Introduction
Liver cirrhosis has clinical features such as high
incidence, difficult curability and high fatality rate (1). Hepatitis B virus-related cirrhosis is
the main type of liver cirrhosis. With the development of hepatitis
B disease, liver cirrhosis occurs. According to statistics, for the
patients with hepatitis B cirrhosis, the 5-year survival rate is
approximately 50%, while the survival rate is lower than 20% in the
decompensated period, leading to severe impact on the patients'
quality of life of the elderly in China (2,3).
The concurrent infection caused by bacteria and
other pathogens is one of the important factors resulting in the
difficulties to successfully treat patients with hepatitis B
cirrhosis, and can lead to death (4). In recent years, it has been reported
that most patients with hepatitis B cirrhosis complicated with
infection are accompanied with a decrease of cortiadrenal function,
and thereby liver function is indirectly affected, which makes the
disease difficult to treat (5–7).
In this study, the cortisol secreted by the adrenal
cortex was the focal point, the changes of the infected and
uninfected cortisol and serum inflammatory factors of patients with
hepatitis B cirrhosis were compared, and the correlation between
cortisol (COR) and concurrent infection as well as the
countermeasures were analyzed.
Materials and methods
Materials
According to the criteria of the Guideline of
Prevention and Treatment for Chronic hepatitis B (2010 version), 86
patients who were diagnosed and treated in the Beijing YouAn
Hospital from March 2014 to March 2017 were selected as the
non-infection group. This group comprised 58 males and 28 females,
aged 25–72 years, with an average age of 54.32±15.41 years.
According to the Child-Pugh grade, there were 7 cases with grade A,
38 cases with grade B and 41 cases with grade C. Thirty-two cases
of patients were included in the infection group, including 21
males and 11 females aged 24–73 years, with an average age of
55.14±16.03 years. There were 2 cases with grade A, 14 cases with
grade B and 16 cases with grade C. Of the 32 cases, 14 cases had
simple abdominal infection, 10 cases had abdominal infection
complicated with sepsis, and 8 cases had abdominal infection
complicated with pulmonary infection.
Exclusion criteria were: i) Patients with other
virus infections except HBV; ii) patients with non-alcoholic
steatohepatitis, tumor, alcoholic liver disease,
cardio-cerebrovascular disease and other major diseases; iii)
patients with abnormal adrenal cortex function and any patients who
had taken drugs affecting the secretion of cortisol within the last
six months.
The differences were not statistically significant
regarding age, sex, weight, disease grading and other indexes
between the two groups of patients (P>0.05), and thus
comparable.
This study was approved by the Hospital Ethics
Committee of the Beijing YouAn Hospital (Beijing, China), and made
an introduction to the selected patients to ensure that informed
consent was signed by them and that they were fully informed.
Reagents
COR chemiluminescence kit (Beckman Coulter, Brea,
CA, USA), cortisol binding globulin (CBG) enzyme-linked
immunosorbent assay (ELISA) kit (Shanghai Jiang Lai Biotechnology
Co., Ltd., Shanghai, China), TRIzol (Thermo Fisher Scientific,
Waltham, MA, USA), chloroform, isopropanol (Beijing Chemical
Factory, Beijing, China), moloney murine leukemia virus (M-MLV)
reverse transcriptase, DNase I (both from Thermo Fisher
Scientific), SYBR® Premix Ex Taq™ II (Takara, Dalian,
China), and synthetic primers (BGI Genomics, Shenzhen, Guangdong,
China) were used in the present study.
Blood routine test
Fasting venous blood (15 ml) was collected from all
the patients in the morning, and 7 ml was used in the tests for
related inflammatory indexes using a Roche C501 automatic
biochemical analyzer (Roche Diagnostics, Basel, Switzerland)
including white blood cells (WBC), neutrophils, procalcitonin
(PCT), endotoxin and C-reactive protein (CRP).
Detection of cortisol
concentration
The collected 7 ml fasting venous blood was
centrifuged at 1,300 g/min for 15 min at 4°C in order to collect
serum. The concentrations of COR and CBG in the serum were measured
by corresponding kits. The concentration of free cortisol (FC) was
calculated using the Coolens formula: FC concentration (mmol/l) =
Z2 + 0.122c) 1/2-Z; and the coefficient Z was
calculated, Z=0.0167+0.182 (T-C), while T was the concentration of
COR, and C was the concentration of CBG (8).
mRNA expression detection of CBG
gene
RNA was extracted from 1 ml whole blood by TRIzol.
