Introduction
Anti-tumour chemotherapy using 5-fluorouracil (5-FU)
and other cytostatics is often complicated by various disorders.
Cellular damage caused by 5-FU is related to its intracellular
transformation into 5-FU deoxynucleotide, which incorporates into
RNA and interferes with RNA processing, and also inhibits
thymidylate synthase enzyme, thereby affecting DNA synthesis and
repair (1). 5-FU is effective
against cancer of the digestive system (esophageal, stomach,
intestinal, colon, pancreatic and liver cancer) and breast cancer
(2). The toxicity of 5-FU is
primarily associated with its effect on rapidly renewing tissues
(those with high cellular turnover); the major dose-limiting
factors are myelosuppression and damage to the gastrointestinal
mucosa (3). Generation of reactive
oxygen species (ROS) is the leading mechanism of the synergistic
effect of polychemotherapeutic schemes incorporating 5-FU, causing
disturbance of antioxidant enzymes (4), and is also one of the leading aspects
in the development of mucositis (5).
An important aim of adjunct treatment in oncology
patients is decreasing chemotherapy toxicity, since side effects
lead to increased intervals between chemotherapy cycles or require
dose reduction, and these changes have a negative effect on
treatment results (6). The options
for treating such disorders remain limited (7,8).
Ideally, medications used to correct toxic effects should not have
side effects themselves, nor should they increase or decrease
treatment effectiveness. Natural plant polyphenols are promising in
this regard; they protect healthy tissues, in particular decreasing
production of intracellular ROS, but do not diminish the effect of
chemotherapies (9). Notably, not
only low-molecular weight polyphenols exhibit antioxidant efficacy,
but polymeric polyphenolic compositions, such as lignin
derivatives, exert it too (10).
The present study examined the effect of BP-C3 on
the development of haematological and intestinal manifestations of
toxicity caused by the administration of high doses of 5-FU to
mice. BP-C3 is a product, which comprises lignin-derived
polyphenolic composition of benzenepolycarboxylic acids (BP-Cx-1)
with iron complex, selenium, ascorbic acid and retinol. BP-Cx-1,
produced through high-temperature alkaline hydrolysis of lignin
conducted in the presence of air oxygen with subsequent isolation
and purification (11), is used for
preparation of medicinal products. Antineoplastic
platinum-containing investigational medicinal product, BP-C1, is
used in clinical trials in patients with metastatic breast cancer
(12). Investigational medicinal
product, BP-C2, a composition of BP-Cx-1 with ammonium molybdate,
inhibits radiation-induced skin damage in mice (13) and improves quality of life of
patients with cancer treated with 5-FU-containing regimens
(14). BP-C3, used in the present
study, was previously demonstrated to exhibit geroprotective
activity (15).
Materials and methods
Animals
A total of 105 outbred 2-month-old male
Swiss-H-derived Rappolovo albino (SHR) mice were purchased from the
Rappolovo Animal Facility (Rappolovo, Russia). The weight of
animals at the commencement of the study was 31.0±2.2 g. Animals
were maintained in a 12-h light/dark cycle at 21±2°C with 20–50%
average humidity and with ad libitum access to laboratory
feed (Laborotorkorm Ltd., Moscow, Russia) and tap water. Study
protocols were approved by the Local Ethics Committee of the N.N.
Petrov National Medical Research Center of Oncology, previously
known as N.N. Petrov Research Institute of Oncology (protocol no. 1
dated 13/02/2014; Saint-Petersburg, Russia). At the end of the
study, the experimental animals were euthanized by trained
personnel using CO2 inhalation.
Preparations
BP-C3 is a composition developed jointly by the N.N.
Petrov Research Institute of Oncology and Nobel Ltd.
(Saint-Petersburg, Russia) (16).
BP-C3 is comprised of polyphenolic composition BP-Cx-1 (RD
Innovation ApS, Copenhagen, Denmark), iron complex, selenium,
ascorbic acid and retinol. BP-C3 composition was supplied by Nobel
Ltd., as a 0.5% aqueous solution. 5-FU Ebewe (Sandoz International
GmbH, Holzkirchen, Germany) concentrate for solution for infusions
(50 mg/ml; batch no. EW4102) was used.
Experimental design
A total of 105 male SHR mice were randomly
subdivided into five groups (n=21/group). The mice in group 1, the
control group, were given tap water by gavage as a placebo. The
mice in groups 2, 3 and 4 were given a single intravenous injection
of 5-FU (150 mg/kg), while the mice in groups 1 and 5 were injected
with 0.9% saline solution on day 0). In addition, the mice in
groups 3, 4, and 5 were gavaged with BP-C3 (80 mg/kg) daily except
for day 0 (a total of 18 times). BP-C3 was administered on the
preventative/therapeutic schedule in group 3, starting 7 days
before the administration of 5-FU (day-7) and continuing through to
day 11, whereas in group 4 BP-C3 was given on the therapeutic
schedule, starting on day 1 and continuing through to day 18 of the
experiment.
The 5-FU dose level selected for the study is known
to induce anemia in mice (17) and
corresponds to dose levels that patients with cancer are exposed
to; for example 500 mg/m2 in CAF regimen
[cyclophosphamide, doxorubicin hydrochloride (Adriamycin) and
5-FU], which corresponds to 165 mg/kg dose in mice. The selected
dose level was <200 mg/kg, which is the maximum tolerated single
dose used in tumour-bearing mice (18). Animals were observed once daily,
including weekends.
Blood count and bone marrow nucleated
cell (BMNC) assay
Blood counts were examined in samples collected on
day 0 before 5-FU administration and on days 4, 7, 11, 17 and 20.
Blood samples (20–40 µl) were taken from the tip of the tail and
collected in test tubes (MiniCollect®; Greiner Bio-One
International GmbH, Kremsmünster, Austria) containing
K3EDTA. Clinical blood analysis was performed on a
Mindray BC-2800Vet Hematology Analyzer (Shenzhen Mindray
Bio-Medical Electronics Co., Ltd, Shenzhen, China). On days 4, 11,
and 20, 5 mice from each group were euthanized, and the cellular
composition of the bone marrow was quantitatively analysed in a
bone marrow cell suspension prepared by mixing of 0.02 ml bone
marrow aspirate with 0.4 ml of a 3% acetic acid solution. BMNCs
were counted using a haemocytometer (19).
