Introduction
Mesenchymal stem cells (MSCs), derived from the
mesoderm, have a strong self-replicative ability and
multi-differentiation potential. MSCs are used in tissue
engineering, cytokines alternative therapy, gene therapy, and other
aspects of organ transplantation (1), and as biomaterials in the repair of
bone, cartilage, tendons, and glia (2). MSCs differentiate into osteoblasts and
then into bone cells, accounting for approximately 95% of bone.
Bone cells can act as mechanical stress receptors, and regulate the
activities and functions of both osteoblasts and osteoclasts in the
form of endocrine cells (3).
A previous study has shown that mechanical stress
stimulation promotes osteogenesis (4). Mechanical stress stimuli can cause cell
morphology changes, cell projection increases, formation of
intercellular gap junctions (GJs), and cytoskeletal structural
changes (5). GJs are composed of two
adjacent hemichannels which anchor to each other, forming a
hydrophilic channel (6) for
intercellular communication via small molecules, such as inorganic
ions (calcium and potassium), amino acids and glucose (7,8). GJs are
dynamic structures, and a variety of factors are involved in
regulating their opening and closing, such as intracellular pH,
calcium ion (Ca2+) concentration, and membrane potential
(9). GJs have functions in cell
metabolism and differentiation, transmission of nerve impulses and
conduction of information, coordination of consistency between
cellular activity, material transport, and electrical excitation
conduction (10).
There are three GJ protein families: i) innexin,
mainly expressed in invertebrates; ii) connexin, mainly expressed
in vertebrates; and iii) a class of genes in vertebrates which are
similar to invertebrate innexin, and named pannexin (Px) by
Panchina et al (11). There
are three Px subtypes: Px1, Px2, and Px3. Px is widely expressed in
human tissues and organs, such as osteoblasts, chondroblasts,
spleen cells, skin tissues, the kidney, and the central nervous
system (12). Px forms hemichannels
in the cell membrane, which can be induced to open by hypoxia, low
permeability, mechanical stress, cell depolarization, and
Ca2+ concentration increases to mediate ATP release and
intercellular Ca2+ wave transmission, regulating blood
flow or the immune response (13,14). Px1
is a newly discovered mechanical stimuli-sensitive channel protein;
however, whether Px1 participates in MSC osteogenic differentiation
and signal transduction after mechanical stimulation has been
rarely reported. This study aimed to explore the relationship
between Px1 channels and MSC differentiation under mechanical
stress stimulation, and examine the specific molecular
mechanism.
Materials and methods
Isolation and culture of rat MSCs
The Ethics Committee and Animal Management Committee
of The First Affiliated Hospital of Dalian Medical University
(LCKY20I3-18) approved all experiments. MSCs were isolated from the
femurs and tibias of Sprague Dawley (SD) rats (SPF grade, 3 weeks
old, weighing 100–120 g). Briefly, rats were euthanized by ether
anesthesia with 1.5% pentobarbital sodium (375 mg/kg) followed by
cervical dislocation. Muscle tissue and cartilage were removed, and
bone marrow suspensions were obtained from marrow cavities using an
injector. Subsequently, the bone marrow suspensions were dispersed
and centrifuged at 200 × g for 5 min. After removing the
supernatant, the pellet was suspended in Dulbecco's modified
Eagle's medium (DMEM; HyClone; GE Healthcare Life Sciences, Logan,
UT, USA) with 100 U/ml penicillin and 100 U/ml streptomycin
(HyClone; GE Healthcare Life Sciences) in a 10-cm dish, and
cultured for 48 h. Cell passaging was performed and the third
generation was used in the following experiments.
Experimental protocols
First, a safe concentration of CBX (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was determined using the CCK8
method according to the instructions provided with the CCK-8 cell
proliferation and cytotoxicity assay kit (cat. no. CA1210; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China). This
concentration (100 µM) was used for subsequent experiments. MSCs
were divided into 6 groups: A control group that was neither
stressed nor treated with CBX; a stress group, which was only
treated with stress; and four groups that were stressed after CBX
treatment for 3, 6, 12, and 24 h. Stress loading was performed
using a four-point bending device, as previously described
(15). The stress loading strength
was 4,000 µ strain with a frequency of 0.5 Hz for 15 min. The
treated MSCs were cultured in DMEM with 10% fetal bovine serum
(Gibco, Carlsbad, CA, USA) in a 37°C incubator (SANYO, Osaka,
Japan).
Detection of alkaline phosphatase
(ALP) activity and intracellular ATP content
MSCs were sonicated with an ultrasonic cell
disruptor (SENXIN, Shanghai, China) and centrifuged at 13,800 × g
for 10 min. The optical density (OD) of the cells was read with a
UV spectrophotometer (USA Thermo Electron Corporation, San Jose,
CA, USA) at 520 nm. ALP activity was calculated using an Alkaline
Phosphatase Assay kit (cat. no. P0321; Beyotime Institute of
Biotechnology, Jiangsu, China), according to the manufacturer's
instructions. Intracellular ATP content was determined utilizing an
ATP bioluminescence kit (cat. no. GN202-01; YPH-Bio, Beijing,
China), according to the manufacturer's instructions.
