Introduction
Deep venous thrombosis (DVT) of the lower extremity
is a haemal disease involving abnormal circumfluence (1). Once the thrombus occurs, a small
fraction of them ablate on their own; however, most thrombus spread
gradually to the venous main body of the limbs (2). If timely clinical diagnosis and
treatment are not provided, the situation will aggravate with
thrombus sequelae, with serious heath consequences. Research
suggests the increase of homocysteine (Hcy) levels in plasma is
closely related to DVT (3,4). Some studies propose that the metabolic
process of hyperplasma Hcy is associated with folic acid and
vitamin B12, and that folic acid and vitamin B12 can lower the
plasma levels of Hcy (5–7). Thus, can folic acid and vitamin B12 be
used to treat hyperhomocysteinemia with DVT? If so, what are the
effects of different doses of folic acid and vitamin B12 on DVT
with hyperhomocysteinemia? In this study, we created a rabbit model
with DVT and hyperhomocysteinemia and used different doses of folic
acid and vitamin B12. We then examined the effect of each treatment
to provide experimental support for the treatment of DVT with
hyperhomocysteinemia with folic acid and vitamin B12.
Materials and methods
Experiment animals and treatments
Sixty male New Zealand rabbits of grade SPF were
provided by the experimental animal center of Lanzhou University,
with body mass of 2.1–3.1 kg. We created the rabbit model of DVT
with hyperhomocysteinemia and randomly divided them into three
groups: control (treated with normal saline), low-dose (treated
with 5 mg/day folic acid + 0.25 mg/day vitamin B12) and high-dose
(treated with 15 mg/day folic acid + 0.5 mg/day vitamin B12).
Normal saline was obtained from Shanghai Di Ran Dang Cheng
Pharmaceutical, folic acid tablets from Fujian Minghua
Pharmaceutical, Fujian, China (National Medicine permission number:
H19993229), and vitamin B12 from Datong Changxing Pharmaceutical,
Shanxi, China (National Medicine permission number: H14022782). The
rabbits were kept in a cage with controlled temperature (24°C) and
free access to water. The study was approved by the Ethics
Committee of the First Hospital of Lanzhou University.
Hemorheology detection
Three days and 10 days after modeling, 5 ml of whole
blood were obtained from the ear artery from each animal and
hemorheology indexes such as plasma viscosity, whole blood reduced
viscosity at low shear, whole blood reduced viscosity at high
shear, and red cell assembling index were detected using a
hemorheology detector (Beijing Succeeder Science and Technology
Development).
Coagulation function detection
Three days and 10 days after modeling, 3 m1 whole
blood were obtained from the ear artery from each animal, and
coagulation function indexes such as fibrinogen, activated partial
thromboplastin time, thrombin time, and prothrombin time were
detected using a coagulation convention detector (Nanjing Perlong
Image Documentation Equipment, Nanjing, China).
Biochemical tests
The fully automated biochemical analyzer (Shanghai
Hengsheng Medical Apparatus and Instruments Limited Company,
Shanghai, China) was used to measure Hcy levels before and 10 days
after DVT modeling by enzymatic cycling assay. Latex agglutination
test was used to measure plasma D-dimer in days 1 and 10 days after
DVT modeling. 10 days after DVT modeling, 5% thipentone of 0.1
ml/100 g body mass was injected to enterocoelia for anesthesia.
Then, rabbits were dissected to expose the saphenous artery, vein,
saphenous vein, artery part of left hind limbs, to check thrombus
and record if there is bump in the lower limbs. After blood vessel
of left hind limb was separated, formalin was applied to fix and
conventional pathological section was conducted, with hematoxylin
and eosin (H&E) stain and pathologic histology change of deep
venous thrombosis was inspected of rabbit lower extremity under
microscopic examination. Color Doppler ultrasound (Jiangsu Jiahua
Electronic Equipment Limited Company, Jiangsu, China) was applied
to observe prognosis of thrombus.
