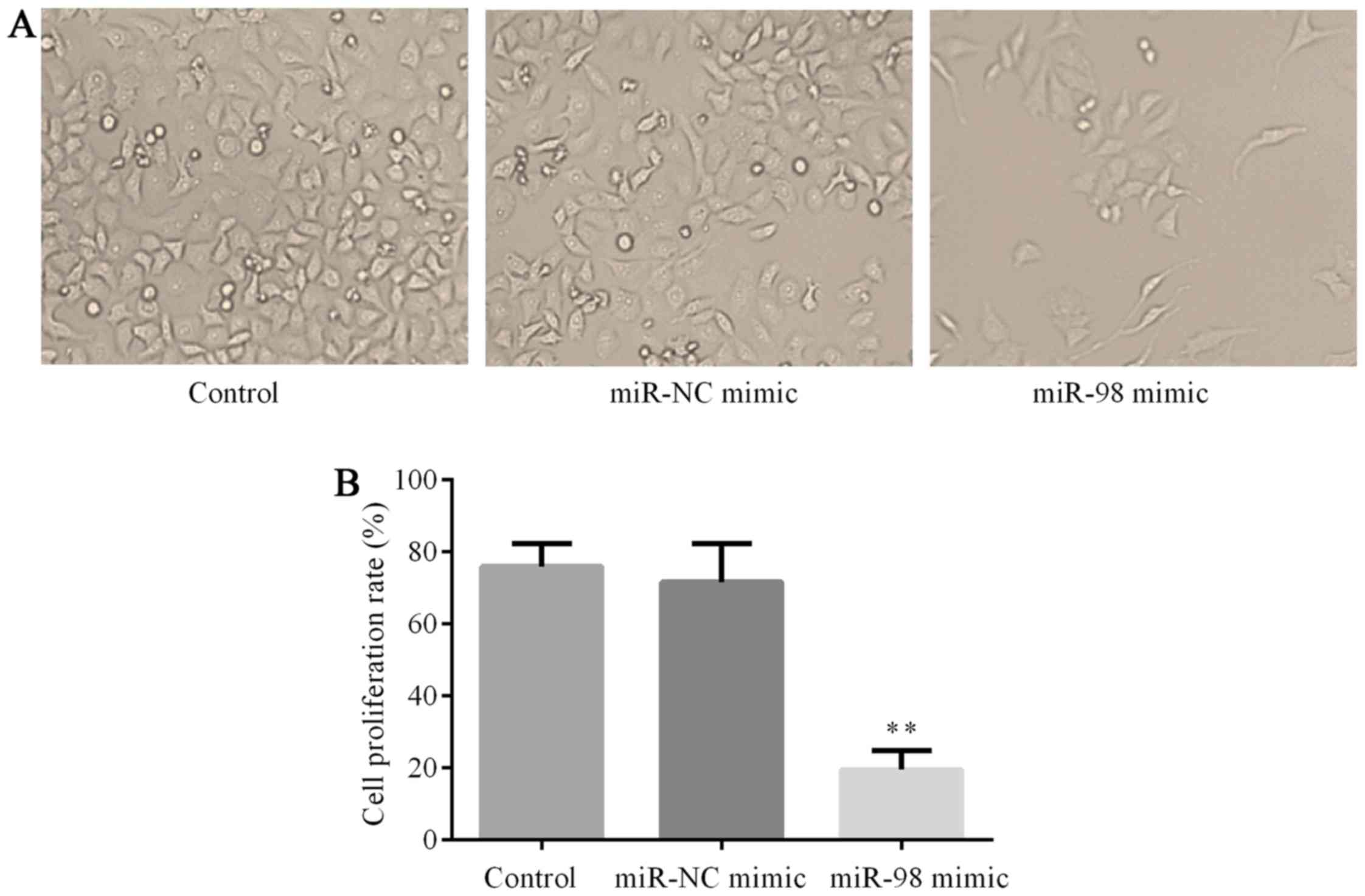
To investigate the potential influences of
hsa-miR-98 on cell proliferation, HUVECs were treated with
hsa-miR-98 mimics or miR-NC mimic at the concentration of 50 nmol/l
for 48 h. Thereafter, MTT assay was used to assess the influences
of hsa-miR-98 on HUVEC proliferation. The results showed that cells
transfected with hsa-miR-98 mimics grew much more slowly than cells
in the control group and miR-NC mimic group (P<0.01; Fig. 4).
