Introduction
Metastatic breast cancer is one of the most common
types of metastatic tumors and affects the health of women around
the world (1). The mortality rate of
patients with breast carcinoma is high due to local invasion and
distant metastasis and >30% of patients with breast carcinoma
develop metastasis during progression of their disease (2,3). The
5-year overall survival rate of patients with breast cancer is
4–28% and reports have indicated that the incidence of breast
cancer in young women is growing and is frequently metastatic
following diagnosis (4,5). Therefore, it is critical to analyze the
potential mechanism(s) associated with local invasion and distant
metastasis to improve the 5-year overall survival rate and
prognosis of patients with breast carcinoma (6,7).
Transforming growth factor (TGF)-β-inducible gene-h3
(βig-h3) is highly expressed in various types of tumors in humans
and is associated with the growth and metastasis of tumor cells
(8–10). βig-h3 encodes a secreted
extracellular matrix (ECM) protein and it has been demonstrated
that this protein is induced by TGF-β in pancreatic cancer cells,
subsequently stimulating the growth and invasion of pancreatic
cancer cells (11). Furthermore,
previous studies have indicated that the ECM protein TGF-βig-h3
promotes colon, gastric and ovarian cancer metastasis by enhancing
cell extravasation (12–14).
The phosphatidylinositol 3-kinase (PI3K)/protein
kinase B (Akt) signaling pathway is associated with cancer cell
growth and metastasis and serves a role in the development of
chemoresistance to platinum-based neoadjuvant chemotherapy
(15). In a previous study,
measurement of the cellular and molecular responses to lapatinib
and Akt inhibitors suggested that they suppress breast cancer
growth and aggressiveness (16). In
addition, inhibiting PI3K/Akt downregulates the breast cancer
resistance protein resensitized MCF7 breast cancer cell line, which
promotes the apoptosis of MCF7 cells induced by mitoxantrone
chemotherapy (17). Furthermore, a
previous study indicated that the binding of matrix
metalloproteinase-9-degraded fibronectin to integrin promotes the
invasion of breast cancer cells via the focal adhesion
kinase-Src-associated extracellular signal-regulated kinase 1/2 and
PI3K/Akt/Smad-1/5/8 pathways (18).
These results indicate that inhibiting the PI3K/Akt signaling
pathway may suppress the growth of breast cancer cells.
The present study investigated the role of βig-h3 in
the progression of breast carcinoma in vitro and in
vivo. The potential mechanism of βig-h3-mediated growth and
aggressiveness of breast carcinoma cells was also investigated. The
results indicated that βig-h3 promotes the growth and metastasis of
breast carcinoma cells. This may increase understanding of the
signaling pathways that are activated during the metastasis of
breast cancer cells and indicate that βig-h3 may be a potential
therapeutic target to treat breast cancer.
Materials and methods
Cell culture
The breast cancer cell lines MCF-7, BT474 and SKBR3
and the normal breast cell line MCF-10A were purchased from the
American Type Culture Collection (Manassas, VA, USA). All tumor
cells were cultured in RPMI-1640 medium supplemented with 10%
heat-inactivated fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 3 mM L-glutamine, 50 µg/ml
gentamicin (Biowhittaker Reagents; Lonza Group, Ltd., Basel,
Switzerland) and 1% penicillin/streptomycin. MCF-10A cells were
cultured in a mammary epithelial cell medium (cat. no. 7611,
MEpiCM; ScienCell Research Laboratories, Inc., San Diego, CA, USA).
MCF-7 is the typical human breast carcinoma cell, therefore, this
cell line was chosen for further experiments. All cells were
cultured at 37°C in 5% CO2.