Immunofluorescence observation of type
I collagen expression
The expression of type I collagen in MSCs was
observed by immunofluorescence. MSCs were fixed in 4%
paraformaldehyde at 25°C for 15 min, and then 0.1% Triton X-100 was
added for 15 min. After blocking with 5% bovine serum albumin
(Beijing Solarbio Science & Technology Co., Ltd.) for 1 h, MSCs
were incubated with a rabbit-anti-mouse polyclonal type I collagen
antibody (1:500; cat. no. AB765P; Merck KGaA, Darmstadt, Germany)
for 2 h and then incubated with DyLight 488 AffiniPure
goat-anti-rabbit IgG (H+L) (1:500; EarthOx, San Francisco, CA, USA)
for 1 h. After washing thrice with phosphate-buffered saline (PBS),
the MSCs were mounted with the anti-quenching sealing agent
Fluoromount-G (Southern Biotech, Birmingham, AL, USA) and observed
under a fluorescence microscope (Nikon, Tokyo, Japan).
Flu-3AM Ca2+ fluorescence
probe to detect the intracellular Ca2+
concentration
Intracellular Ca2+ concentrations were
detected using the Flu-3AM Ca2+ fluorescence probe.
Briefly, the treated cells were incubated with 3 µM Flu-3AM reagent
(Beyotime Institute of Biotechnology, Jiangsu, China, cat. no.
S1056) for 15–60 min at 37°C. After washing thrice with PBS, the
cells were reincubated once to ensure the complete conversion of
Flu-3AM to Flu-3, and observed using a fluorescence microscope
(Nikon, Tokyo, Japan).
Western blot analysis
Total proteins were extracted in cell lysis buffer
[1% Triton X-100, 100 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA,
1 mM EGTA, 0.2 mM Na3VO4, 10 mM NaF, Protease
Inhibitor (Sigma-Aldrich; Merck KGaA), 0.5% NP-40]. Following
centrifugation at 13,800 × g at 4°C for 10 min, the supernatant was
collected. The protein concentration was estimated by Bradford
assay utilizing a Bio-Rad Protein Assay Kit II (cat. no. 5000002;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). Further, 50 µg of
total protein was separated on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Subsequently, the proteins were transferred onto polyvinylidene
difluoride membranes and blocked with 5% skim milk for 1 h at 25°C.
Rabbit Px1 polyclonal antibody (1:1,000; cat. no. ab124131; Abcam,
Cambridge, MA, USA), rabbit p38 phosphorylation polyclonal antibody
(1:1,000; cat. no. ab47363; Abcam), rabbit ERK phosphorylation
polyclonal antibody (1:1,000; cat. no. ab214362; Abcam), rabbit
anti-mouse polyclonal type I collagen antibody (1:1,000), and
β-actin mouse monoclonal antibody (1:5,000; cat. no. AF0003;
Beyotime Institute of Biotechnology) were used as primary
antibodies, and were incubated overnight with the membranes at 4°C.
Subsequently, membranes were incubated with goat anti-rabbit IgG
(1:5,000; cat. no. A0208; Beyotime Institute of Biotechnology) or
goat anti-rat horseradish peroxidase-conjugated secondary
antibodies (1:5,000; cat. no. A0192; Beyotime Institute of
Biotechnology) for 2 h at 25°C. Finally, proteins were detected
using the ECL Plus Western Blotting Detection System (GE
Healthcare, Little Chalfont, UK) and visualized using the Bio-Rad
gel imaging system (Bio-Rad Laboratories, Inc.). Bands were
analyzed using Labwork 4.6 software (UVP Products, Upland, CA,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
(SD). Differences within groups were analyzed utilizing a one-way
repeated measures analysis of variance. P<0.05 was considered to
indicate a statistically significant difference. SPSS 16.0 software
(SPSS, Inc., Chicago, IL, USA) was used for all statistical
analysis.