Effects of overexpression of miR-98 on
cell apoptosis
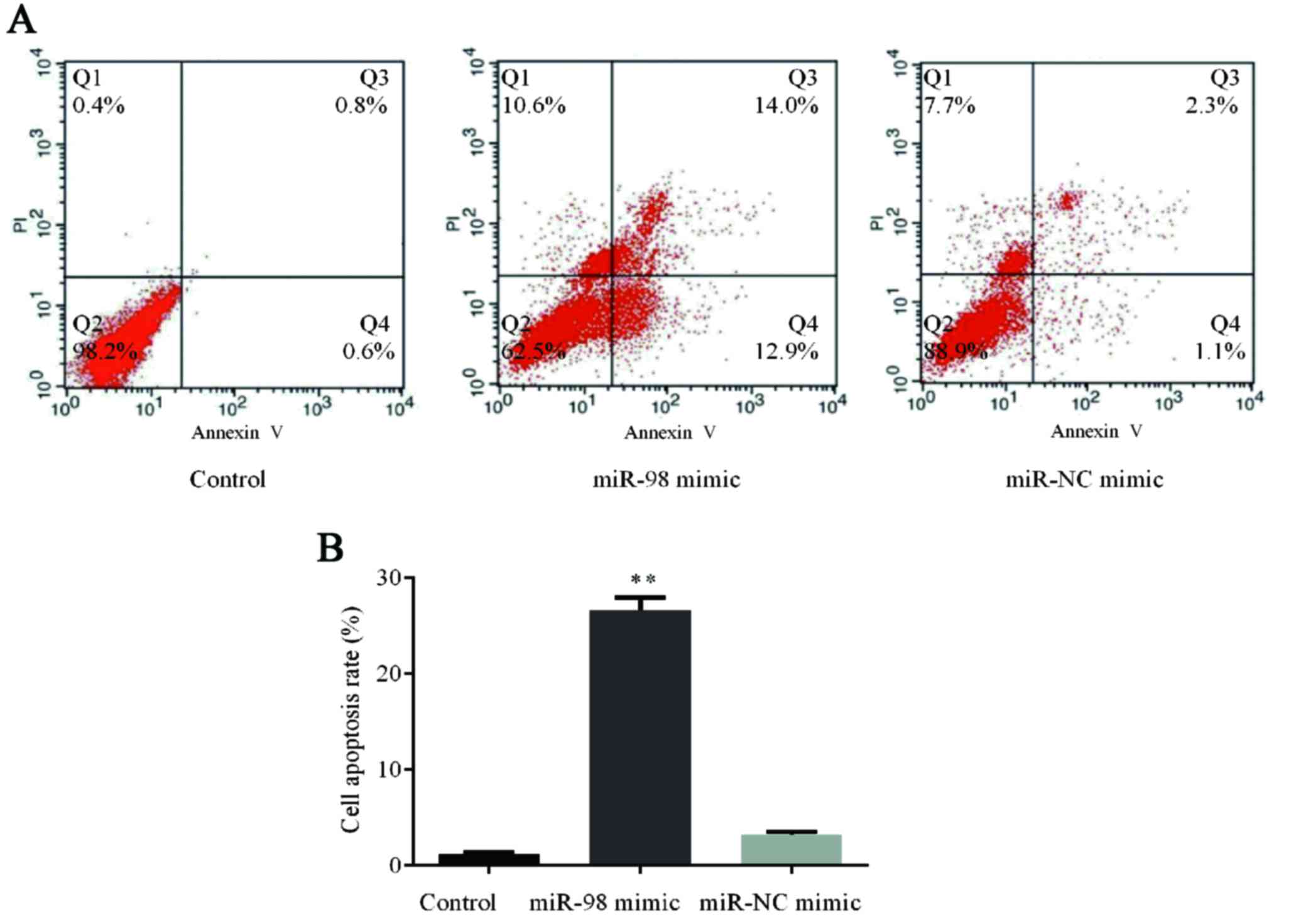
To explore the functions of hsa-miR-98 in HUVECs,
the cells were transfected with 100 nmol/l hsa-miR-98 mimic or
miR-NC mimic and cultured for 48 h. Afterwards, flow cytometry
assay of cell apoptosis was carried out which demonstrated that
overexpression of hsa-miR-98 in HUVECs induced an apoptosis rate
higher than in those in the control group and miR-NC mimic group
(P<0.01; Fig. 5).