Transfection of small interfering RNA
(siRNA)
A total of 1×108 cells (MCF-7, BT474,
SKBR3 and MCF-10A) were transfected with either si-Rβig-h3 for
knockdown of CHOP (Kd-CHOP) or si-Rvector (Invitrogen; Thermo
Fisher Scientific, Inc.). MCF-7 cells (1×106) were
transfected with 120 pmol si-Rβig-h3 (5′-CCUUUACGAGACCCUGGGATT-3′
and 5′-UCCCAGGGUCUCGUAAAGGTT-3′) targeting βig-h3 or si-R vector as
a control (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using a Cell Line Nucleofector kit L (Lonza Group, Ltd.). Following
48 h transfection, cells were used for further analysis.
Overexpression of PI3K or βig-h3
MCF-7 (1×106) cells were cultured until
85% confluence was reached; media was then removed and cells were
washed three times with PBS. MCF-7 cells were then transfected with
pLentivirus-PI3K (pPI3K), plentivirus-βig-h3 (pβig-h3, 100 pmol) or
plentivirus-vector (pVector, 100 pmol) using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific., Inc.) according to
manufacturer's instructions. Following 72 h transfection, cells
were used for further analysis.
MTT assay
PI3K- and βig-h3-overexpressed, pVector-transfected,
si-Rβig-h3-treated or si-Rvector-treated MCF-7 cells
(1×103 per well) were cultured in 96-well plates for 48
h at 37°C in triplicate for each condition and pVector-transfected
MCF-7 was used as a control. Following incubation, 20 µl MTT (5
mg/ml) in PBS solution was added and dissolved in dimethyl
sulfoxide for a further 2 h at 37°C. Optical density was measured
using an ELISA reader at a wavelength of 450 nm.
Apoptosis assay
βig-h3-overexpressed, pVector-transfected,
si-Rβig-h3-treated or si-Rvector-treated MCF-7 cells were grown at
37°C with 5% CO2 until 90% confluence was reached. Cells
were then incubated with cisplatin (2.0 mg/ml) or PBS for 48 h at
37°C, trypsinized and collected. Cells were then washed in cold
PBS, adjusted to 1×106 cells/ml with PBS, labeled with
Annexin V-fluoroscein isothiocyanate and propidium iodide (Annexin
V-fluoroscein isothiocyanate kit; BD Biosciences, Franklin Lakes,
NJ, USA) for 1 h at 37°C and analyzed using a FACScan flow
cytometer (BD Biosciences) to assess apoptosis. Treatments were
performed in triplicate and the percentage of labeled cells
undergoing apoptosis in each group was determined and calculated
using a Coulter EPICS XL Flow Cytometer and the results were
analyzed using Expo32-ADC v. 1.2B software (Beckman Coulter Inc.,
Brea, CA, USA).
Cell migration and invasion
assays
Sable PI3K-overexpressed, βig-h-overexpressed,
pVector-transfected, si-Rβig-h3-treated or si-Rvector-treated MCF-7
cells were cultured in serum-free medium for 72 h at 37°C.
Migration and invasion assays were conducted in a 6-well culture
plate with Transwell inserts (BD Biosciences). A total of
1×104 MCF-7 cells/well were placed into the upper
chamber in DMEM supplemented with 5% FBS for migration assays. For
invasion assays, MCF-7 cells (1×104/well) were placed
into the upper chamber with a Matrigel-coated membrane in DMEM
supplement with 5% FBS for 72 h at 37°C. The cells that invaded
through the membrane were fixed with 3% formaldehyde for 15 min at
37°C and stained with 0.5% crystal violet for 10 min at 37°C. The
invasion and migration of tumor cells were assessed in a minimum of
three randomly selected fields using an inverted microscope
(Olympus BX51; Olympus Corporation, Tokyo, Japan).