Pathomorphology studies
In mice euthanized on day 4, the morphology of
crypts in the small intestine was evaluated. At autopsy, the
jejunum was taken and fixed in 10% buffered formalin at room
temperature for 48 h. Following fixation, 10 rings of 4–5 mm in
height were dissected from each lymphoid-free intestinal sample.
After routine histological processing, histological sections 4-µm
thick were prepared and stained with Mayer's haematoxylin (8 min at
room temperature) with subsequent differentiation and bluing and
with eosin (30 sec at room temperature), dehydrated and mounted
with xylene-based mounting medium. The survival of intestinal
crypts was evaluated as described by Withers and Elkind (20), by counting the number of crypts (no
fewer than 10 cells with basophilic nuclei lying next to each
other) in at least six transverse cuts using a light microscope
(Nikon Eclipse Ni-U, Nikon Corporation, Tokyo, Japan) at a
magnification of ×400 with NIS-Elements Br software (version
4.30.00; Nikon Corporation).
To evaluate the organ-specific toxic effect of 5-FU,
the organ/body weight ratios of the spleen, thymus, heart, liver
and kidneys were determined in animals euthanized on days 4, 11 and
20. At the autopsy the organs were dissected free of adhering
tissue and weighed using Sartorius Research R 160 P (Sartorius AG,
Gottingen, Germany). Organ/body weight (mg) ratio was multiplied by
10 and the mean absolute organ: Body weight ratios were calculated
and compared.
Statistical analysis
Data were presented as the mean ± standard error of
the mean. SPSS (version 16.0; SPSS Inc., Chicago, IL, USA) was used
to evaluate the data. Results were assessed by a two-way analysis
of variance and Tukey's multiple comparisons test for 3–4 repeated
measurements. P<0.05 was considered to indicate a statistically
significant difference.
Results
Survival of animals exposed to
5-FU
Evaluating survival was not within the scope of the
study; however, there was some mortality observed among the mice,
indicating that 5-FU had a toxic effect (Table I). On day 7, 6 experimental animals
succumbed in group 2 (the group receiving 5-FU alone). The
mortality in group 2 was significantly greater than that observed
in the control group (29% in group 2 vs. 0% in the control group;
P<0.05; Table I). The maximum
weight loss of the 6 animals that succumbed in group 2 was 3–16%.
Due to this, on the same day the remaining mice were weighed and a
daily weighing of animals with body weight loss >15% (6 animals
in total) was introduced in the protocol. In group 3, 2 mice with
20% and 1 with 12% body weight loss found recumbent were
euthanized. Additionally, 3 mice with a body weight loss of 17–20%
and absent of clinical manifestations at the previous observation
succumbed. In the group with preventative/therapeutic use of BP-C3
(group 3), the total dropout rate was also 29%; dropouts in group 3
occurred 1–11 days later than those in group 2. The mortality in
the group with therapeutic BP-C3 regimen (commenced 24 h after 5-FU
administration; group 4) was decreased to 5% (1 mouse with 17%
weight loss died at day 8; P=0.093 vs. group 2; Table I). Enteritis was identified as the
primary macroscopic change in all dropout animals. Adverse events
may develop rapidly in mice; therefore, more frequent inspection
(i.e., twice-daily) of animals receiving 5-FU at doses starting at
150 mg/kg will be introduced in future protocols.
 | Table I.Animal dropout rate during the
experiment. |
Table I.
Animal dropout rate during the
experiment.
|
| Experiment day |
|
|---|
|
|
|
|
|---|
| Group | 4 | 11 | 20 | Dropout, % |
|---|
| 1. Control | 0 (5) | 0 (5) | 0 (11) | 0 |
| 2. 5-FU | 0 (5) | 6a (5) | 0 (5) | 29c |
| 3. ВР-С3 + 5-FU +
ВР-С3 | 0 (5) | 1a (5) | 5b (5) | 29c |
| 4. 5-FU +
ВР-С3 | 0 (5) | 1a (5) | 0 (10) |
5d |
| 5. ВР-С3 | 0 (5) | 0 (5) | 0 (11) | 0 |
Dynamics of body weight of the
animals
The toxic effect of 5-FU was manifested by the
decreased body weight of the animals (average loss of 7% as of day
4). There was no significant difference in mean body weight of
animals treated with 5-FU alone, and animals treated with a
combination of 5-FU and BP-C3 (Table
II). Body weight loss is a general sign of toxicity and is
commonly ~10% in animal studies evaluating cytostatic agents such
as 5-FU (21). The maximum body
weight loss registered in individual mice on day 4 was −16, −11 and
−10% of the initial body weight in the 5-FU (group 2), BP-C3 + 5-FU
+ BP-C3 (group 3) and 5-FU + BP-C3 (group 4) groups, respectively.
The average body weight loss was transient and by day 11 no
statistically significant differences between the experimental
groups were present.
 | Table II.Body weight of mice exposed to 5-FU
with or without BP-C3. |
Table II.
Body weight of mice exposed to 5-FU
with or without BP-C3.
|
| Body weight in each
group, g |
|---|
|
|
|
|---|
| Experiment day | 1. Control | 2. 5-FU | 3. ВР-С3 + 5-FU +
ВР-С3 | 4. 5-FU +
ВР-С3 | 5. ВР-С3 |
|---|
| 0 |
31.66±0.49 |
32.45±0.38 |
32.01±0.48 |
31.89±0.34 |
32.27±0.46 |
| 4 |
32.34±0.65 |
30.14±0.63a,b |
30.39±0.47b |
30.13±0.43a,b |
32.60±0.54 |
| 11 |
32.07±0.56 |
32.62±0.50 |
31.15±0.63 |
32.11±0.61 |
32.55±0.53 |
| 20 |
32.62±0.55 |
33.76±0.61 |
32.61±0.68 |
33.26±0.2 |
32.83±0.45 |
Organ/body weight ratios of the
animals
To evaluate the organ-specific toxic effect of 5-FU,
the organ/body weight ratios of the spleen, thymus, heart, liver
and kidneys were determined (Table
III). The spleen and thymus were the main organs that exhibited
a toxic effect in response to 5-FU. The toxic effect was
demonstrated by the significant decrease of the organ/body weight
ratios of these organs on day 4 post 5-FU administration compared
with those observed in the control group (P<0.05). The use of
BP-C3 in the therapeutic (group 4) and preventative/therapeutic
(group 3) schedules was protective against the toxic effect that
5-FU exerted on the lymphopoietic organs.
 | Table III.The effect of BP-C3 on 5-FU-induced
changes in organ/body weight ratios. |
Table III.