Statistical analysis
Software SPSS 20.0 (IBM, New York, NY, USA) was used
to process data. Enumeration data are shown by rate, χ2
was used to detect and measurement data are presented by mean ±
standard deviation. Comparison between two groups was done by
t-test and comparison among several groups applied F in detection.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Hemorheology indexes
We measured hemorheology indexes at days 3 and 10
days after DVT modeling. At day 3, plasma viscosity, whole blood
reduced viscosity at low shear, whole blood reduced viscosity at
high shear, and red cell assembling index were significantly lower
in the low-dose and high-dose groups compared to controls (Table I). At day 10, all the values improved
in the three groups, but the low- and high-dose groups still showed
lower values than the control group (Table II). Additionally, the hemorheology
indexes for the high-dose group were superior to those of low-dose
group at days 3 and 10, suggesting the benefits of the higher dose
(Tables I and II).
 | Table I.Hemorheology indexes 3 days after
modeling. |
Table I.
Hemorheology indexes 3 days after
modeling.
| Groups | No. | Plasma viscosity
(mPa·s) | Whole blood reduced
viscosity at low shear (mPa·s) | Whole blood reduced
viscosity at high shear (mPa·s) | Red cell assembling
index |
|---|
| Control | 20 |
1.72±0.75 |
41.35±1.35 |
5.83±0.05 |
4.68±0.06 |
| Low-dose | 20 |
1.53±0.36a |
35.74±0.85a |
5.66±0.07a |
4.44±0.08a |
| High-dose | 20 |
1.42±0.27a,b |
32.57±0.62a,b |
5.41±0.04a,b |
4.13±0.11a,b |
 | Table II.Hemorheology indexes 10 days after
modeling. |
Table II.
Hemorheology indexes 10 days after
modeling.
| Groups | No. | Plasma viscosity
(mPa·s) | Whole blood reduced
viscosity at low shear (mPa·s) | Whole blood reduced
viscosity at high shear (mPa·s) | Red cell assembling
index |
|---|
| Control | 20 |
1.70±0.65 |
40.07±1.25 |
5.76±0.06 |
4.62±0.04 |
| Low-dose | 20 |
1.45±0.31a |
33.32±0.54a |
5.42±0.04a |
4.26±0.05a |
| High-dose | 20 |
1.27±0.23a,b |
28.55±0.52a,b |
5.01±0.06a,b |
3.97±0.07a,b |
Coagulation function
We next measured coagulation indexes in the rabbits
3 and 10 days after DVT modeling. The levels of fibrinogen at days
3 and 10 were significantly lower in the two treatment groups
compared with controls (Tables III
and IV). The coagulation indexes
APTT, PT, and TT were significantly higher in both treatment groups
compared controls (Tables III and
IV). The coagulation function
indexes of the high-dose group were superior to those of the
low-dose group (Tables III and
IV).
 | Table III.Coagulation function 3 days after
modeling. |
Table III.
Coagulation function 3 days after
modeling.
| Groups | No. | Fibrinogen (g/l) | Activated partial
thromboplastin time (sec) | Thrombin time
(sec) | Prothrombin time
(sec) |
|---|
| Control | 20 |
4.23±0.35 |
15.12±0.85 |
27.75±0.31 |
8.76±0.47 |
| Low-dose | 20 |
3.64±0.75a |
17.83±1.12a |
31.57±0.52a |
10.03±0.13a |
| High-dose | 20 |
3.32±0.54a,b |
19.05±0.76a,b |
33.56±0.35a,b |
11.94±0.13a,b |
 | Table IV.Coagulation function indexes 10 days
after modeling. |
Table IV.
Coagulation function indexes 10 days
after modeling.