Western blot analysis
MCF-7 cells were harvested by scraping and were
subsequently lysed in radioimmunoprecipitation buffer
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) followed by
homogenization at 4°C for 10 min. Protein concentration was
measured by a BCA protein assay kit (Thermo Scientific, Pittsburgh
PA, USA). Protein (20 µg) was analyzed using 12% SDS-PAGE and then
transferred onto a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA). The membranes were incubated in blocking
buffer (5% milk) for 2 h at 37°C prior to incubation with primary
antibodies at 4°C overnight. Proteins were incubated with rabbit
anti-human βig-h3 (cat no. ab170874, 1:1,000 dilution, Abcam,
Cambridge, MA, USA), PI3K (cat no. ab86714, 1:1,000 dilution,
Abcam), pAKT (cat no. ab81283, 1:1,000 dilution, Abcam), Akt (cat
no. ab8805, 1:1,000 dilution, Abcam) and β-actin (1:2,000 dilution,
cat no. ab5694; Abcam, Cambridge, UK) antibodies for 12 h at 4°C.
Proteins were the incubated with horseradish peroxide (HRP)-labeled
secondary goat anti-rabbit antibodies (1:5,000 dilution, ab205718,
Abcam) for 2 h at 37°C and protein expression was analyzed using a
chemiluminescence detection system (Version 3.0, Sigma-Aldrich,
Merck KGaA). The density of the bands was analyzed by Quantity One
software (version 4.62, Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Animal study
A total of 30 specific pathogen-free (SPF) female
nude Balb/c mice (6–8 weeks old) were purchased from Shanghai
Laboratory Animal Center (Shanghai, China). All rats were housed in
a temperature-controlled facility at 23±1°C with a relative
humidity of 50±5% and were exposed to a 12-h light/dark cycle. The
mammary glands of mice were subcutaneously implanted with
βig-h3-overexpressed, si-Rβig-h3-treated or si-Rvector-transfected
MCF-7 cells (1×107) and were subsequently divided into
three groups (n=10 in each group). Mice were observed for 40 days
following tumor inoculation. Tumor diameters were recorded every 2
days and tumor volume was calculated using the following formula:
0.52 × smallest diameter2 × largest diameter (17). Experimental mice were euthanized when
the tumor diameter reached 10 mm. The present study was performed
in strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of China (18). The present study was also approved by
the Ethics Committee of Pingdu People's Hospital (Qingdao, China).
All surgery was performed following intraperitoneal injection of
sodium pentobarbital (40 mg/kg, Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) to induce anesthesia and all efforts were made
to minimize suffering.
Tumor metastasis assay
Animals were euthanized by overdose with
pentobarbital (intraperitoneal, 120 mg/kg) on day 40. Mice were
dissected and the distribution of the tumor mass was observed in
the lung, liver, bowel, bone, brain and pleura. The tumor
metastasis rate was recorded in each group (n=6) according to the
numbers of tumors as described previously (19).
Immunohistochemistry
Tumor tissues from xenografted mice were fixed using
10% formaldehyde for 30 min at 37°C, washed with PBS and followed
with embedding in paraffin wax. Tissues were deparaffinized in
xylene and rehydrated in grade alcohols. They were then cut into
4-µm thick serial sections. Antigen retrieval was then performed on
tumor sections using Antigen Retrieval Reagents (cat. no. #CTS015,
Bio-Rad Laboratories, Inc.). Tumor sections were blocked with 5%
milk for 2 h at 37°C and then incubated with goat anti-human βig-h3
(cat no. ab170874, 1:1,000 dilution), PI3K (1:1,000 dilution, cat
no. ab86714) pAKT (cat no. ab81283, 1:1,000 dilution) or Akt
(1:1,000 dilution, cat no. ab8805; all Abcam) antibody for 12 h at
37°C. Sections were then incubated with HRP-labeled secondary goat
anti-rabbit antibodies (1:2,000 dilution, ab150077, Abcam) and
visualized using ZEISS LSM 510 confocal microscope at a
magnification of ×40.
Statistical analysis
All data are expressed as the mean ± standard
deviation of triplicate dependent experiments. The results were
analyzed using Student's t test or one-way analysis of variance
followed by a Tukey's honest significant difference test. All data
were analyzed using SPSS Statistics 19.0 (IBM Corp., Armonk, NY,
USA) and Graphpad Prism version 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA), as well as Microsoft Excel (version 2010,
Microsoft Corporation, Redmond, WA, USA). P<0.05 was determined
to indicate statistically significant difference.