Effects of overexpression of miR-98 on
Bax, Bcl-2, caspase-3 and MAPK6 protein levels
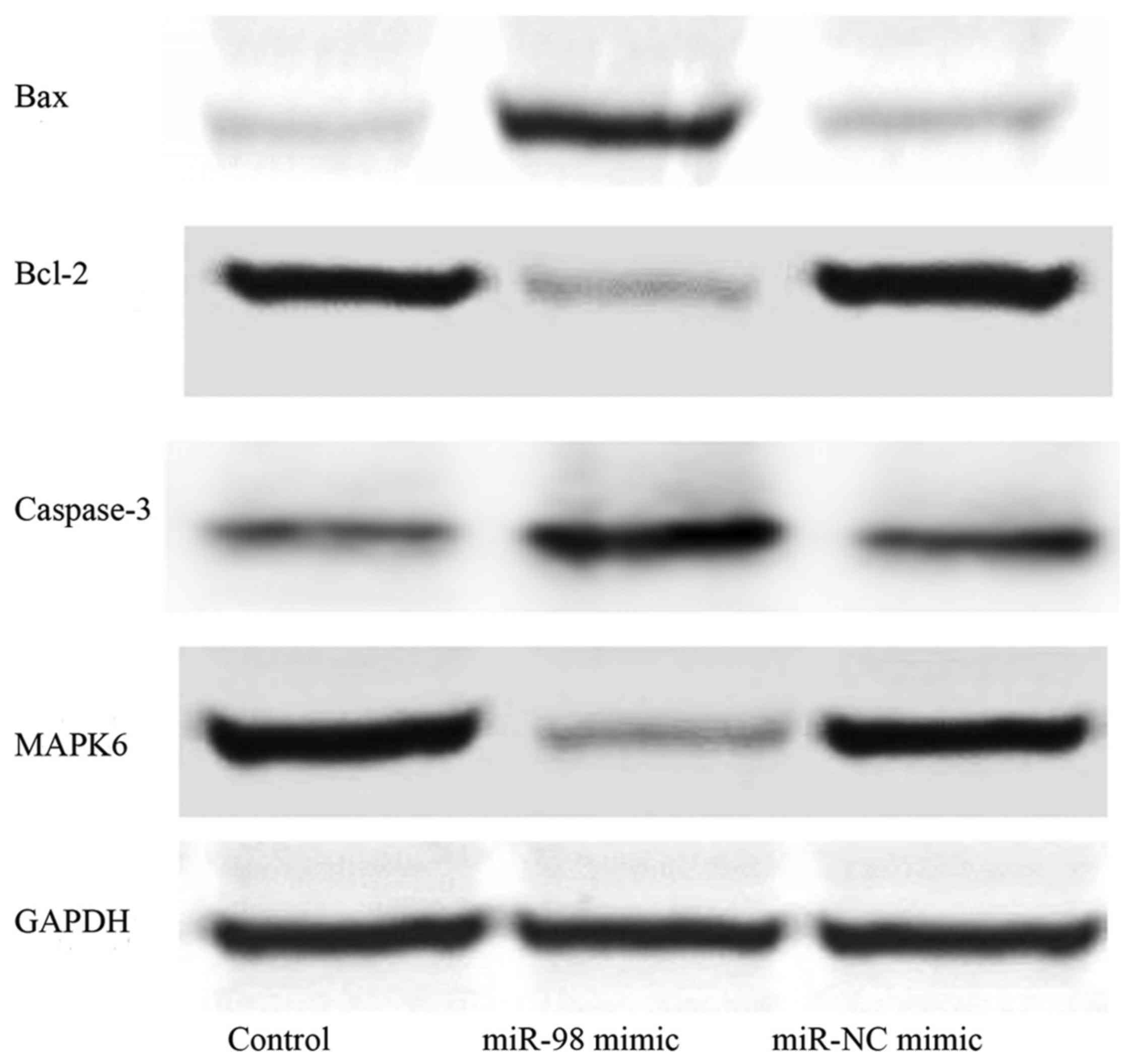
Western blotting was performed to explore the
protein levels of Bax, Bcl-2, caspase-3 and MAPK6 following the
administration of hsa-miR-98 mimic or miR-NC mimic. We found that
the levels of MAPK6 and Bcl-2 were lower, while those of Bax and