The effect of BP-C3 on 5-FU-induced
changes in organ/body weight ratios.
| A, Spleen |
|---|
|
|---|
|
| Organ/body weight
ratio on each experiment day, mg/10 g |
|---|
|
|
|
|---|
| Group | 4 | 11 | 20 |
|---|
| 1. Control |
36.39±3.01 |
38.43±2.83 |
37.11±5.41 |
| 2. 5-FU |
16.57±1.55a |
45.10±6.66b |
88.96±5.02a,c |
| 3. ВР-С3 + 5-FU +
ВР-С3 |
23.89±2.67 |
54.95±10.31b,c |
95.02±2.77a,c |
| 4. 5-FU +
ВР-С3 |
36.31±2.00 |
92.92±9.81a,c |
80.80±5.21a,c |
| 5. ВР-С3 |
35.86±5.10 |
30.75±0.65 |
35.38±1.78 |
|
| B,
Thymus |
|
|
| Organ/body
weight ratio on each experiment day, mg/10 g |
|
|
|
| Group | 4 | 11 | 20 |
|
| 1. Control |
22.26±2.79 |
22.21±1.45 |
20.60±0.65 |
| 2. 5-FU |
14.01±1.10a |
18.44±2.39 |
20.39±1.61 |
| 3. ВР-С3 + 5-FU +
ВР-С3 |
18.90±3.76 |
21.02±1.47 |
21.16±2.25 |
| 4. 5-FU +
ВР-С3 |
16.21±1.06 |
15.88±1.23 |
17.16±1.21 |
| 5. ВР-С3 |
19.51±2.63 |
22.62±1.53 |
20.98±1.03 |
|
| C,
Heart |
|
|
| Organ/body
weight ratio on each experiment day, mg/10 g |
|
|
|
| Group | 4 | 11 | 20 |
|
| 1. Control |
66.23±8.99 |
66.13±6.66 |
64.64±3.85 |
| 2. 5-FU |
63.10±4.28 |
52.64±2.57 |
56.23±1.37 |
| 3. ВР-С3 + 5-FU +
ВР-С3 |
56.90±3.59 |
64.93±9.11 |
57.87±2.17 |
| 4. 5-FU +
ВР-С3 |
55.12±2.57 |
64.24±5.31 |
55.82±2.73 |
| 5. ВР-С3 |
66.88±6.60 |
60.73±4.05 |
60.58±2.61 |
|
| D,
Liver |
|
|
| Organ/body
weight ratio on each experiment day, mg/10 g |
|
|
|
| Group | 4 | 11 | 20 |
|
| 1. Control |
594.38±54.71 |
605.24±46.98 |
541.53±19.14 |
| 2. 5-FU |
475.70±13.77 |
472.04±26.63 |
509.56±26.22 |
| 3. ВР-С3 + 5-FU +
ВР-С3 |
595.79±24.40 |
545.28±40.06 |
576.07±30.38 |
| 4. 5-FU +
ВР-С3 |
540.68±39.11 |
590.89±48.13 |
511.19±33.71 |
| 5. ВР-С3 |
600.78±69.93 |
541.90±31.40 |
552.30±35.96 |
|
| E, Kidney
(left) |
|
|
| Organ/body
weight ratio on each experiment day, mg/10 g |
|
|
|
| Group | 4 | 11 | 20 |
|
| 1. Control |
83.14±7.72 |
89.56±8.51 |
77.76±3.74 |
| 2. 5-FU |
88.18±8.92 |
80.58±6.00 |
69.26±6.07 |
| 3. ВР-С3 + 5-FU +
ВР-С3 |
79.40±3.36 |
86.12±2.71 |
71.84±3.79 |
| 4. 5-FU +
ВР-С3 |
75.43±5.35 |
70.95±3.74 |
67.10±2.97 |
| 5. ВР-С3 |
79.40±3.36 |
86.12±2.71 |
71.84±3.79 |
Notably, by day 20 there was a significant increase
in spleen weight in all groups that received 5-FU compared with
that observed in the control group (P<0.05; Table IIIA). Administration of BP-C3
affected this process. By day 11 the spleen/body weight ratio in
animals treated with BP-C3 on the therapeutic schedule (24 h after
exposure to 5-FU) was greater than that in the animals that
received BP-C3 on the preventative/therapeutic schedule. On day 20,
the spleen/body weight ratio was reached the values observed in all
groups where 5-FU was administered.