The procedure was strictly performed as per the protocol following
the instructions, and the concentration was measured. RNA (1µg) was
taken to be used for reverse transcription reaction by reverse
transcriptase kit to obtain cDNA. The concentration of cDNA was
adjusted, and the levels of mRNA and relative expression of
different groups were measured using Bio-Rad CFX 96 PCR (Hercules,
CA, USA) instrument in accordance with the instructions of the
SYBR® Premix Ex Taq™ II kit. The corresponding primer
sequences are shown in Table I
(9).
 | Table I.RT-PCR primer sequences of CBG and
β-actin mRNA. |
Table I.
RT-PCR primer sequences of CBG and
β-actin mRNA.
| Gene names | Primer sequences |
|---|
| CBG |
5′-TAGCCCAGCCATCCTC-3′ |
|
|
3′-GTGCTCGACTGCAACATC-5′ |
| β-actin |
5′-TCAGGTCATCACTATCGGCAAT-3′ |
|
|
3′-AAAGAAAGGGTGTAAAACGCA-5′ |
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 (Hangzhou NewGrand Software Co., Ltd., Hangzhou, China) was
used for data processing. Measurement data are shown as mean ± SD,
and were detected by student's t-test. The Kruskal-Wallis test was
used for the enumeration data and multiple comparisons (Tables I and II). Analysis of variance was performed,
and then pairwise comparison. Pearsons test was used for the
correlation analysis between factors. P<0.05 was considered
statistically significant (10).
 | Table II.Comparisons of cortisol levels for the
three Child-Pugh grades in the two groups, mean ± SD. |
Table II.
Comparisons of cortisol levels for the
three Child-Pugh grades in the two groups, mean ± SD.
|
| Non-infection
group | Infection group |
|---|
|
|
|
|
|---|
| Observation