caspase-3 were higher in the hsa-miR-98 mimic group compared with
the control group and miR-NC mimic group (Fig. 6).
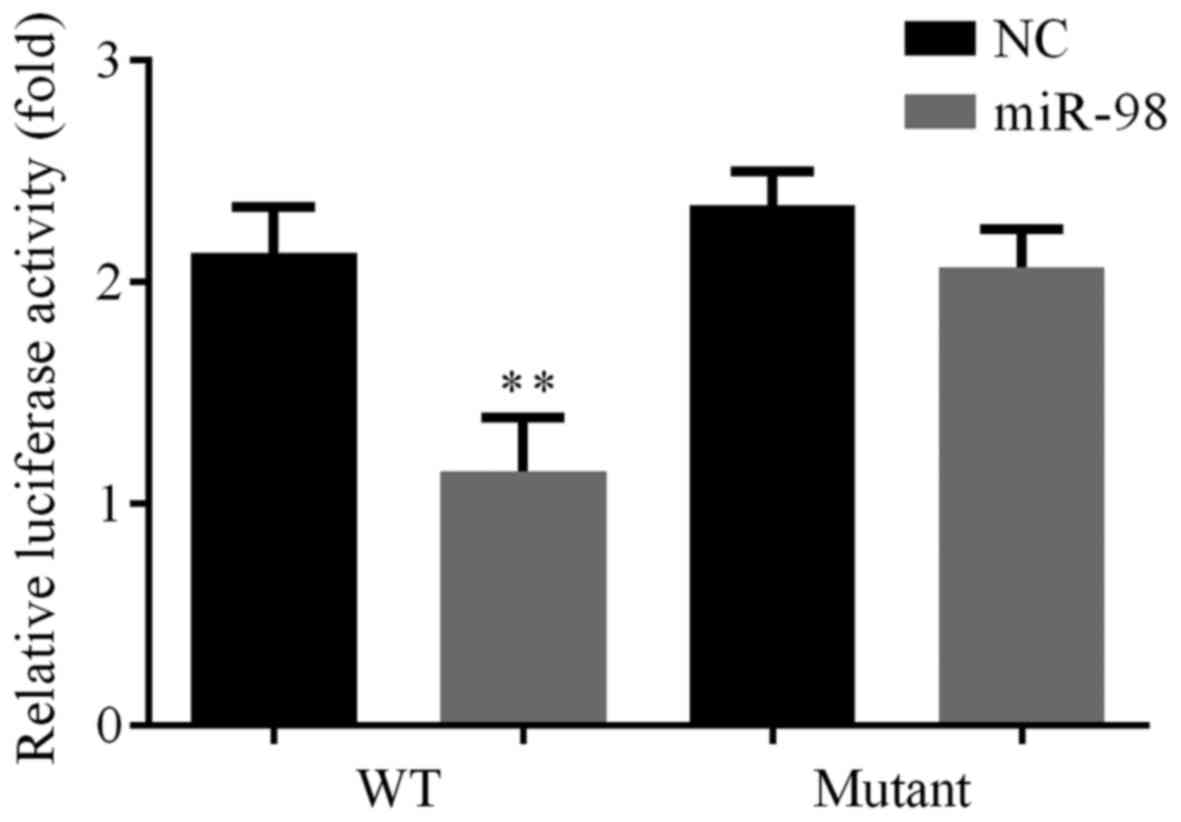
Verification of an interaction between
miR-98 and MAPK6
Luciferase assays showed that miR-98 repressed
wild-type (WT) MAPK6-3′-UTR-luciferase activity but not mutant
MAPK6-3′-UTR-luciferease reporter. This result verified that
hsa-miR-98 targeted MAPK6 in HUVECs (Fig. 7).