BP-C3 effects on 5-FU-induced
hematological toxicity
The modification of the myelotoxic effect of 5-FU by
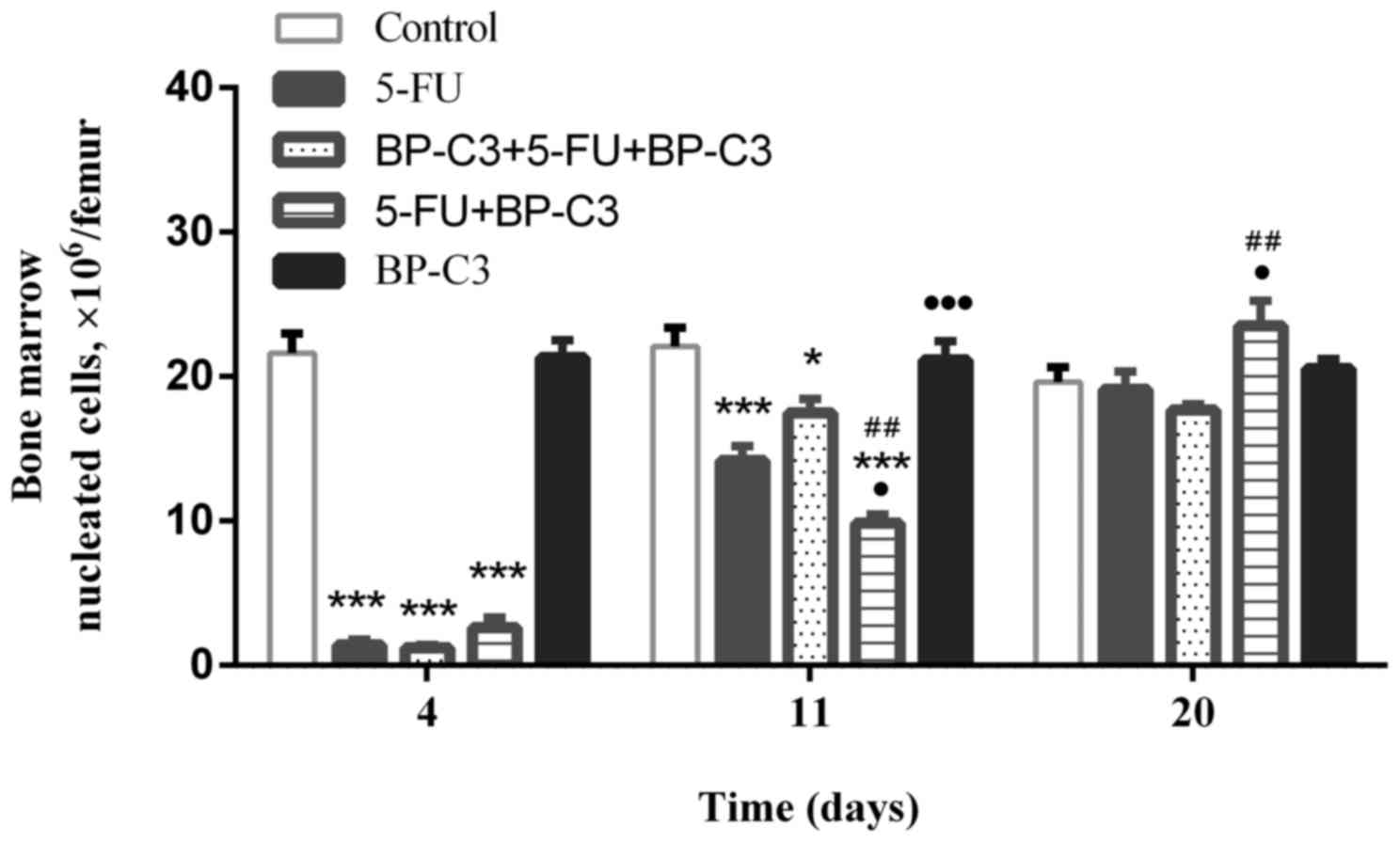
co-administration of BP-C3 was evaluated (Fig. 1). The use of BP-C3 did not improve
the 5-FU-induced suppression of bone marrow haematopoiesis that was
observed on day 4. However, during the time that the BMNC count was
recovering, there were differences in the effects of ВР-С3 used on
the therapeutic and preventative/therapeutic schedules. On day 11,
significantly fewer bone marrow nucleated cells were observed in
group 4 compared with group 2 (P<0.05) and group 3 (P<0.01).
However, on day 20 the BMNC count in group 4 was significantly
greater compared with group 2 (P<0.05) and group 3
(P<0.01).
When blood counts were analysed, the following
changes were noted (Table IV): Mice
in groups 2, 3 and 4, which all received 5-FU, exhibited pronounced
leukopenia on days 4 and 7, which changed to leukocytosis in groups
2 and 3 by days 14–17; however, this phenomenon was not reproduced
in group 4. The changes in the populations of lymphocytes,
granulocytes and monocytes were similar.
 | Table IV.The effect of BP-C3 on 5-FU-induced
changes in white and red blood cell counts. |
Table IV.
The effect of BP-C3 on 5-FU-induced
changes in white and red blood cell counts.
| A, Lymphocytes,
109/l |
|---|
|
|---|
|
| Experiment day |
|---|
|
|
|
|---|
| Group | 0 | 4 | 7 | 11 | 14 | 17 | 20 |
|---|
| 1. Control |
5.99±0.94 |
6.18±0.55 |
8.08±0.76 |
6.40±0.99 |
6.71±0.55 |
6.77±1.07 |
5.44±0.65 |
| 2. 5-FU |
5.61±0.65 |
2.79±0.37a,b |
5.02±1.17 |
10.60±1.24 |
22.02±5.94a,b |
13.54±3.50b |
7.72±3.24 |
| 3. ВР-С3 + 5-FU +
ВР-С3 |
5.96±0.69 |
2.90±0.48a,b |
2.45±0.49a,b |
7.51±1.31 |
16.63±3.15a–c |
18.18±3.95a,b |
10.06±0.97 |
| 4. 5-FU +
ВР-С3 |
6.07±0.52 |
2.48±0.15a |
2.52±0.38a,b |
6.87±1.98 |
8.68±1.30 |
11.64±1.41 |
5.94±2.27 |
| 5. ВР-С3 |
5.61±1.03 |
5.82±0.75 |
6.50±0.59 |
5.70±0.81 |
5.43±1.47 |
4.10±0.65 |
4.03±1.19 |
|
| B, Granulocytes,
109/l |
|
|
| Experiment
day |
|
|
|
| Group | 0 | 4 | 7 | 11 | 14 | 17 | 20 |
|
| 1. Control |
4.88±0.37 |
4.44±0.29 |
4.71±0.84 |
3.97±0.41 |
4.54±0.60 |
4.39±0.96 |
4.20±0.64 |
| 2. 5-FU |
4.29±0.35 |
1.95±0.27a,b |
0.58±0.13a,b |
3.65±0.63 |
12.04±2.75 |
16.98±7.93a,b |
8.74±2.09 |
| 3. ВР-С3 + 5-FU +
ВР-С3 |
4.14±0.30 |
1.46±0.15a,b |
0.41±0.09a,b |
3.84±0.61 |
21.00±6.93a–c |
19.54±8.25a–c |
9.34±1.83 |
| 4. 5-FU +
ВР-С3 |
4.22±0.59 |
1.08±0.28a,b |
0.40±0.06a,b |
3.68±1.17 |
7.58±0.85 |
8.44±1.00 |
5.44±0.87 |
| 5. ВР-С3 |
3.80±0.42 |
3.32±0.24 |
3.82±0.65 |
3.57±0.34 |
3.73±0.38 |
2.88±0.51 |
2.45±0.34 |
|
| C, Monocytes,
109/l |
|
|
| Experiment
day |
|
|
|
| Group | 0 | 4 | 7 | 11 | 14 | 17 | 20 |
|
| 1. Control |
0.58±0.04 |
0.63±0.08 |
0.64±0.13 |
0.52±0.06 |
0.51±0.09 |
0.54±0.12 |
0.60±0.12 |
| 2. 5-FU |
0.47±0.04 |
0.19±0.03a,b |
0.15±0.03a,b |
0.60±0.21 |
2.26±0.22a–c |
1.58±0.36a,b |
0.94±0.22 |
| 3. ВР-С3 + 5-FU +
ВР-С3 |
0.44±0.04 |
0.16±0.03a,b |
0.11±0.03a,b |
0.79±0.11 |
2.37±0.39a–c |
2.07±0.30a–c |
1.46±0.15a–c |
| 4. 5-FU +
ВР-С3 |
0.38±0.07 |
0.12±0.04a,b |
0.1±0.03a,b |
0.74±0.23 |
1.38±0.17a,b |
0.98±0.26 |
0.48±0.09 |
| 5. ВР-С3 |
0.43±0.06 |
0.44±0.04 |
0.52±0.10 |
0.43±0.04 |
0.40±0.04 |
0.50±0.20 |
0.25±0.06 |
| D, Red blood
cells, 1012/l |
|
|
| Experiment
day |
|
|
|
| Group | 0 | 4 | 7 | 11 | 14 | 17 | 20 |
|
| 1. Control |
10.7±0.3 |
10.4±0.2 |
10.6±0.3 |
10.5±0.2 |
9.9±0.4 |
10.0±0.3 |