| Groups | No. | Fibrinogen (g/l) | Activated partial
thromboplastin time (s) | Thrombin time
(s) | Prothrombin time
(s) |
|---|
| Control | 20 |
4.16±0.41 |
15.05±0.62 |
28.11±0.54 |
9.01±0.47 |
| Low-dose | 20 |
3.64±0.75a |
17.83±1.12a |
35.45±0.43a |
12.16±0.22a |
| High-dose | 20 |
3.32±0.54a,b |
19.05±0.76a,b |
38.33±0.46a,b |
14.43±0.55a,b |
Hcy level
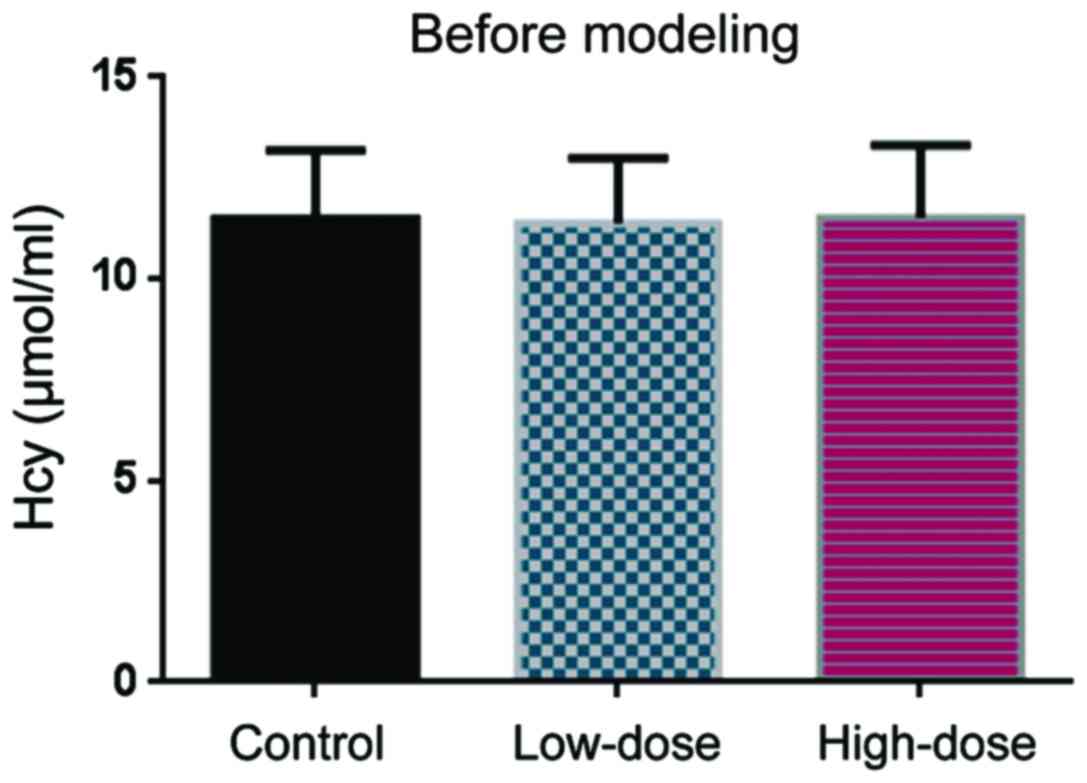
Before DVT modeling, the Hcy levels were comparable