Results
Effects of βig-h3 on breast carcinoma
cell growth
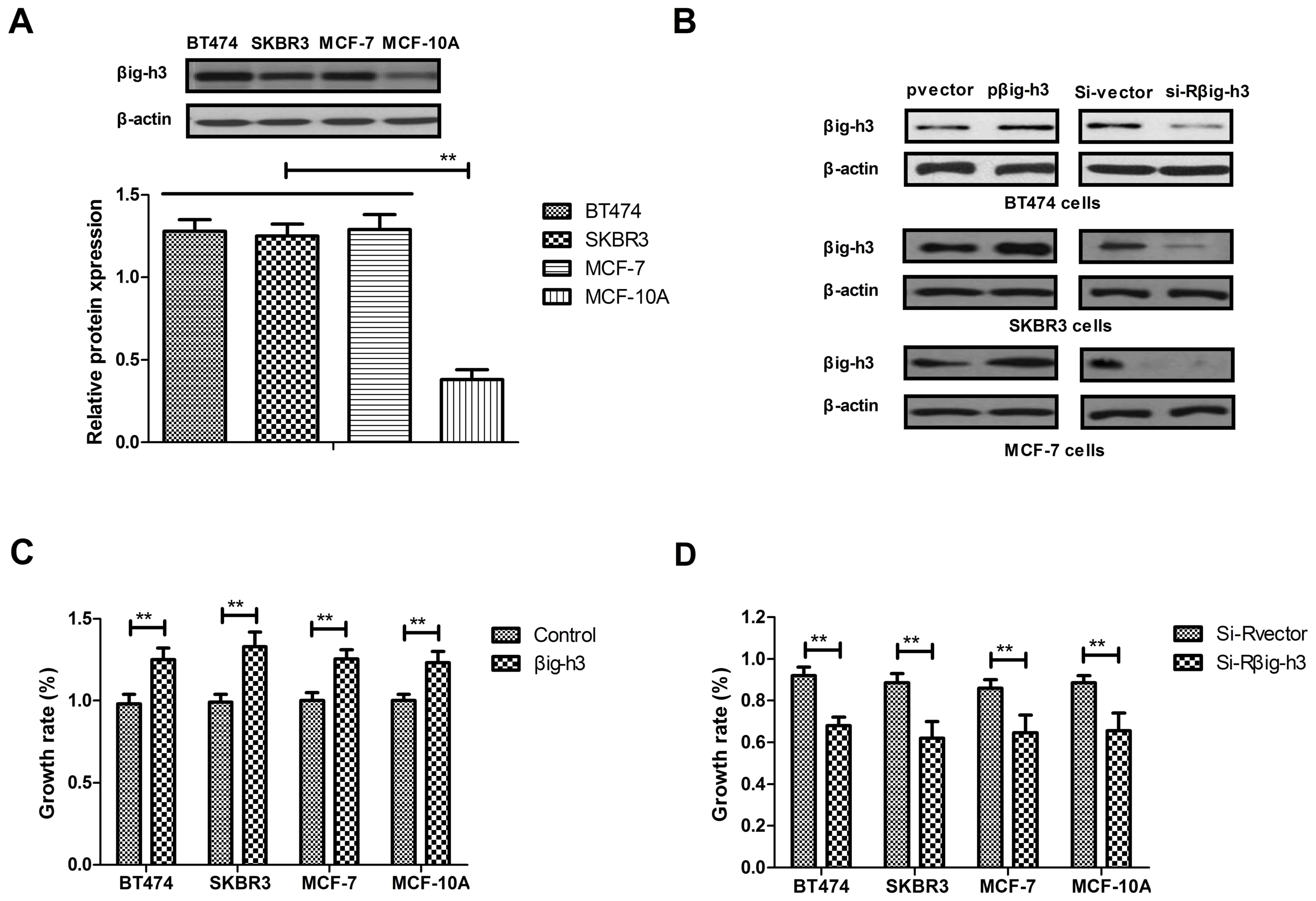
The expression of βig-h3 was analyzed in the breast
cancer cell lines MCF-7, BT474 and SKBR3 and in the normal breast
cell line MCF-10A, which acted as the control. The expression of
βig-h3 was significantly increased in MCF-7, BT474 and SKBR3 cells
compared with MCF-10A cells (Fig.