9.9±0.2 |
| 2. 5-FU |
11.0±0.3 |
10.7±0.3 |
8.9±0.5a–c |
8.5±0.5a,b |
9.5±0.3 |
10.2±0.4 |
10.5±0.4 |
| 3. ВР-С3 + 5-FU +
ВР-С3 |
11.1±0.2 |
10.7±0.4 |
9.5±0.4 |
8.1±0.2a,b |
8.4±0.2a,b |
9.4±0.1 |
9.8±0.4 |
| 4. 5-FU +
ВР-С3 |
11.4±0.3 |
11.4±0.3 |
10.5±0.4 |
9.4±0.8 |
8.8±0.3 |
9.5±0.2 |
9.2±0.3 |
| 5. ВР-С3 |
10.7±0.2 |
10.3±0.2 |
10.7±0.3 |
10.7±0.3 |
10.5±0.3 |
10.7±0.3 |
10.7±0.1 |
The lymphocyte count on day 4 in group 2 was 55%
lower than that of the control group (P<0.05; Table IVA). By day 7, the lymphocyte count
in group 2 returned to a normal level. Although the lymphocyte
count in group 2 was 38% lower compared with the control group on
day 7, no significant difference was identified. On day 14, the
lymphocyte count in group 2 was significantly increased by 228%
(P<0.05) compared with the control group, followed by a decrease
to the normal level by day 20. The lymphocyte count in group 3 was
reduced by 53% on day 4 and by 70% on day 7 compared with the
control group at the respective time points (both P<0.05), and
thus this reduction lasted longer than that of group 2. On day 14
and 17 the lymphocyte count in group 3 increased significantly by
148 and 167%, respectively (P<0.05 vs. the control group). In
group 4, the lymphocyte count decreased by 60% on day 4 and by 69%
on day 7 (both P<0.05 vs. control group).
Contrary to groups 2 and 3, no increase in
lymphocyte count was observed in group 4 at later time points
(between day 14 and 20). In groups 2, 3 and 4, the granulocyte
count (Table IVB) decreased by ~80%
on day 4 (P<0.05 vs. the control group). The decrease in the
granulocyte count of ~90% was most pronounced in groups 2, 3 and 4
on day 7 (P<0.05 vs. the control group). Differences between the
groups 2, 3 and 4 were not statistically significant on day 7. At
later time points, the granulocyte count significantly increased in
group 2 by day 17 (P<0.05 vs. the control group), and in group 3
by day 14 and 17 (all P<0.05 vs. the control group). In group 4,
no increase of the granulocyte count was observed on day 14 and 17
compared with the control group at the same time points.
The monocyte count significantly decreased on day 4
and 11 after 5-FU exposure in groups 2, 3 and 4 (all P<0.05 vs.
the control group; Table IVC). At
later time points, the monocyte count increased significantly on
day 14 and 17 in group 2, and days 14–20 in group 3 (P<0.05 vs.
the control group). In group 4, a significant increase in the
monocyte count was observed only on day 14 (P<0.05 vs. the
control group) and was normalised by day 17. In group 4, the
recovery of leukopoiesis without a pronounced stage of enhanced
haematological response can be attributed to the anti-inflammatory
effect of BP-C3, likely due to a shorter pro-inflammatory response
of monocytes. Administration of BP-C3 without 5-FU did not have a
significant effect on white blood cell differentiation in the
mice.
Mild anaemia developed in the mice in group 2
between day 7 and 11, and in group 3 between day 11 and 14 as the
red blood cell count significantly decreased at those time points
in the aforementioned groups (all P<0.05 vs. the control group;
Table IVD). ВР-C3 was administered
24 h after 5-FU to mice in group 4, which prevented the development
of anaemia in the mice. Thus, BP-C3, depending on the
administration schedule, has different effects on the
haematological parameters of the haematopoietic organs and the
peripheral blood.