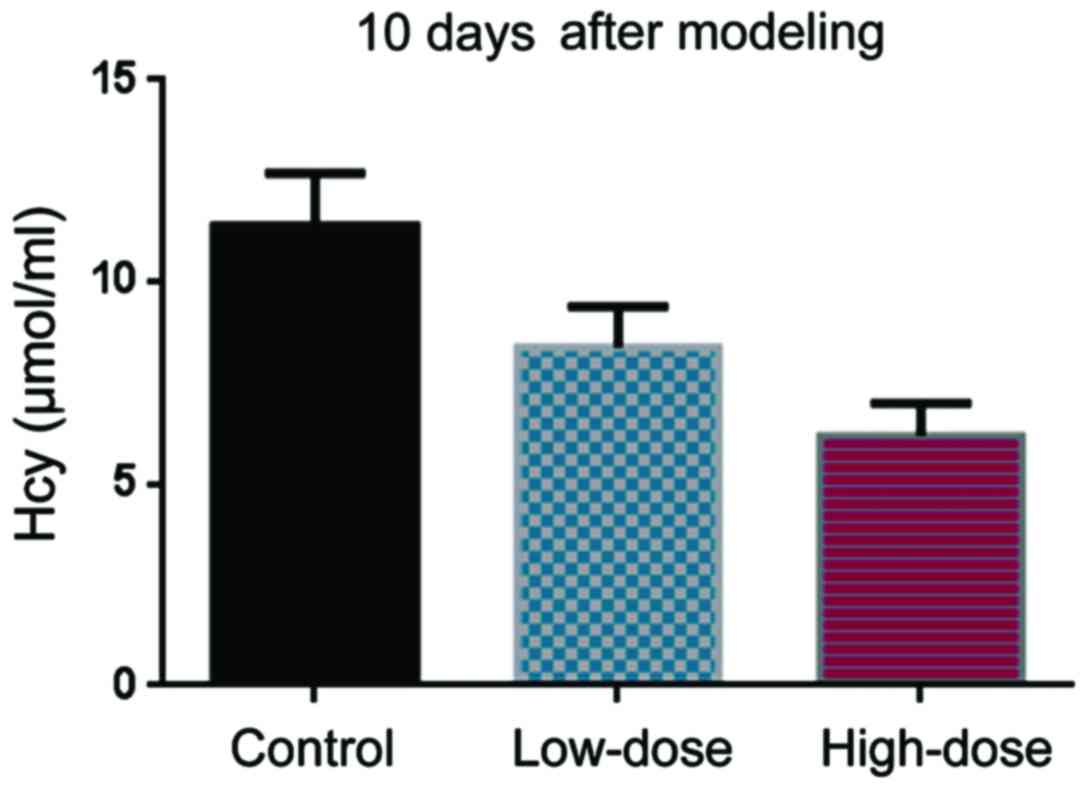
in the control, low-dose, and high-dose groups (Fig. 1). 10 days after DVT modeling, Hcy
levels in the low-dose group were lower than in the control group
(Fig. 2). The Hcy levels in the
high-dose group were even lower than those in the low-dose group
(Fig. 2).
D-dimer
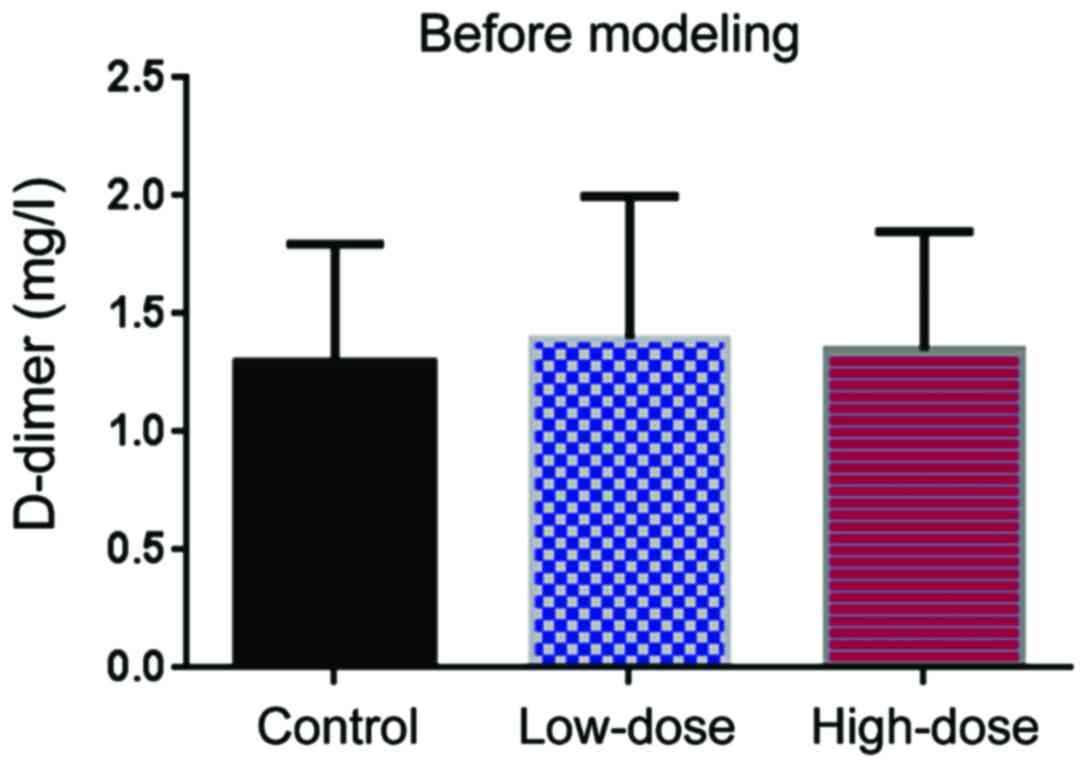
Before DVT modeling, the level of D-dimer level was
comparable in the three groups (Fig.