Results
MSCs morphology
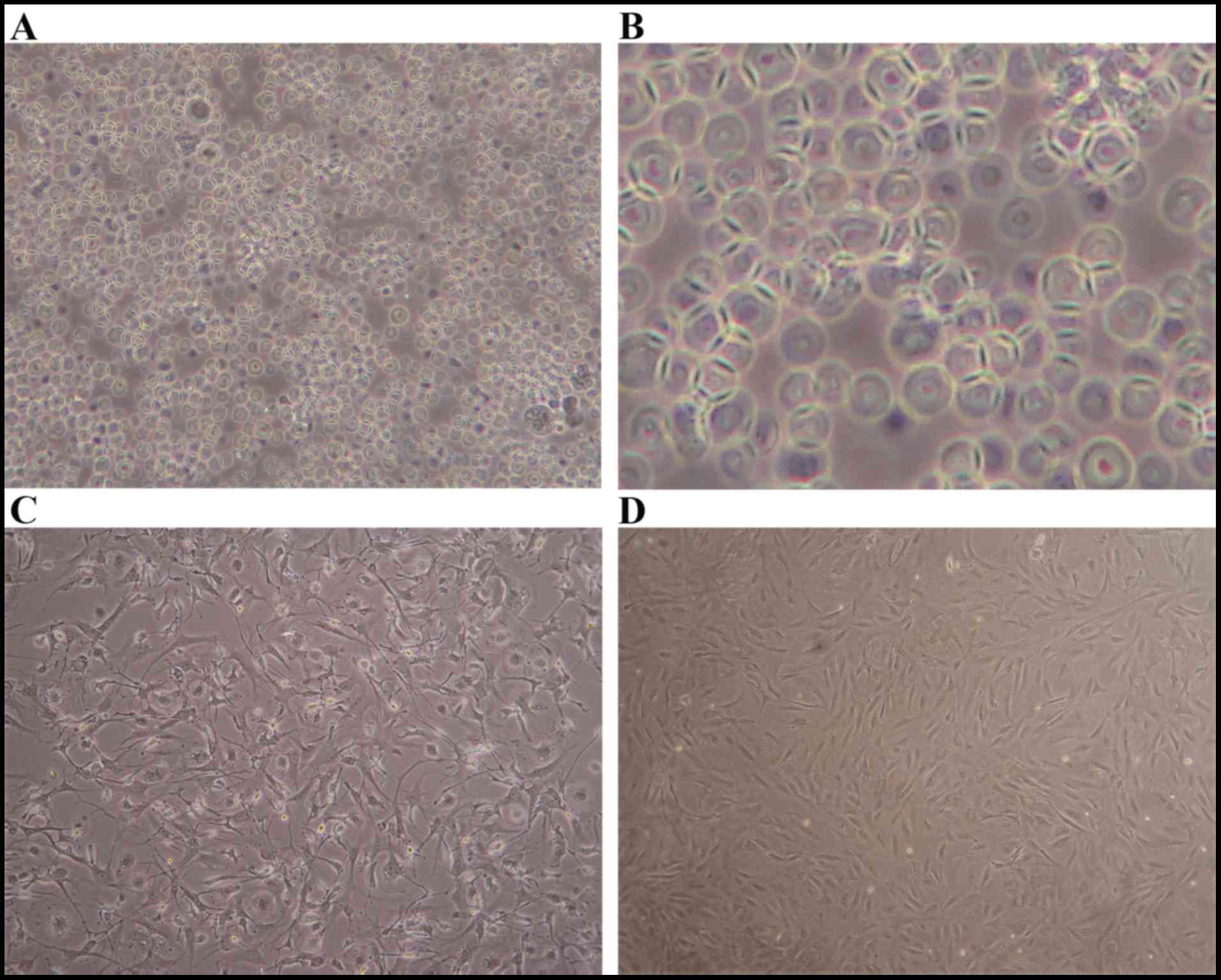
Fig. 1 shows the
morphology of the MSCs extracted and cultured in whole bone marrow
culture. Primary MSCs were initially rounded (Fig. 1A) and then gradually attached and
became fusiform (Fig. 1B and C).
After the cells were passaged thrice and the confluence reached 80%
(Fig. 1D), they were used in
experiments.