indexes | Grade A | Grade B | Grade C | Grade A | Grade B | Grade C |
|---|
| COR (µg/dl) |
14.98±4.34 |
13.87±4.78a |
11.98±4.07a,b |
13.76±5.13c |
12.12±4.97a,c |
10.43±3.76a–c |
| CGB (µg/ml) |
47.43±11.21 |
36.54±9.87a |
28.42±9.06a,b |
38.21±14.32c |
29.56±12.01a,c |
22.36±9.65a–c |
| FC (nmol/l) |
95.21±3.21 |
94.72±4.54a |
94.77±3.12a |
89.21±4.21c |
88.67±3.87a,c |
87.32±2.63a,c |
Results
Comparisons of cortisol levels for the
three Child-Pugh grades in the two groups
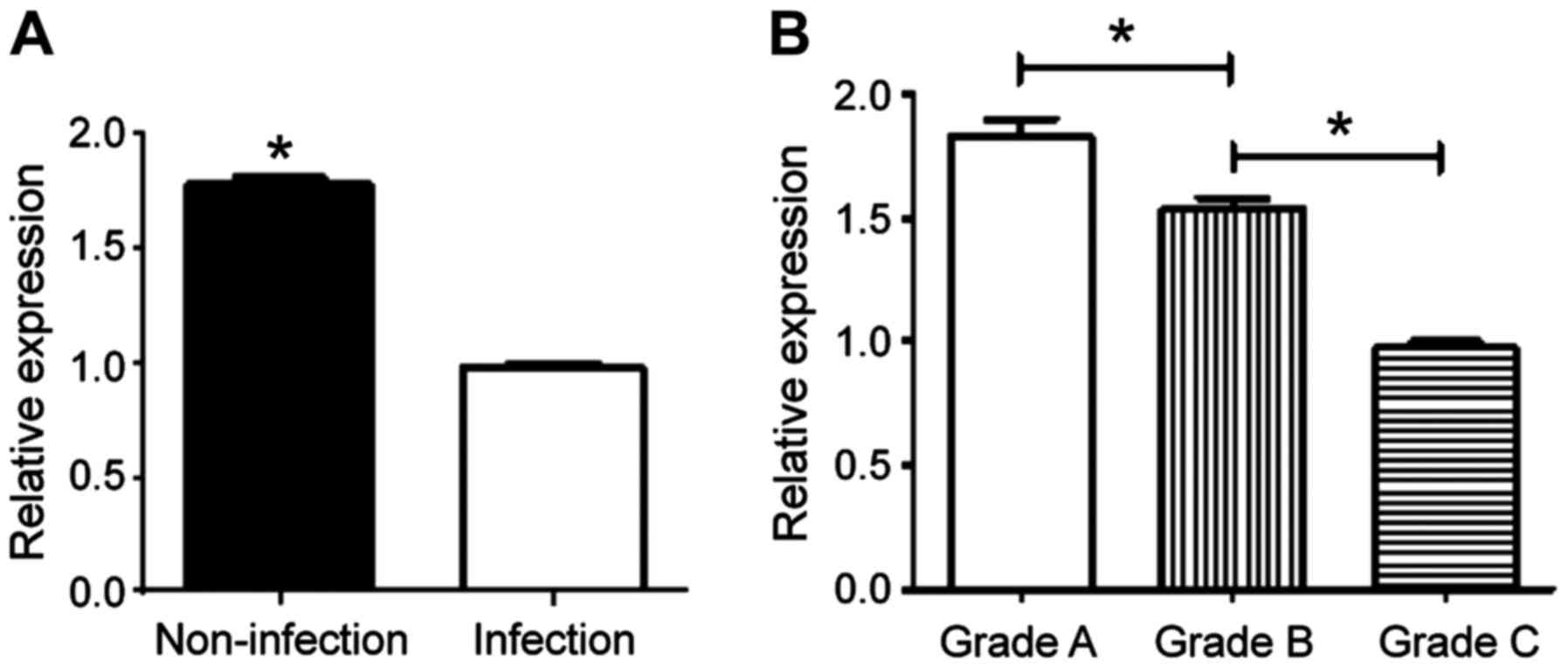
The concentrations of COR and CBG decreased with the
increase of Child-Pugh grades in the infection and non-infection
groups, and the differences were statistically significant
(P<0.05). The concentrations of COR, CBG and FC at the same
grade in the non-infection group were higher than those in the
infection group (P<0.05) (Table
II). The relative expression of CBG was detected by RT-qPCR,
which also showed that grade A > grade B > grade C
(P<0.05), and non-infection group > infection group
(P<0.05) (Fig. 1).
Comparisons of cortisol levels among
different infection types in the infection group
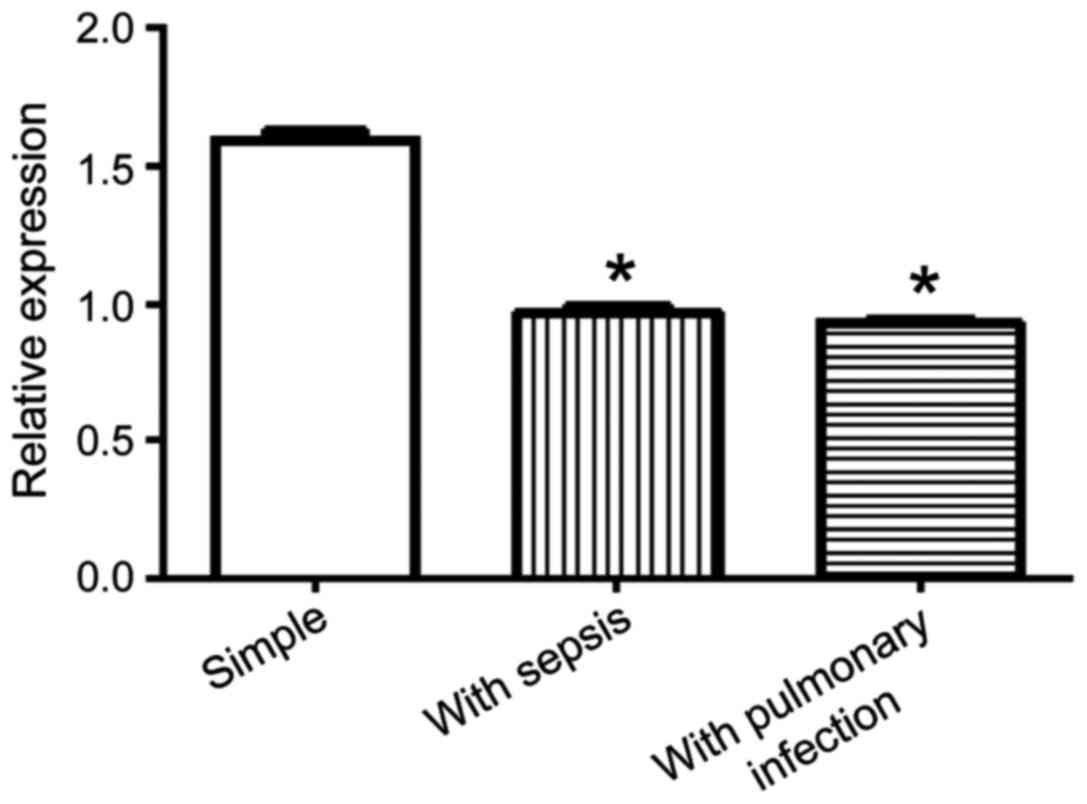
The concentrations of COR, CBG and FC for abdominal
infections complicated with sepsis or pulmonary infection were
lower than those for simple abdominal infection (Table III). The relative expression of CBG
was detected by RT-qPCR, which showed that the group of abdominal
infection complicated with sepsis and the group of abdominal
infection complicated with pulmonary infection were lower than the
group of simple abdominal infection (P<0.05) (Fig. 2).
 | Table III.Comparisons of cortisol levels among