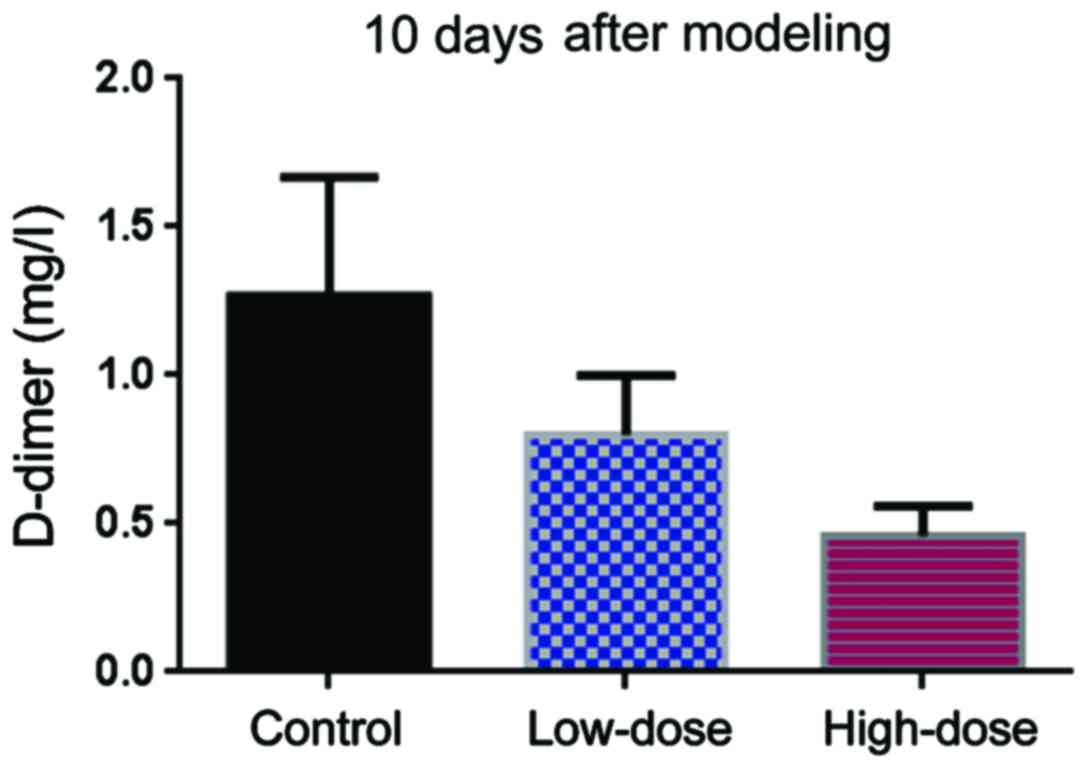
3). Ten days after modeling, the level of D-dimer in the
low-dose group was lower than in the control group (Fig. 4). The Hcy levels in the high-dose
group were even lower than those in the low-dose group (Fig. 4).