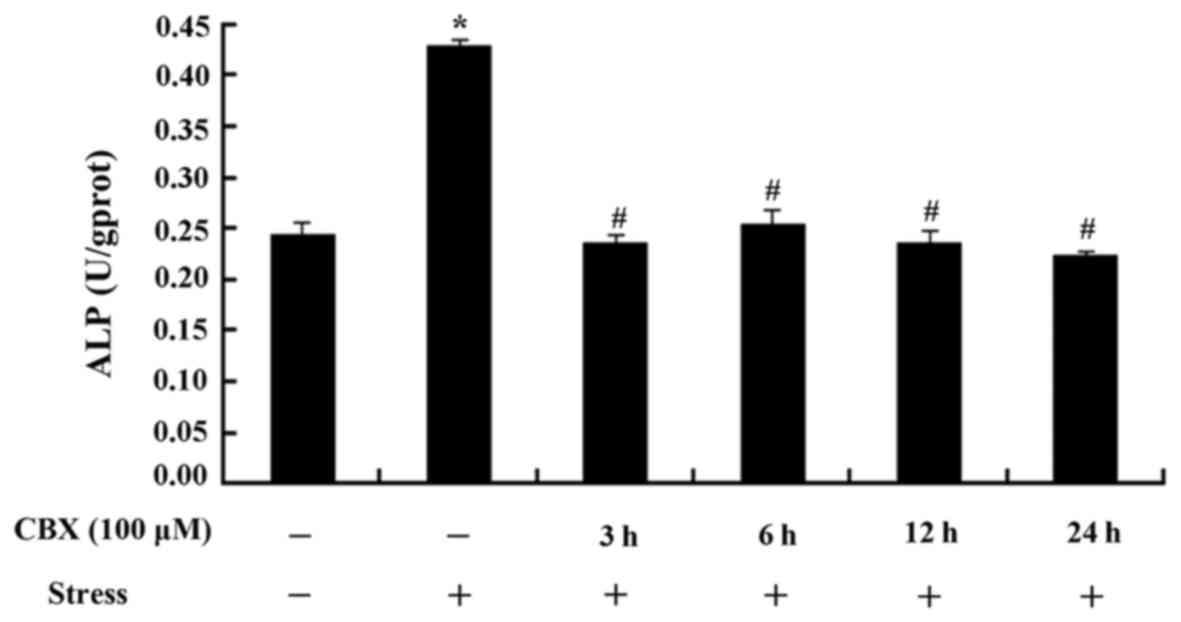
MSCs ALP activity after treatment with
CBX
The stress group showed a significantly higher level
of ALP than the control group (P<0.05, Fig. 2). Cells pretreated with CBX showed
significantly lower levels of ALP than the stress group (P<0.05,
Fig. 2); however, no time-dependent
trends were observed.
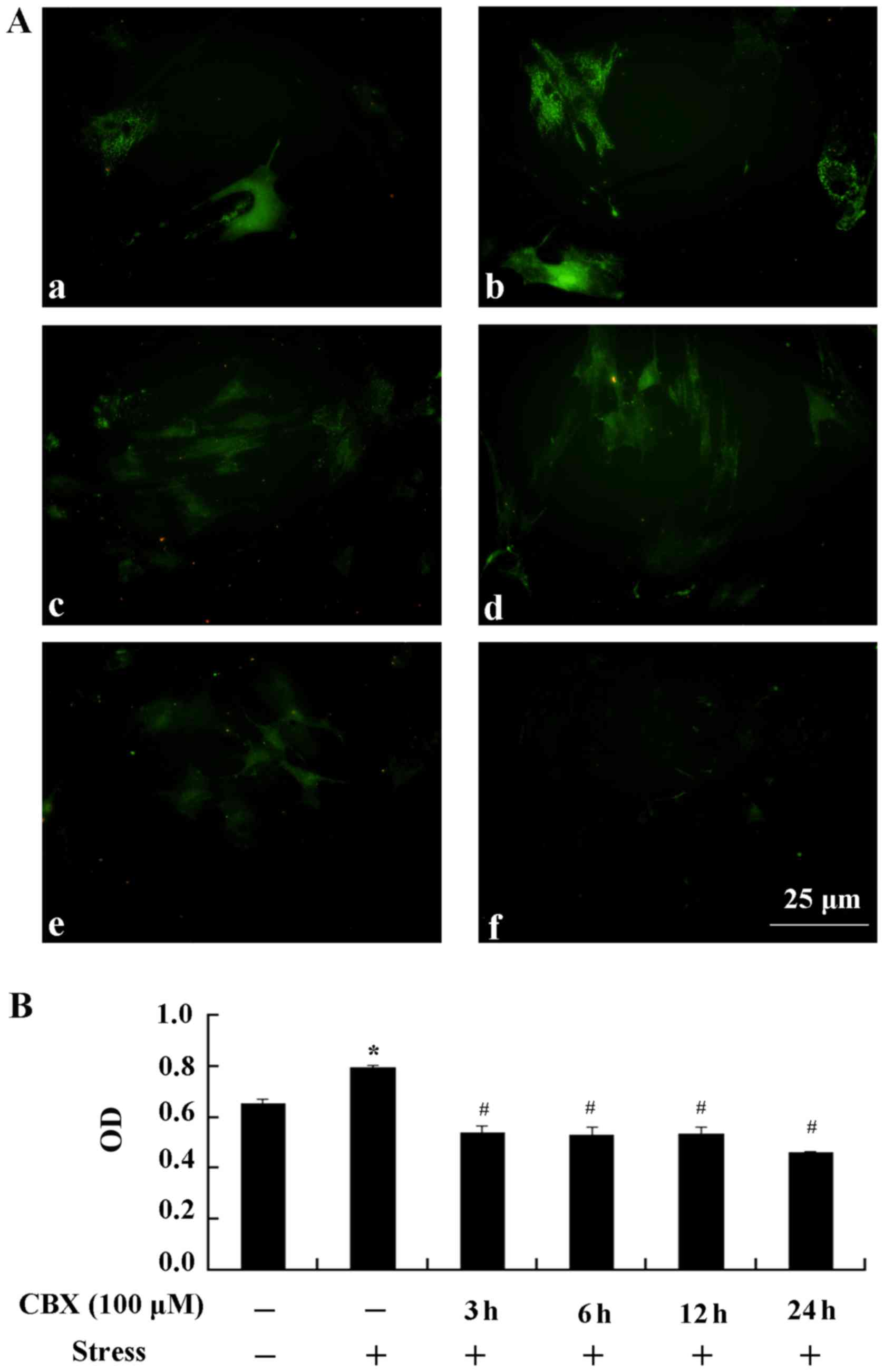
Expression of type I collagen
Stress stimulation significantly promoted the
expression of type I collagen (P<0.05, Fig. 3). When pretreated with 100 µM CBX,
type I collagen expression was significantly lower than in the
stress group (P<0.05). These results suggest that CBX inhibits
the expression of type I collagen.