Discussion
Atherosclerosis presents as arterial wall thickening
(1,2). Endothelial dysfunction occurs initially
during the progression of atherosclerosis (19), and is followed by the proliferation
and migration of smooth muscle cells (20).
MAPK6 modulates the cell proliferation and
angiogenesis of primary HUVECs, and maintains the physiological
condition of VSMC (15), whereas its
knockdown damages the tube formation of VSMC (16). We first examined MAPK6 mRNA levels in
HVECs from healthy and atherosclerotic subjects, and found that
MAPK6 was significantly higher in the HVECs of patients with
atherosclerosis compared with those of healthy volunteers (Fig. 1).
mRNAs have the ability to bind to the 5′UTR or 3′UTR
of target genes to inhibit their expression (5–7).
Circulating miRNAs are potentially functional in the
atherosclerosis associated dysfunction of gene regulatory networks
(21) as well as biological
processes, for instance, cell proliferation (10). However, it remains unclear whether
MAPK6 could be regulated by miRNA in the functioning of the
vascular endothelium. miRanda was adopted to identify miRNAs that
could target MAPK6, and a potential seed sequence of hsa-miR-98 was
exhibited in the MAPK6-3′-UTR (Fig.
2). Thereafter, we identified the expression levels of
hsa-miR-98 in HVECs from healthy and atherosclerotic subjects, and
found that hsa-miR-98 was significantly lower in the HVECs of
patients with atherosclerosis compared with healthy individuals
(Fig. 3), which was consistent with
a previous study (18).
The potential influences of hsa-miR-98 on HUVEC cell
proliferation and apoptosis were tested by MTT assay and flow
cytometry assay, respectively. Results showed slower growth
(Fig. 4) and a higher apoptotic rate
(Fig. 5) in HUVECs transfected with
hsa-miR-98 mimic than in control group and miR-NC mimic group.
Bcl-2 and caspase families participate in the
apoptotic process. Bcl-2 protein families include Bax
(pro-apoptotic) and Bcl-2 (antiapoptotic) (22). Moreover, since caspase-3 is the most
reliable determining factor during apoptosis (23), Bcl-2 regulates apoptosis via a
caspase-3 dependent pathway (24).
Western blotting results showed that MAPK6 and Bcl-2 levels were
lower, while Bax and caspase-3 levels were higher in the hsa-miR-98
mimic group than in control group and miR-NC mimic group (Fig. 6).
Finally, the interaction between miR-98 and MAPK6
was tested by luciferase assay. The results verified that
hsa-miR-98 targeted MAPK6 in HUVECs (Fig. 7).
In conclusion, miR-98 inhibited HUVEC proliferation,
promoted HUVEC apoptosis, lowered MAPK6/Bcl-2 and upregulated
Bax/caspase-3 via targeting MAPK6. Thus miR-98 might be a
therapeutic target for atherosclerosis.