different infection types in the infection group (mean ± SD). |
Table III.
Comparisons of cortisol levels among
different infection types in the infection group (mean ± SD).
| Infection types | No. (A/B/C) | COR (µg/dl) | CGB (µg/ml) | FC (nmol/l) |
|---|
| Abdominal
infection | 14 (2/6/6) |
13.81±4.54 |
40.32±13.12 |
91.43±4.08 |
| Abdominal +
sepsis | 10 (0/5/5) |
10.97±3.54a |
23.32±9.87a |
86.67±3.65a |
| Abdominal +
pulmonary | 8
(0/3/5) |
10.43±3.54a |
22.98±11.08a |
86.18±3.76a |
Comparisons of related infection
indexes between the two groups
The values of WBC, neutrophils, CRP, PCT and
endotoxin in the infection group were higher than those in the
non-infection group, and the differences were statistically
significant (P<0.05) (Table
IV).
 | Table IV.Comparisons of related infection
indexes between the two groups (mean ± SD). |
Table IV.
Comparisons of related infection
indexes between the two groups (mean ± SD).
| Infection
indexes | Non-infection
group | Infection group | t-values | P-value |
|---|
| WBC
(×109/l) |
5.58±2.89 |
8.49±4.99 | 3.345 | 0.002 |
| Neutrophils (%) |
67.87±6.76 |
77.87±9.98 | 5.012 | 0 |
| CRP (µg/l) |
14.61±3.76 |
20.51±5.32 | 3.145 | 0.036 |
| PCT (ng/ml) |
1.29±0.64 |
1.79±0.82 | 3.169 | 0.041 |
| Endotoxin
(pg/ml) |
60.76±29.87 |
271.08±138.76 | 3.132 | 0.024 |
Correlation analysis of cortisol and
related infection indexes
COR, CGB and FC were negatively correlated with such
indexes as WBC, neutrophils, CRP, PCT and endotoxin. The specific
Pearsons coefficients are shown in Table
V.
 | Table V.Correlation analysis of cortisol and
related infection indexes. |
Table V.
Correlation analysis of cortisol and
related infection indexes.