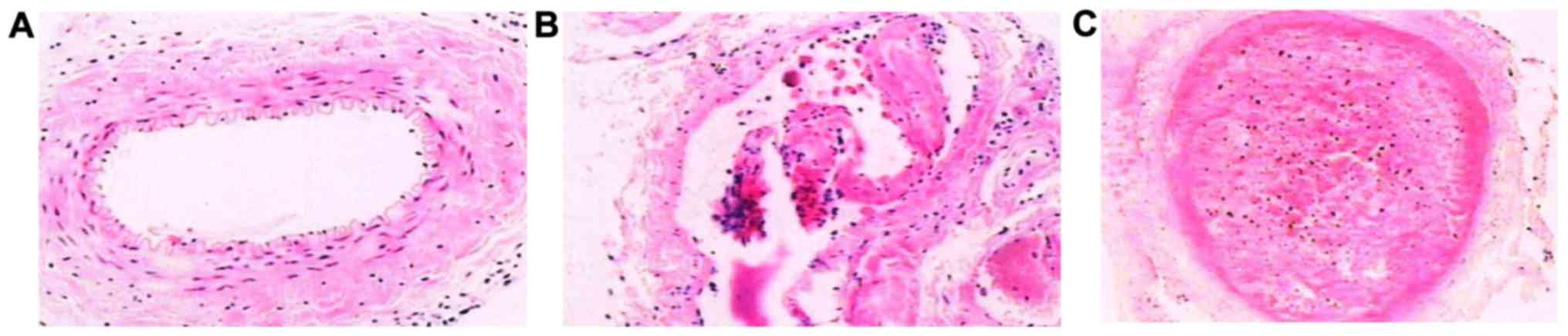
Vein pathology
Ten days after DVT modeling, we compared the change
of lower extremity deep venous thrombosis of high-dose group and
low-dose group were significantly reduced, while the improvement
level of high-dose group was the best. Each layer of the normal
femoral vein under light microscope recovered well in the high-dose
group, without edema in loose connective tissue of adventitia
(Fig. 5A). Partial serious intima
damage was visible in the low-dose group, with endothelial falling
partially (Fig. 5B). In the control
group, endothelial cells of vein blood vessel fell off, with
obvious adventitia edema, a great amount of fibrous protein
aggregation in ‘latticed’ fibrin, full of red cells and a little
deciduous endothelial cells, forming thrombus filling the whole
lumen (Fig. 5C).
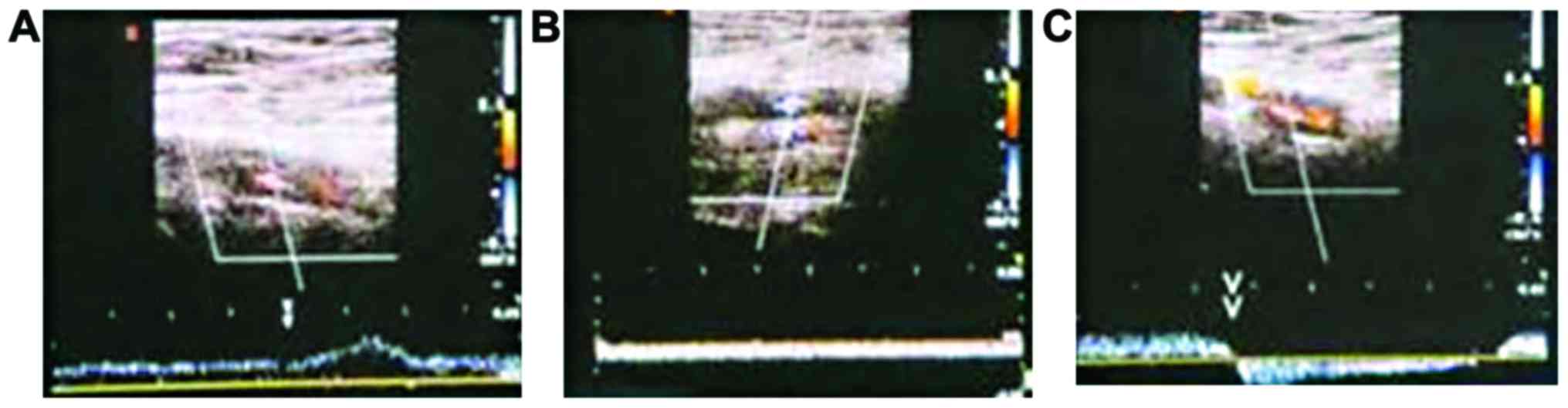
Thrombus recovery
Ten days after DVT modeling, the effective rate of
treating lower extremity deep venous thrombosis with the high doses
of folic acid and vitamin B12 was 100%, whereas the effective rate
of the low-dose treatment was 75% (Table
V). The control group showed no benefits and had an effective
rate of 0% (Table V). Ten days after
DVT modeling, the femoral venous wall echo was enhanced in the
control group, with uneven intima echo incrassation and a little
blood flow signal in the femoral vein (Fig. 6A). The femoral venous wall of the
low-dose group thickened, with full solid and medium echo, blood
flow signal in lumen around, dark color suggesting that flow rate
was low (Fig. 6B). The femoral
venous wall of the high-dose group thickened with strong echo in
lumen, colorful blood flow presenting irregular fullness (Fig. 6C).
 | Table V.Thrombus recovery. |
Table V.