References
|
1
|
Ross R: The pathogenesis of
atherosclerosis: A perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hansson GK and Hermansson A: The immune
system in atherosclerosis. Nat Immunol. 12:204–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lüscher TF and Barton M: Biology of the
endothelium. Clin Cardiol. 20(11 Suppl 2): II-3-101997.PubMed/NCBI
|
|
4
|
Olejarz W, Bryk D, Zapolska-Downar D,
Małecki M, Stachurska A and Sitkiewicz D: Mycophenolic acid
attenuates the tumour necrosis factor α-mediated proinflammatory
response in endothelial cells by blocking the MAPK/NF-κB and ROS
pathways. Eur J Clin Invest. 44:54–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pillai RS, Bhattacharyya SN and Filipowicz
W: Repression of protein synthesis by miRNAs: How many mechanisms?
Trends Cell Biol. 17:118–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forman JJ and Coller HA: The code within
the code: microRNAs target coding regions. Cell Cycle. 9:1533–1541.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lytle JR, Yario TA and Steitz JA: Target
mRNAs are repressed as efficiently by microRNA-binding sites in the
5′ UTR as in the 3′UTR. Proc Natl Acad Sci USA. 104:pp. 9667–9672.
2007; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McManus DD and Ambros V: Circulating
MicroRNAs in cardiovascular disease. Circulation. 124:1908–1910.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kosaka N, Iguchi H and Ochiya T:
Circulating microRNA in body fluid: A new potential biomarker for
cancer diagnosis and prognosis. Cancer Sci. 101:2087–2092. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cirera-Salinas D, Pauta M, Allen RM,
Salerno AG, Ramírez CM, Chamorro-Jorganes A, Wanschel AC, Lasuncion
MA, Morales-Ruiz M, Suarez Y, et al: mir-33 regulates cell
proliferation and cell cycle progression. Cell Cycle. 11:922–933.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen WJ, Yin K, Zhao GJ, Fu YC and Tang
CK: The magic and mystery of microRNA-27 in atherosclerosis.
Atherosclerosis. 222:314–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Torella D, Iaconetti C, Catalucci D,
Ellison GM, Leone A, Waring CD, Bochicchio A, Vicinanza C, Aquila
I, Curcio A, et al: MicroRNA-133 controls vascular smooth muscle
cell phenotypic switch in vitro and vascular remodeling in vivo.
Circ Res. 109:880–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meloche S, Beatty BG and Pellerin J:
Primary structure, expression and chromosomal locus of a human
homolog of rat ERK3. Oncogene. 13:1575–1579. 1996.PubMed/NCBI
|
|
15
|
Tan J, Yang L, Liu C and Yan Z:
MicroRNA-26a targets MAPK6 to inhibit smooth muscle cell
proliferation and vein graft neointimal hyperplasia. Sci Rep.
7:466022017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tak H, Jang E, Kim SB, Park J, Suk J, Yoon
YS, Ahn JK, Lee JH and Joe CO: 14-3-3epsilon inhibits MK5-mediated
cell migration by disrupting F-actin polymerization. Cell Signal.
19:2379–2387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Small EM and Olson EN: Pervasive roles of
microRNAs in cardiovascular biology. Nature. 469:336–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song Z and Li G: Role of specific
microRNAs in regulation of vascular smooth muscle cell
differentiation and the response to injury. J Cardiovasc Transl
Res. 3:246–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mudau M, Genis A, Lochner A and Strijdom
H: Endothelial dysfunction: The early predictor of atherosclerosis.
Cardiovasc J Afr. 23:222–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fish JE, Santoro MM, Morton SU, Yu S, Yeh
RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY and Srivastava D:
MiR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaufmann SH, Lee SH, Meng XW, Loegering
DA, Kottke TJ, Henzing AJ, Ruchaud S, Samejima K and Earnshaw WC:
Apoptosis-associated caspase activation assays. Methods.
44:262–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marsden VS, O'Connor L, O'Reilly LA, Silke
J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ,
et al: Apoptosis initiated by Bcl-2-regulated caspase activation
independently of the cytochrome c/Apaf-1/caspase-9 apoptosome.
Nature. 419:634–637. 2002. View Article : Google Scholar : PubMed/NCBI
|