| Indexes | WBC
(×109/l) | Neutrophils, sec
(%) | CRP (µg/l) | PCT (ng/ml) | Endotoxin
(pg/ml) |
|---|
| COR (µg/dl) | −0.657 | −0.598 | −0.712 | −0.698 | −0.812 |
| CGB (µg/ml) | −0.712 | −0.608 | −0.743 | −0.765 | −0.784 |
| FC (nmol/l) | −0.687 | −0.654 | −0.733 | −0.654 | −0.765 |
Correlation analysis of COR and
FC
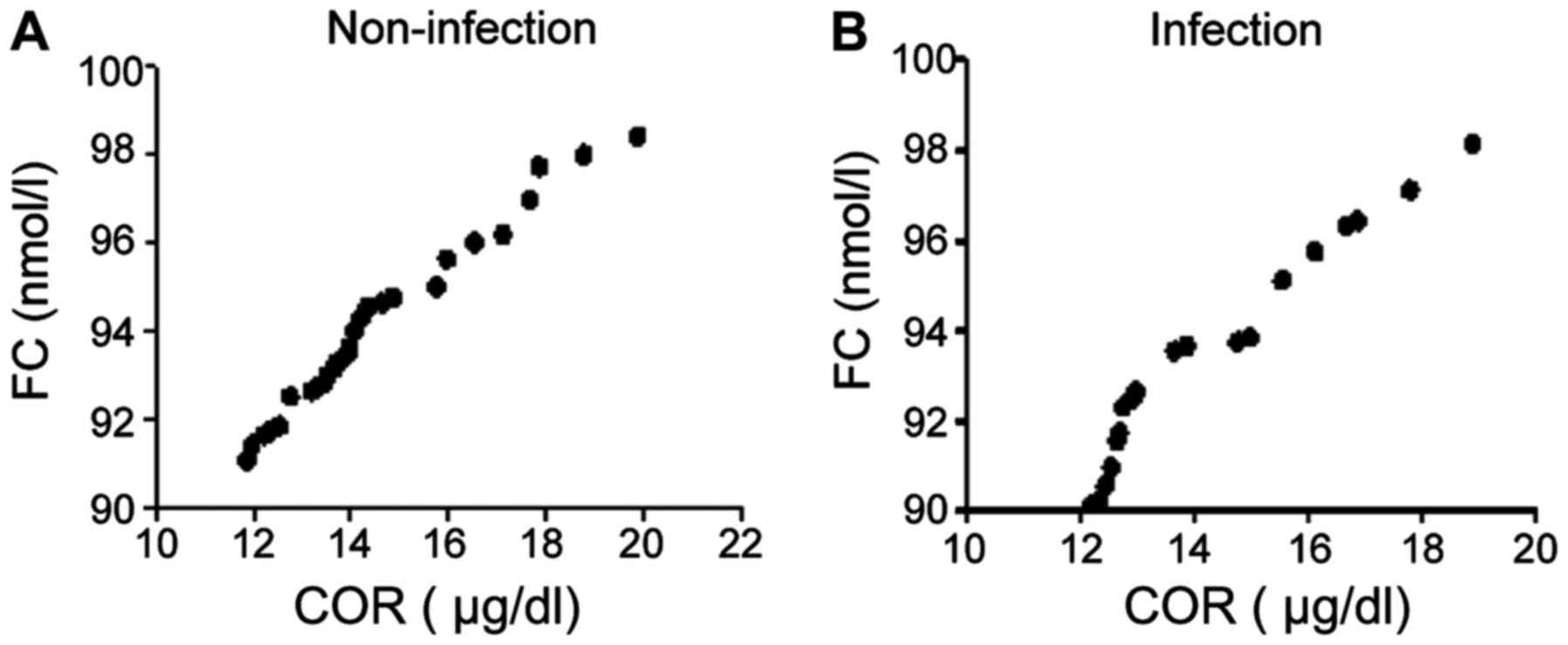
The r value of COR and FC in the non-infection group
was 0.678, while that of of COR and FC in the infection group was
0.787; infection group was positively correlated with non-infection
group, and COR was positively correlated with FC (Fig. 3).
Discussion
Hepatitis induced by HBV is one of the most common
forms of hepatitis induced by various viruses. Liver cirrhosis
caused by this is one of the major diseases in the world. Liver
cirrhosis is commonly complicated with infection, liver ascites and
liver cancer (11). Infection is the
common complication in liver cirrhosis. Liver cirrhosis complicated
with infection often has long recovery time and poor prognostic
result, and the mortality rate is 60% (12). Cortisol, also known as hydrocortisone
and which is produced by the adrenal gland, mainly exists in the
form of binding state and free state. Cortisol has a particularly
important effect in controlling mood and health, immune cells and
inflammation, connection between blood vessel and blood pressure,
as well as maintaining bones, muscles, skin and other organs.