Thrombus recovery.
| Groups | No. | Excellent | Effective | Non-effective | Effective rate of
treatment (%) |
|---|
| Control | 20 | 0 | 0 | 20 | 0 |
| Low-dose | 20 | 13 | 2 | 5 | 75a |
| High-dose | 20 | 12 | 8 | 0 | 100a,b |
Discussion
Thrombus is caused by blood solidification and
abnormal viscosity due to obstacles affecting its fluidity; this
results in the formation of thrombocytes and activation of blood
coagulation factor (8–10). DVT is one of the results where
abnormal hemorheology and viscosity induce ischemia and anoxia of
organs (11–13). Therefore, correcting blood viscosity
and improving blood coagulation function indexes have an extremely
important role in the prevention and treatment of DVT.
Our study showed that for 3 days and 10 days after
DVT modeling, hemorheology and coagulation indexes were the best in
the high-dose folic acid and vitamin B12 group. Some reports have
proposed that Hcy is a thrombus-forming agent, and high Hcy could
induce deep venous thrombosis (14–17). In
addition, plasma D-dimer has been proposed to have a forewarning
function for thrombus and hypercoagulable state (18,19). Ten
days after DVT modeling, we found that the levels of Hcy and
D-dimer improved the most in the high-dose group.
Through histopathologic examination, we found that
10 days after DVT modeling the high-dose and low-dose groups showed
significant improvement in the change of lower extremity deep
venous thrombosis. Deep venous thrombosis of lower extremity with
color Doppler ultrasound diagnosis has some advantages, such as
simple operation, safety and high efficiency, low cost,
repeatability and direct observation, and the detection of related
lesions and bleeding features around blood vessels (20). Our results also showed that after DVT
modeling, the effective rate of lower extremity deep venous
thrombosis treatment of high-dose group was 100%. The results of
color Doppler ultrasound diagnosis of DVT corresponded to our
findings in histopathologic examination. This validated the
feasibility and high efficiency of color Doppler ultrasound
diagnosis for deep venous thrombosis of the lower extremity.
In summary, folic acid and vitamin B12 have clear
therapeutic effects in a rabbit model of DVT with
hyperhomocysteinemia. The high-doses we tried improved various
relevant symptoms, including the levels of Hcy and D-dimer,
hemorheology, coagulation function, and vein pathology. Overall,
these studies support the use of high doses of folic acid and
vitamin B12 to treat DVT.
References
|
1
|
Mouravas H, Verettas D, Kazakos K, Xarhas
K, Panagiotou N and Ellinas P: Homocysteine and its relationship to
deep venous thrombosis in patients undergoing total knee or hip
arthroplasty. Hippokratia. 14:185–188. 2010.PubMed/NCBI
|
|
2
|
Hosseini S, Kalantar E, Hosseini MS,
Tabibian S, Shamsizadeh M and Dorgalaleh A: Genetic risk factors in
patients with deep venous thrombosis, a retrospective case control
study on Iranian population. Thromb J. 13:352015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hosseini S, Kalantar E, Hosseini MS,
Tabibian S, Shamsizadeh M and Dorgalaleh A: Genetic risk factors in
patients with deep venous thrombosis, a retrospective case control
study on Iranian population. Thromb J. 13:352015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinelli I, Cattaneo M, Panzeri D,
Taioli E and Mannucci PM: Risk factors for deep venous thrombosis
of the upper extremities. Ann Intern Med. 126:707–711. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roy S, Sable P, Khaire A, Randhir K, Kale
A and Joshi S: Effect of maternal micronutrients (folic acid and
vitamin B12) and omega 3 fatty acids on indices of brain oxidative
stress in the offspring. Brain Dev. 36:219–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shu XJ, Li ZF, Chang YW, Liu SY and Wang