Detection of intracellular
Ca2+
Intracellular Ca2+ fluorescence intensity
significantly increased in the stress group compared with the
control group (P<0.05, Fig. 4),
while the fluorescence intensity dramatically decreased with CBX
treatment compared with the stress group (P<0.05, Fig. 4). Of these, the group treated with
100 µM CBX for 24 h showed the lowest Ca2+ level. These
results suggest that intracellular Ca2+ exchange is
blocked by CBX.
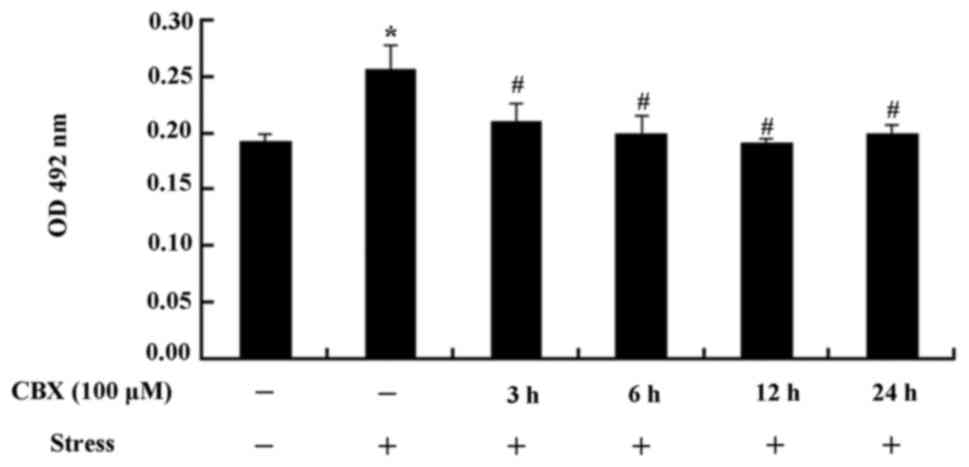
ATP release from MSCs
The release of ATP significantly increased in the
stress group compared with the control group (P<0.01, Fig. 5). However, after 6–24 h treatment
with CBX, ATP release was significantly lower than in the stress
group (P<0.05, Fig. 5). These
results demonstrate that ATP release can be reduced by CBX.
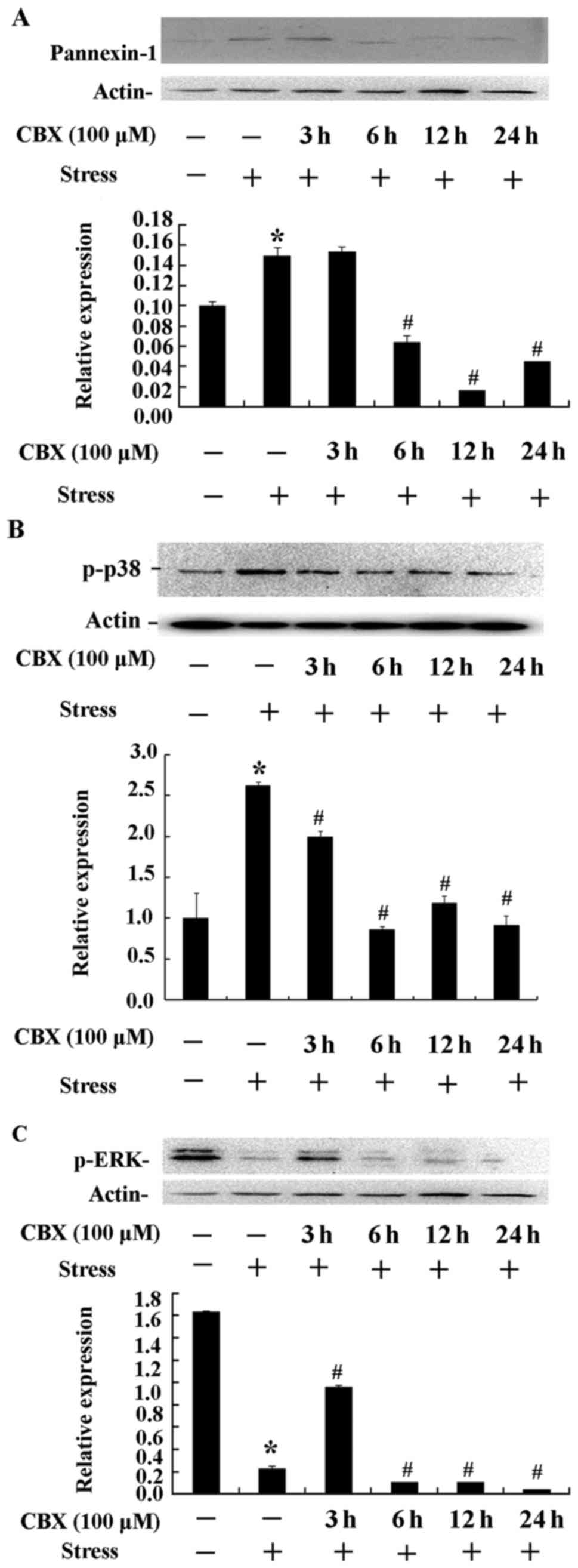
Px1 expression and p38 and ERK
phosphorylation
As shown in Fig. 6,
the expression of Px1 and the phosphorylation of p38 and ERK
protein kinases were estimated by western blot analysis. There was
a dramatically increased expression of Px1 in the stress group
(P<0.01, Fig. 6A). However,
stress combined with 6–24 h pretreatment with CBX showed
significantly lower Px1 expression than with stress alone
(P<0.05, Fig. 6A). These results
further confirm that Px1 expression is inhibited by CBX. The stress
and stress combined with 3 h CBX treatment groups showed higher p38
phosphorylation (P<0.01, Fig.
6B), while longer CBX treatment (6–24 h) resulted in
significantly lower p38 phosphorylation (P<0.05, Fig. 6B). These results indicate that CBX
inhibits the phosphorylation of p38. ERK phosphorylation was
significantly reduced in the stress group compared with the control
group (P<0.01, Fig. 6C). Stress
combined with 3 h CBX treatment resulted in dramatically higher ERK
phosphorylation compared with the stress group (P<0.01, Fig. 6C), but significantly reduced
phosphorylation with longer CBX treatment (6–24 h; P<0.05,
Fig. 6C). This suggests that there
is no direct link between Px1 and ERK signaling.
Discussion
This study indicates that Px1 closely participates
in the process of osteogenic differentiation under mechanical
stress. Mechanical stress stimulation increased ALP activity, type