Cortisol generally maintains the stability of blood pressure and
controls excessive inflammation in the case of pressure state
(13,14).
Cirrhosis can be divided into grade A, B and C
according to Child-Pugh score from low to high in accordance with
its development degree. Especially in later period, the secretion
of COR often becomes abnormal, which is largely related to the
synthesis and expression of CBG being affected (15,16). In
this study, the results showed that the concentrations of COR and
CBG decreased with the increase of Child-Pugh grade in either the
infection group or non-infection group, and the differences were
statistically significant (P<0.05). The relative expression of
CBG was detected by RT-qPCR, which showed that: grade A > grade
B > grade C (P<0.05). Previous findings have shown that
50–70% of the patients suffering from shock due to cirrhosis
combined with infection have adrenal insufficiency, and the damaged
adrenal cortical function is bound to affect the level of cortisol
in vivo (17). In this study,
the results also verified that the concentrations of COR, CBG and
FC in the infection group were lower than those in the
non-infection group whether on the whole or at the same Child-Pugh
grade, and RT-qPCR also confirmed that the expression of CBG
gene in the infection group was lower than that in the
non-infection group, which was consistent with the findings of
Hamrahian et al, who identified that, the expression of
CBG gene declined rapidly with the deepening of infection
extent (18).
This study also showed that the concentrations of
COR, CBG and FC for abdominal infection complicated with sepsis or
pulmonary infection were lower than those for simple abdominal
infection (P<0.05). The relative expression of CBG was detected
by RT-qPCR showing that the group of abdominal infection
complicated with sepsis or pulmonary infection were lower than the
simple abdominal infection (P<0.05). The results suggests that
different infection sites and types can also lead to the decrease
of the cortisol level in vivo, especially for more than two
concurrent infections, which may be greatly associated with that of
more serious infection. Thus, the metastasis of abdominal infection
to infect other organs exacerbates the disease.
WBC, neutrophil percentage, CRP, PCT and endotoxin
are the most common and sensitive indexes used to measure infection
and the most intuitive indexes for infection extent of blood
routine at present, and their elevated degree is directly
proportional to disease degree of inflammation (19). The present study also verified that
the above indexes of the hepatitis B cirrhosis patients complicated
with infection were higher than those of the hepatitis B cirrhosis
patients without infection. In addition COR, CGB and FC were
negatively correlated with such indexes as WBC, neutrophils, CRP,
PCT and endotoxin by further study for the correlation between
infection indexes and cortisol. Results show that the cortisol
levels of hepatitis B cirrhosis patients are significantly
correlated whether infected or not, and grades of condition and
infection types can be used as sensitive indexes to control
hepatitis B cirrhosis infection.
In conclusion, the content of cortisol in
vivo can be used as an important index to treat and prevent
infection. Therefore, to reduce the probability of infection,
grading of hepatitis B cirrhosis should be predicted by the
detection of cortisol concentrations. Medical treatment should be
taken as early as possible according to different disease grades,
and nursing measures should be reinforced. Adrenocortical function
should be improved, and the cortisol level should be kept at a
reasonable range, in order to indirectly reduce the occurrence of
infection. In addition, nursing care for cirrhosis patients should
be enhanced, in order to reduce the occurrence rates of
psychological fear and unforeseen circumstances.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen GC, Yu T, Min XH, Zhao LN, Qing Q,
Yuan YH, Su H, Zhan J, Huang KH and Chen QK: Prognosis of 153
patients with decompensated hepatitis B virus-related cirrhosis is
improved after 3-year continuous lamivudine treatment. Chin Med J
(Engl). 126:1538–1543. 2013.PubMed/NCBI
|
|
2
|
Lv GC, Yao JM, Yang YD, Zheng L, Sheng JF,
Chen Y and Li LJ: Efficacy of combined therapy in patients with
hepatitis B virus-related decompensated cirrhosis. World J
Gastroenterol. 19:3481–3486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou YM, Zhang XF, Li B, Sui CJ and Yang
JM: Prognosis after resection of hepatitis B virus-related
hepatocellular carcinoma originating from non-cirrhotic liver. Ann
Surg Oncol. 21:2406–2412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ming D, Yu X, Guo R, Deng Y, Li J, Lin C,
Su M, Lin Z and Su Z: Elevated TGF-β1/IL-31 pathway is associated
with the disease severity of hepatitis B virus-related liver
cirrhosis. Viral Immunol. 28:209–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun LL, Li YH, Chen F, Wang S and Shi H:
Study on correlation between liver ultrasonic appearance of
patients with chronic hepatitis B and cirrhosis and hydrothorax.