WH: Effects of folic acid combined with vitamin B12 on DVT in
patients with homocysteine cerebral infarction. Eur Rev Med
Pharmacol Sci. 21:2538–2544. 2017.PubMed/NCBI
|
|
7
|
Ravari H, Zafarghandi MR, Alvandfar D and
Saadat S: Serum homocysteine in deep venous thrombosis, peripheral
atherosclerosis and healthy Iranians: A case-control study. Pak J
Biol Sci. 12:1019–1024. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lungren MP, Ward TJ, Patel MN, Racadio JM
and Kukreja K: Endovascular thrombolysis to salvage central venous
access in children with catheter-associated upper extremity deep
vein thrombosis: Technique and initial results. J Thromb
Thrombolysis. 40:274–279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prandoni P, Tormene D, Dalla Valle F,
Concolato A and Pesavento R: D-dimer as an adjunct to compression
ultrasonography in patients with suspected recurrent deep vein
thrombosis. J Thromb Haemost. 5:1076–1077. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodrigues CA, Morelli VM, DA Silveira RC,
D'Almeida V and Lourenço DM: Homocysteine reduction by B-vitamin
supplementation increases t-PA and PAI-1 levels in patients with
venous thromboembolism. J Thromb Haemost. 5:195–198. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duplessis M, Girard CL, Santschi DE and
Pellerin D: An economic model evaluating the supplementation of
folic acid and vitamin B12 given around parturition and
in early lactation on dairy farms in Québec, Canada. Can J Anim
Sci. 94:737–747. 2014. View Article : Google Scholar
|
|
12
|
Malý R, Masopust J, Hosák L and
Konupcíková K: Assessment of risk of venous thromboembolism and its
possible prevention in psychiatric patients. Psychiatry Clin
Neurosci. 62:3–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Negrão L and Nunes P; Portuguese Group for
the Study of Peripheral Neuropathy, : Uridine monophosphate, folic
acid and vitamin B12 in patients with symptomatic peripheral
entrapment neuropathies. Pain Manag. 6:25–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cebeci F, Onsun N, Ulusal HA and Inan B:
The relationship between deep vein thrombosis and erythema nodosum
in male patients with Behçet's disease. Eur Rev Med Pharmacol Sci.
18:3145–3148. 2014.PubMed/NCBI
|
|
15
|
Negrão L, Almeida P, Alcino S, Duro H,
Libório T, Melo Silva U, Figueira R, Gonçalves S and Neto Parra L:
Effect of the combination of uridine nucleotides, folic acid and
vitamin B12 on the clinical expression of peripheral neuropathies.
Pain Manag. 4:191–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Criado PR, Alavi A and Kirsner RS:
Elevated levels of coagulation factor VIII in patients with venous
leg ulcers. Int J Low Extrem Wounds. 13:130–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Falcone M and Serra P: Massive pulmonary
embolism in a woman with leiomyomatous uterus causing pelvic deep
venous thrombosis. Ann Ital Med Int. 20:104–107. 2005.PubMed/NCBI
|
|
18
|
Shammas NW, Shammas G, Bryan D, Rauba J,
Dippel E and Jerin M: Predictors of target lesion revascularization
in patients undergoing lower extremity percutaneous interventions.
J Invasive Cardiol. 21:266–269. 2009.PubMed/NCBI
|
|
19
|
Sagban TA, Scharf RE, Wagenhäuser MU,
Oberhuber A, Schelzig H, Grabitz K and Duran M: Elevated risk of
thrombophilia in agenesis of the vena cava as a factor for deep
vein thrombosis. Orphanet J Rare Dis. 10:32015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Al-Hameed F, Al-Dorzi HM, Shamy A, Qadi A,
Bakhsh E, Aboelnazar E, Abdelaal M, Al Khuwaitir T, Al-Moamary MS,
Al-Hajjaj MS, et al: The Saudi clinical practice guideline for the
diagnosis of the first deep venous thrombosis of the lower
extremity. Ann Thorac Med. 10:3–15. 2015.PubMed/NCBI
|