I collagen expression, and ATP and Ca2+ release,
promoting osteogenic differentiation, and these effects were
prevented by the addition of the Px1 channel inhibitor CBX. In
addition, a positive relationship between Px1 and p38 MAPK
signaling was noted.
ALP, also known as mineralization-associated
protein, is critical for mineralization during bone formation
(16). Akbari and colleagues
demonstrated that ALP can serve as a sign of osteogenic
differentiation (17).
Ca2+ deposition is regarded as another marker of
osteogenic differentiation (18). A
rapid increase in intracellular osteoblast Ca2+
concentration occurs in response to external forces (19). Osteoblast cytoskeletal reorganization
and gene expression changes are associated with increased
stimulation, and are mainly dependent on internal inositol
triphosphate-mediated release (20).
This increase in Ca2+ results from intracellular
Ca2+ release and extracellular Ca2+ flow. In
this study, under the stimulation of mechanical stress, ALP
activity and cellular Ca2+ concentration were
significantly increased in MSCs, indicating the pro-osteogenic
effects of mechanical stress. When the Px1 channel was blocked by
CBX, increases in ALP activity and cellular Ca2+
concentration were significantly prevented, which might be related
to a block in Ca2+ exchange.
ATP is an important energy molecule in cells under
physiological conditions, and an important cell-cell signaling
molecule. Under conditions of hypoxia, low permeability, cell
deformation, and other stimuli such as depolarization, ATP release
increases. There are two explanations for this: ATP, like other
neurotransmitters, or in conjunction with other neurotransmitters,
could be released via vesicles; ATP release could also be mediated
through some kind of a channel. Lu et al suggested that
connexin-containing hemichannels are involved in ATP release
(21). The Px1 channel has been
reported to mediate ATP release from taste cells, airway epithelial
cells, red blood cells, and other cell types (22–24).
Under physiological conditions, certain stimuli, such as mechanical
stress and hypotonic solutions, can induce normal cells to release
ATP (25). Previous study indicated
that Px hemichannels may mediate the release of both ATP and
Ca2+ waves (26).