BP-C3 effects on 5-FU-induced
intestinal toxicity
As one of the primary manifestations of the toxic
effect of 5-FU is enteritis, the number of surviving intestinal
crypts following exposure to 5-FU was evaluated. Histological
examination of samples, collected on day 4 from all mice treated
with 5-FU, identified the death of crypts, shortening or loss of
the villi and a chronic inflammatory response in the intestinal
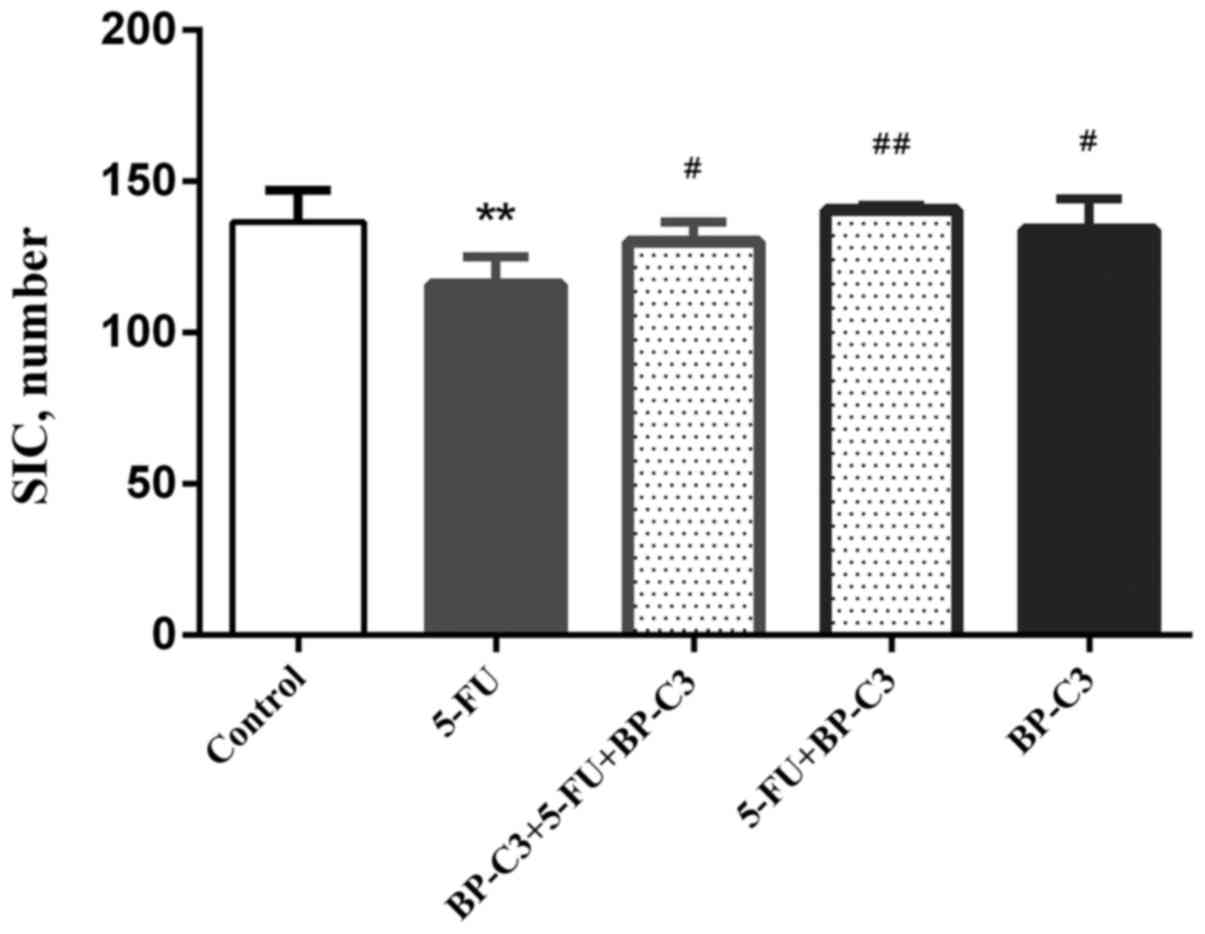
mucosa. The counts of the surviving crypts are demonstrated in
Fig. 2. The number of crypts per
intestinal cross-section decreased significantly in group 2
(P<0.01 vs. the control group). By contrast, BP-C3 promoted
intestinal crypt survival when administered on either the
preventative/therapeutic (group 3; P<0.05 vs. group 2) or the
therapeutic (group 4; P<0.01 vs. group 2) schedule. BP-C3
treatment alone (group 5) did not affect number of surviving
intestinal crypts. In summary, BP-C3 protects the epithelium of the
small intestine against damage induced by 5-FU.
Discussion
Substances of plant origin, polyphenols in
particular, are promising chemotherapy adjuncts for several
reasons. First, they have low toxicity and cause essentially no
side effects, as opposed to the synthetic drugs that are currently
used in clinical practice to decrease chemotherapy side effects
(22). Second, various compounds in
this chemical group have been demonstrated to have their own
anti-tumour activity. Polyphenol compounds slow the growth of
various cancer cell lines and also inhibit carcinogenesis in
vivo (23–25). Various plant agents, including
genistein, curcumin, epigallocatechin gallate, resveratrol and
proanthocyanidin, are able to potentiate the effectiveness of
traditional chemotherapeutic drugs (22).
In the cell, polyphenols bind to numerous targets,
neutralise free radicals and affect numerous signalling pathways,
including the epigenetic regulation of gene expression and
mitochondrial function (26).
Oxidative stress is one of the mechanisms of action of various
anti-tumour substances; therefore, the effectiveness of
chemotherapy may be modulated by substances with antioxidant
properties. Some of the most common exogenous antioxidants are
polyphenolic compounds, which protect us from developing
cardiovascular diseases, diabetes, cancer and infectious diseases
(27). Antioxidants may protect
normal tissue without affecting the effectiveness of anti-tumour
chemotherapeutic drugs (28,29). Paradoxically, polyphenols exert an
antioxidant effect on healthy cells, while in cancer cells they
increase free radicals, causing cellular aging, cell cycle arrest
and apoptosis (30). In the presence
of copper ions, of which there are more in cancer cells than in
normal cells (31), at least some
polyphenols (luteolin, apigenin, epigallocatechin-3-gallate and
resveratrol) act as pro-oxidants. The anti-tumour effect of these
polyphenols decreases significantly in the presence of copper
chelators (32).
Polyphenols reduce toxic effects on normal tissues,
thus quercetin protects normal renal tubular cells from drug
toxicity and attenuates drug-induced nausea and vomiting (22). Traditional Japanese medicines have
been demonstrated to protect intestinal epithelium during 5-FU use
(33). Besides their direct
antioxidant effect, vegetable and fruit flavonoids are able to
increase the activity of detoxifying enzymes (34).
Notably, various investigational drugs are
traditional medicines, also known as multi-component mixtures, and
their various constituents possibly potentiate one another. For
example, experiments in mice exposed to γ radiation have
demonstrated that the mixtures of polyphenols of green tea were
more protective than the tea's individual components (35). Although such traditional medicines
are effective, lack of sufficient standardisation is their
drawback.