Eur Rev Med Pharmacol Sci. 20:50322016.PubMed/NCBI
|
|
6
|
Srivastava M, Rungta S, Dixit VK, Shukla
SK, Singh TB and Jain AK: Predictors of survival in hepatitis B
virus related decompensated cirrhosis on tenofovir therapy: An
Indian perspective. Antiviral Res. 100:300–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang N, Cao Y, Song W, He K, Li T, Wang J,
Xu B, Si HY, Hu CJ and Li AL: Serum peptide pattern that
differentially diagnoses hepatitis B virus-related hepatocellular
carcinoma from liver cirrhosis. J Gastroenterol Hepatol.
29:1544–1550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Öcal S, Korkmaz M, Harmancı Ö, Ensaroğlu
F, Akdur A, Selçuk H, Moray G and Haberal M: Hepatitis B- and
hepatitis D-virus-related liver transplant: Single-center data. Exp
Clin Transplant. 13 Suppl 1:133–138. 2015.PubMed/NCBI
|
|
9
|
Niro GA, Ippolito AM, Fontana R, Valvano
MR, Gioffreda D, Iacobellis A, Merla A, Durazzo M, Lotti G, Di
Mauro L, et al: Long-term outcome of hepatitis B virus-related
chronic hepatitis under protracted nucleos(t)ide analogues. J Viral
Hepat. 20:502–509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu ZW, Lu HF, Wu J, Zuo J, Chen P, Sheng
JF, Zheng SS and Li LJ: Assessment of the fecal lactobacilli
population in patients with hepatitis B virus-related decompensated
cirrhosis and hepatitis B cirrhosis treated with liver transplant.
Microb Ecol. 63:929–937. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim IS, Mun JI, Koo JH, Kang CJ, Bak JK,
Cheong JY and Cho SW: Entecavir therapy for patients with hepatitis
B virus-related decompensated cirrhosis. Korean J Gastroenterol.
59:224–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z,
Wang JF, Zhang Z, Lu S, Huang X, et al: Plasma microRNA panel to
diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin
Oncol. 29:4781–4788. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Y, Lu Y, Xie L, Deng Y, Li S and Qin
X: Interferon gamma polymorphisms and hepatitis B virus-related
liver cirrhosis risk in a Chinese population. Cancer Cell Int.
15:352015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saichan X, Wei C, Qinglong F, Jun W and
Lei X: Plasma cortisol as a noninvasive biomarker to assess
severity and prognosis of patients with craniocerebral injury. Eur
Rev Med Pharmacol Sci. 20:3835–3838. 2016.PubMed/NCBI
|
|
15
|
Karra VK, Gumma PK, Chowdhury SJ, Ruttala
R, Polipalli SK, Chakravarti A and Kar P: IL-18 polymorphisms in
hepatitis B virus related liver disease. Cytokine. 73:277–282.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bae JS, Kim JH, Pasaje CF, Cheong HS, Lee
TH, Koh IS, Lee HS, Kim YJ and Shin HD: Association study of
genetic variations in microRNAs with the risk of hepatitis
B-related liver diseases. Dig Liver Dis. 44:849–854. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chu CM and Liaw YF: Hepatitis B
virus-related cirrhosis: Natural history and treatment. Semin Liver
Dis. 26:142–152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hamrahian AH, Oseni TS and Arafah BM:
Measurements ofserum free cortisol in critically ill patients. N
EnglJ Med. 350:1629–1638. 2004. View Article : Google Scholar
|
|
19
|
Bárcena R, Domínguez-Antonaya M,
López-Sanromán A, Martínez-Turnes A, Urman J, del Campo S and
Moreno N: Lamivudine therapy of hepatitis B virus-related liver
disease: Cirrhosis, post-transplantation recurrence, and de novo
infection. Transplant Proc. 31:pp. 2457–2458. 1999; View Article : Google Scholar : PubMed/NCBI
|