Recent studies have reported the presence of
functional GJs between osteoblasts (27,28). The
process of phosphorylation and dephosphorylation of GJ proteins
acts as a switch for GJ function. In vitro cultured bone
cells under stress display increased phosphorylation levels,
indicating increased connections between osteoblasts. In addition,
GJs participate in the regulation of osteoblast function and
signaling. Px hemichannels can be induced to open by hypoxia, low
permeability, mechanical stress, cell depolarization, increased
intracellular Ca2+ concentration, and other factors
(26).
MAPK signaling pathways are important components of
intracellular signal transduction and are involved in a variety of
cellular processes, and p38 MAPK in particular plays an important
role in the process of differentiation into bone cells (29). The p38 MAPK signal transduction
pathway controls three stages in the early differentiation of bone
cells, including proliferation, extracellular matrix maturation,
and matrix mineralization. In the matrix maturation stage, ALP gene
expression levels peak as an early differentiation marker. After
the onset of osteoblast differentiation, type I collagen secretion
peaks, further promoting bone cell differentiation. The p38 MAPK
pathway transduces cellular stress signals to promote osteoblast
maturation, including the regulation of ALP expression during
osteoblast differentiation (29).
This study found that mechanical stress could increase the
phosphorylation of p38, but this was decreased after pretreatment
with a Px1 inhibitor. Thus we speculate that p38 signaling is
important for MSC osteoblast differentiation. In contrast, no
changes in ERK phosphorylation were observed after Px1 inhibition,
indicating that there was no direct relationship between Px1
channels and ERK signaling.
Altogether, our results indicate that Px1 channels
might play a crucial role in the process of osteogenic
differentiation. Notably, this may involve signal transduction
between Px1 channels and the p38 MAPK phosphorylation.
Acknowledgements
We thank the Central Laboratory at Dalian Medical
University for experimental support. This study was funded by the
Shanghai Pudong New Area Science and Technology Development Fund
(grant no. PKJ2013-Y40) and the National Natural Science Foundation
of China (grant no. 30973071).
References
|
1
|
Awad HA, Butler DL, Boivin GP, Smith FN,
Malaviya P, Huibregtse B and Caplan AI: Autologous mesenchymal stem
cell-mediated repair of tendon. Tissue Eng. 5:267–277. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kopen GC, Prockop DJ and Phinney DG:
Marrow stromal cells migrate throughout forebrain and cerebellum,
and they differentiate into astrocytes after injection into
neonatal mouse brains. Proc Natl Acad Sci USA. 96:pp. 10711–10716.
1999; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang W, Ma A, Wang T, Han K, Liu Y, Zhang
Y, Zhao X, Dong A, Du Y, Huang X, et al: Intravenous
transplantation of mesenchymal stem cells improves cardiac
performance after acute myocardial ischemia in female rats. Transpl
Int. 19:570–580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Triplett JW, O'Riley R, Tekulve K, Norvell
SM and Pavalko FM: Mechanical loading by fluid shear stress
enhances IGF-1 receptor signaling in osteoblasts in A
PKCzeta-dependent manner. Mol Cell Biomech. 4:13–25.
2007.PubMed/NCBI
|
|
5
|
Klein-Nulend J, van der Plas A, Semeins
CM, Ajubi NE, Frangos JA, Nijweide PJ and Burger EH: Sensitivity of
osteocytes to biomechanical stress in vitro. FASEB J. 9:441–445.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trexler EB, Bennett M, Bargiello TA and
Verselis VK: Voltage gating and permeation in a gap junction
hemichannel. Proc Natl Acad Sci USA. 93:pp. 5836–5841. 1996;
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iyyathurai J, Himpens B, Bultynck G and
D'hondt C: Calcium wave propagation triggered by local mechanical
stimulation as a method for studying gap junctions and
hemichannels. Methods Mol Biol. 1437:203–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Ma M, Locovei S, Keane RW and Dahl
G: Modulation of membrane channel currents by gap junction protein
mimetic peptides: Size matters. Am J Physiol Cell Physiol.
293:C1112–C1119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Escalona Y, Garate JA, Araya-Secchi R,
Huynh T, Zhou R and Perez-Acle T: Exploring the membrane potential
of simple dual-membrane systems as models for gap-junction
channels. Biophys J. 110:2678–2688. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meda P and Spray DC: Gap junction
function. Adv Mol Cell Biology. 30:263–322. 2000. View Article : Google Scholar
|
|
11
|
Panchina Y, Kelmanson I, Matz M, Lukyanov
K, Usman N and Lukyanov S: A ubiquitous family of putative gap
junction molecules. Curr Biol. 10:R473–474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Penuela S, Celetti SJ, Bhalla R, Shao Q
and Laird DW: Diverse subcellular distribution profiles of pannexin
1 and pannexin 3. Cell Commun Adhes. 15:133–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Penuela S, Bhalla R, Gong XQ, Cowan KN,
Celetti SJ, Cowan BJ, Bai D, Shao Q and Laird DW: Pannexin 1 and
pannexin 3 are glycoproteins that exhibit many distinct
characteristics from the connexin family of gap junction proteins.