Polyphenol substances exert anti-tumour effects
through other mechanisms as well. Curcumin, a polyphenol of plant
origin, has numerous molecular effects; in particular, it
suppresses the transcription of nuclear factor-κB, and affects the
receptors of various growth factors and cell adhesion molecules
involved in tumour growth, angiogenesis and metastasis (36). Curcumin has a radioprotective or
radiosensitising effect, depending on the dose, and these effects
are now being actively studied to support the use of this product
as an adjunct therapy for radio and chemotherapy (37). Thus, preparations that contain
various polyphenols may be considered as promising adjunct
therapies for oncology patients.
The use of such substances in oncology patients may
improve quality of life and overall survival, particularly in
palliative patients. The results of previous study on BP-C2,
administered from week 2–26 in combination with a FOLFIRINOX
regimen in a patient with inoperable pancreatic cancer, indicated
that during treatment no neutropenia developed for 18 weeks, and
blood biochemistry normalised by week 25 (14). In a long-term study performed in
female SHR mice, the mean life span increased by ~50 days in
animals treated with BP-C3 compared with the untreated control
group (15). A greater number of
tumour-free mice and delayed age-related switch-off of the oestrus
function were observed, possibly due to immunomodulatory effect of
BP-C3 (15).
Thus, the findings from the present study, which
demonstrate the protective effects of BP-C3 on the immune system
and other systems, and intestinal epithelium, are in line with and
complement the results of previous studies by our group, though
molecular mechanisms underlying these effects remain to be further
elucidated. Future studies to assess the potential of BP-C3 in
combination with certain chemotherapy agents and regimens will
allow the identification of optimal combinations, doses and dosing
schedules.
The experimental data of the present study extends
current knowledge on the biological activity of lignin-derived
polyphenolic compositions, which are a group of natural compounds.
The extensive therapeutic potential of lignin-derived polyphenolic
compositions is yet to be explored. The present study demonstrated
the feasibility of using a lignin-derived polyphenolic composition
to diminish the toxic effects of chemotherapy, in particular, to
reduce the haematological and gastrointestinal adverse effects.
Acknowledgements
The present study was supported by a grant from the
Russian Science Foundation (grant no. 16-15-00142 to VNA).
References
|
1
|
Santi DV, McHenry CS and Sommer H:
Mechanism of interaction of thymidylate synthetase with
5-fluorodeoxyuridylate. Biochemistry. 13:471–481. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nair KL, Jagadeeshan S, Nair SA and Kumar
GV: Biological evaluation of 5-fluorouracil nanoparticles for
cancer chemotherapy and its dependence on the carrier, PLGA. Int J
Nanomedicine. 6:1685–1697. 2011.PubMed/NCBI
|
|
3
|
Poon MA, O'Connell MJ, Moertel CG, Wieand
HS, Cullinan SA, Everson LK, Krook JE, Mailliard JA, Laurie JA,
Tschetter LK, et al: Biochemical modulation of fluorouracil:
Evidence of significant improvement of survival and quality of life
in patients with advanced colorectal carcinoma. J Clin Oncol.
7:1407–1418. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsunaga T, Tsuji Y, Kaai K, Kohno S,
Hirayama R, Alpers DH, Komoda T and Hara A: Toxicity against
gastric cancer cells by combined treatment with 5-fluorouracil and
mitomycin c: Implication in oxidative stress. Cancer Chemother
Pharmacol. 66:517–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leitão RFC, Ribeiro RA, Bellaguarda EA,
Macedo FD, Silva LR, Oriá RB, Vale ML, Cunha FQ and Brito GA: Role
of nitric oxide on pathogenesis of 5-fluorouracil induced
experimental oral mucositis in hamster. Cancer Chemother Pharmacol.
59:603–612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Braun MS and Seymour MT: Balancing the
efficacy and toxicity of chemotherapy in colorectal cancer. Ther
Adv Med Oncol. 3:43–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ribeiro RA, Wanderley CW, Wong DV, Mota
JM, Leite CA, Souza MH, Cunha FQ and Lima-Júnior RC: Irinotecan-
and 5-fluorouracil-induced intestinal mucositis: Insights into
pathogenesis and therapeutic perspectives. Cancer Chemother
Pharmacol. 78:881–893. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
You JS, Chen JP, Chan JS, Lee HF, Wong MK,
Yeung WF and Lao LX: Effect of YH0618 soup on chemotherapy-induced
toxicity in patients with cancer who have completed chemotherapy:
Study protocol for a randomized controlled trial. Trials.
17:3542016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coccia A, Mosca L, Puca R, Mangino G,
Rossi A and Lendaro E: Extra-virgin olive oil phenols block cell
cycle progression and modulate chemotherapeutic toxicity in bladder
cancer cells. Oncol Rep. 36:3095–3104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qazi SS, Li D, Briens C, Berruti F and
Abou-Zaid MM: Antioxidant activity of the lignins derived from
fluidized-bed fast pyrolysis. Molecules. 22:pii: E3722017.
View Article : Google Scholar
|
|
11
|
Shipov VP, Pigarev ES and Fedoros EI:
Benzene polycarboxylic acid compounds and their use as drug Patent
WO2013143549 A1. April 2–2013, issued October 3, 2013.
|
|
12
|
Larsen S, Butthongkomvong K, Manikhas A,
Trishkina E, Poddubuskaya E, Matrosova M, Srimuninnimit V and
Lindkær-Jensen S: BP-C1 in the treatment of patients with stage IV
breast cancer: A randomized, double-blind, placebo-controlled
multicenter study and an additional open-label treatment phase.
Breast Cancer (Dove Med Press). 6:179–189. 2014.PubMed/NCBI
|
|
13
|
Fares F, Fares B, Azzam N, Nashashibi M,
Nevelsky A, Larsen S and Lindkær-Jensen S: An innovative complex of
benzene-poly-carboxylic acid and molybdenum, for prevention and
treatment of radiation dermatitis. Med Chem. 5:447–451. 2015.