1A). It was also demonstrated that pβig-h3 promoted βig-h3
expression, while si-Rβig-h3 decreased βig-h3 expression in MCF-7,
BT474 and SKBR3 cells (Fig. 1B). The
results also demonstrated that βig-h3 transfection significantly
promoted the growth of MCF-7, BT474, SKBR3 and MCF-10A cells
compared with their vector transfection controls (Fig. 1C). However, knockdown of βig-h3
expression by si-Rβig-h3 significantly inhibited the growth of
MCF-7, BT474 and SKBR3 cells compared with the controls transfected
with si-R vector (Fig. 1D). These
results suggest that βig-h3 promotes the growth of breast carcinoma
cells.
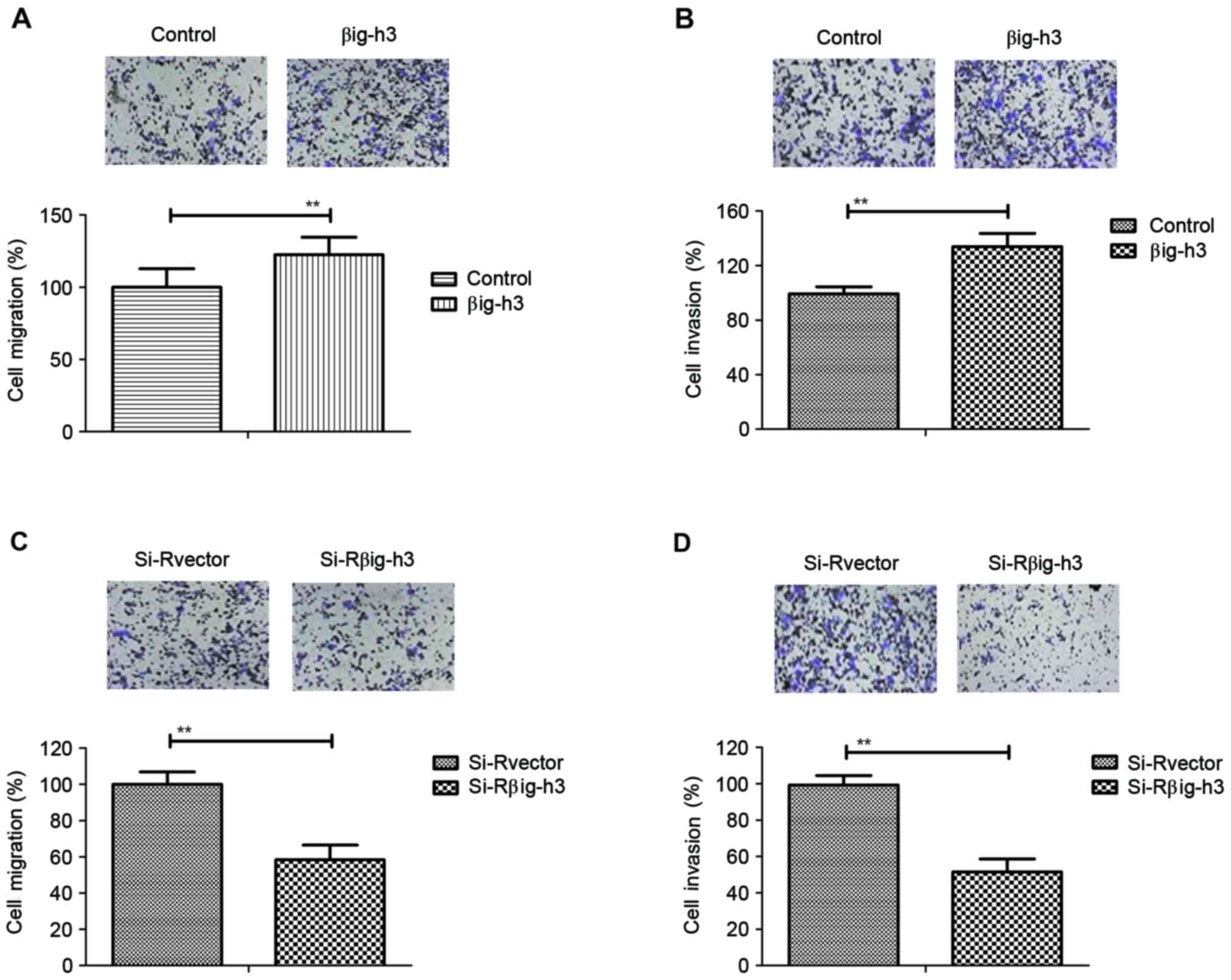
Transfection of βig-h3 promotes the
migration and invasion of breast carcinoma cells