J Cell Sci. 120:3772–3783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Romanov RA, Bystrova MF, Rogachevskaya OA
and Kolesnikov SS: Channel activity of recombinant pannexin 1.
Biochem Suppl. 5:343–349. 2011.
|
|
15
|
Owan I, Burr DB, Turner CH, Qiu J, Tu Y,
Onyia JE and Duncan RL: Mechanotransduction in bone: Osteoblasts
are more responsive to fluid forces than mechanical strain. Am J
Physiol. 273:C810–C815. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Genge BR, Sauer GR, Wu LN, McLean FM and
Wuthier RE: Correlation between loss of alkaline phosphatase
activity and accumulation of calcium during matrix vesicle-mediated
mineralization. J Biol Chem. 263:18513–18519. 1989.
|
|
17
|
Akbari M, Nikbakht M and Sobhani A:
Expression of alkaline phosphatase during osteogenic
differentiation of rat bone marrow stromal cells. Cell J.
43:94–106. 2001.
|
|
18
|
Rodríguez JP, González M, Ríos S and
Cambiazo V: Cytoskeletal organization of human mesenchymal stem
cells (MSC) changes during their osteogenic differentiation. J Cell
Biochem. 93:721–731. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ryder KD and Duncan RL: Parathyroid
hormone enhances fluid shear-induced [Ca2+]i signaling in
osteoblastic cells through activation of mechanosensitive and
voltage-sensitive Ca2+ channels. J Bone Miner Res. 16:240–248.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng JJ, Kronbergs A, Fomin VP, Usatyuk
PV, Natarajan V and Duncan RL: Protein Kinase C (PKC)-mediated
actin disruption regulates [Ca2+]i responses to mechanical load in
osteoblasts. FASEB J. 21:A9132007.
|
|
21
|
Lu L, Tu J and Ballard HJ:
Connexin-hemichannels are involved in acidosis-induced ATP release
from skeletal myocytes. Proceedings of the 17th Annual Scientific
Meeting of the Institute of Cardiovascular Science and Medicine
(ICSM 2013), Hong Kong, China. Journal of the Hong Kong College of
Cardiology. pp. 672013;
|
|
22
|
Huang YJ, Maruyama Y, Dvoryanchikov G,
Pereira E, Chaudhari N and Roper SD: The role of pannexin 1
hemichannels in ATP release and cell-cell communication in mouse
taste buds. Proc Natl Acad Sci USA. 104:pp. 6436–6441. 2007;
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ransford GA, Fregien NF, Qiu F, Dahl G,
Conner GE and Salathe M: Pannexin 1 contributes to ATP release in
airway epithelia. Am J Respir Cell Mol Biol. 41:525–534. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krick S, Wang J, St-Pierre M, Gonzalez C,
Dahl G and Salathe M: Dual oxidase 2 (Duox2) regulates pannexin
1-mediated ATP release in primary human airway epithelial cells via
changes in intracellular pH and not H2O2 production. J Biol Chem.
291:6423–6432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orellana JA, Froger N, Ezan P, Jiang JX,
Bennett MV, Naus CC, Giaume C and Sáez JC: ATP and glutamate
released via astroglial connexin 43 hemichannels mediate neuronal
death through activation of pannexin 1 hemichannels. J Neurochem.
118:826–840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ishikawa M, Iwamoto T, Nakamura T, Doyle
A, Fukumoto S and Yamada Y: Pannexin 3 functions as an ER Ca(2+)
channel, hemichannel, and gap junction to promote osteoblast
differentiation. J Cell Biol. 193:1257–1274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saunders MM, You J, Zhou Z, Li Z,
Yellowley CE, Kunze EL, Jacobs CR and Donahue HJ: Fluid
flow-induced prostaglandin E2 response of osteoblastic ROS 17/2.8
cells is gap junction-mediated and independent of cytosolic
calcium. Bone. 32:350–356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jørgensen NR, Teilmann SC, Henriksen Z,
Meier E, Hansen SS, Jensen JE, Sørensen OH and Petersen JS: The
antiarrhythmic peptide analog rotigaptide (ZP123) stimulates gap
junction intercellular communication in human osteoblasts and
prevents decrease in femoral trabecular bone strength in
ovariectomized rats. Endocrinology. 146:4745–4754. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jaiswal RK, Jaiswal N, Bruder SP,
Mbalaviele G, Marshak DR and Pittenger MF: Adult human mesenchymal
stem cell differentiation to the osteogenic or adipogenic lineage
is regulated by mitogen-activated protein kinase. J Biol Chem.
275:9645–9652. 2000. View Article : Google Scholar : PubMed/NCBI
|