View Article : Google Scholar
|
|
14
|
Ibrahim T, Larsen S, Jensen NHL and
Lindkær-Jensen S: BP-C2 improves functional status, quality of life
and corrects biochemical imbalances as adjuvant therapy to
FOLFIRINOX treatment: A case of advanced inoperable pancreatic
cancer. J Clin Case Rep. 5:5142015.
|
|
15
|
Anisimov VN, Popovich IG, Zabezhinski MA,
Yurova MN, Tyndyk ML, Anikin IV, Egormin PA, Baldueva IA, Fedoros
EI, Pigarev SE and Panchenko AV: Polyphenolic drug composition
based on benzenepolycarboxylic acids (BP-C3) increases life span
and inhibits spontaneous tumorigenesis in female SHR mice. Aging
(Albany NY). 8:1866–1875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anisimov VN, Zabezhinski MA, Popovich IG,
Tyndyk ML, Anikin IV, Egormin PA, Yurova MN, Piskunova TS,
Panchenko AV, Shipov VP, et al N.N. Petrov Research Institute of
Oncology, Nobel Ltd., : Pharmacological geroprotective composition
and method of obtaining thereof Russian patent RU 2522547. 2014
|
|
17
|
Tian Y, Xiang Y, Wan G, Wan D, Zhu H, Wang
T and Yang X: Effects and mechanisms of Bazhen decoction, Siwu
decoction and Sijunzi decoction on 5-fluorouracil-induced anemia in
mice. J Tradit Chin Med. 36:486–495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vershinina SF and Stukov AN: Handbook of
experimental therapy of tumours. FGU RSCRST and FGU RIO n.a. NN
Petrov of the FAHTMC of Russian Federation, Reprint LLC,
Saint-Petersburg. pp. 252008, (In Russian).
|
|
19
|
Mcculloch EA and Till JE: The radiation
sensitivity of normal mouse bone marrow cells, determined by
quantitative marrow transplantation into irradiated mice. Radiat
Res. 13:115–125. 1960. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Withers HR and Elkind MM: Microcolony
survival assay for cells of mouse intestinal mucosa exposed to
radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 17:261–267.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coronado-Cerda EE, Franco-Molina MA,
Mendoza-Gamboa E, Prado-García H, Rivera-Morales LG,
Zapata-Benavides P, Rodríguez-Salazar Mdel C, Caballero-Hernandez
D, Tamez-Guerra RS and Rodríguez-Padilla C: In vivo chemoprotective
activity of bovine dialyzable leukocyte extract in mouse bone
marrow cells against damage induced by 5-fluorouracil. J Immunol
Res. 2016:69423212016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sak K: Chemotherapy and dietary
phytochemical agents. Chemother Res Pract.
2012:2825702012.PubMed/NCBI
|
|
23
|
Katiyar SK, Mohan RR, Agarwal R and
Mukhtar H: Protection against induction of mouse skin papillomas
with low and high risk of conversion to malignancy by green tea
polyphenols. Carcinogenesis. 18:497–502. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kubatka P, Kapinová A, Kello M, Kruzliak
P, Kajo K, Výbohová D, Mahmood S, Murin R, Viera T, Mojžiš J, et
al: Fruit peel polyphenols demonstrate substantial anti-tumour
effects in the model of breast cancer. Eur J Nutr. 55:955–965.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mileo AM and Miccadei S: Polyphenols as
modulator of oxidative stress in cancer disease: New therapeutic
strategies. Oxid Med Cell Longev. 2016:64756242016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barrajón-Catalán E, Herranz-López M, Joven
J, Segura-Carretero A, Alonso-Villaverde C, Menéndez JA and Micol
V: Molecular promiscuity of plant polyphenols in the management of
age-related diseases: Far beyond their antioxidant properties. Adv
Exp Med Biol. 824:141–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mut-Salud N, Álvarez PJ, Garrido JM,
Carrasco E, Aránega A and Rodríguez-Serrano F: Antioxidant intake
and antitumor therapy: Toward nutritional recommendations for
optimal results. Oxid Med Cell Longev. 2016:67195342016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lamson DW and Brignall MS: Antioxidants in
cancer therapy; their actions and interactions with oncologic
therapies. Altern Med Rev. 4:304–329. 1999.PubMed/NCBI
|
|
29
|
Lawenda BD, Kelly KM, Ladas EJ, Sagar SM,
Vickers A and Blumberg JB: Should supplemental antioxidant
administration be avoided during chemotherapy and radiation
therapy? J Natl Cancer Inst. 100:773–783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mileo AM and Miccadei S: Polyphenols as
modulator of oxidative stress in cancer disease: New therapeutic
strategies. Oxid Med Cell Longev. 2016:64756242016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gupte A and Mumper RJ: Elevated copper and
oxidative stress in cancer cells as a target for cancer treatment.
Cancer Treat Rev. 35:32–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan HY, Zubair H, Faisal M, Ullah MF,
Farhan M, Sarkar FH, Ahmad A and Hadi SM: Plant polyphenol induced
cell death in human cancer cells involves mobilization of
intracellular copper ions and reactive oxygen species generation: A
mechanism for cancer chemopreventive action. Mol Nutr Food Res.
58:437–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kato S, Hayashi S, Kitahara Y, Nagasawa K,
Aono H, Shibata J, Utsumi D, Amagase K and Kadowaki M: Saireito
(TJ-114), a Japanese traditional herbal medicine, reduces
5-fluorouracil-induced intestinal mucositis in mice by inhibiting
cytokine-mediated apoptosis in intestinal crypt cells. PLoS One.
10:e01162132015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liska DJ: The detoxification enzyme
systems. Altern Med Rev. 3:187–198. 1998.PubMed/NCBI
|
|
35
|
Lee HJ, Kim JS, Moon C, Kim JC, Lee YS,
Jang JS, Jo SK and Kim SH: Modification of gamma-radiation response
in mice by green tea polyphenols. Phytother Res. 22:1380–1383.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wilken R, Veena MS, Wang MB and Srivatsan
ES: Curcumin: A review of anti-cancer properties and therapeutic
activity in head and neck squamous cell carcinoma. Mol Cancer.
10:122011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hejazi J, Rastmanesh R, Taleban FA, Molana
SH, Hejazi E, Ehtejab G and Hara N: Effect of curcumin
supplementation during radiotherapy on oxidative status of patients
with prostate cancer: A double blinded, randomized,
placebo-controlled study. Nutr Cancer. 68:77–85. 2016. View Article : Google Scholar : PubMed/NCBI
|