The effects of βig-h3 on the migration and invasion
of breast carcinoma cells were investigated in MCF-7 cells
following transfection. The results demonstrated that the migration
and invasion ability of MCF-7 cells was significantly increased in
cells transfected with pβig-h3 compared with control cells
(Fig. 2A and B). By contrast,
knockdown of βig-h3 by si-Rβig-h3 significantly inhibited the
migration and invasion of MCF-7 cells (Fig. 2C and D). These results suggest that
βig-h3 promotes the migration and invasion of breast carcinoma
cells.
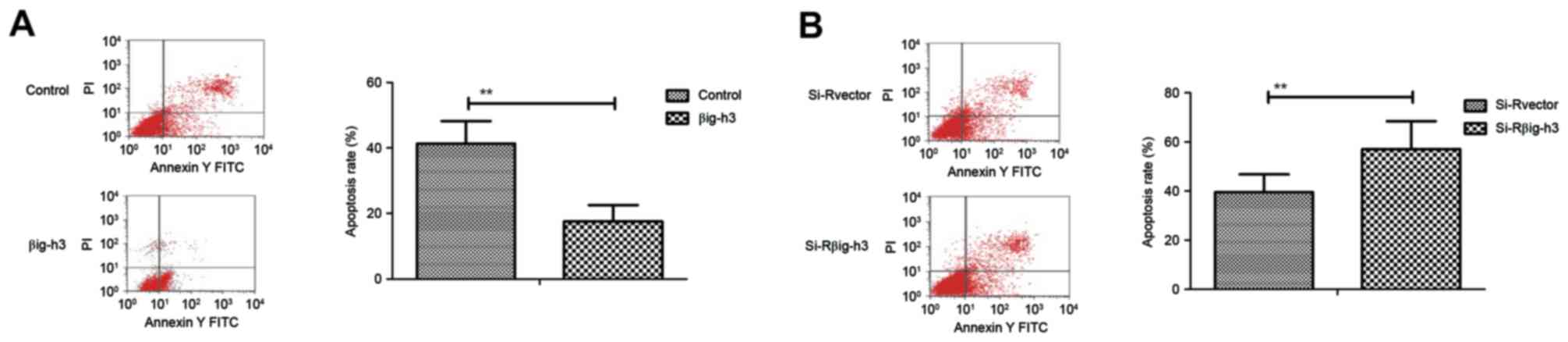
Transfection of βig-h3 inhibits the
apoptosis of breast carcinoma cells induced by cisplatin
Following transfection, the apoptosis of breast
carcinoma cells was analyzed by incubation with cisplatin. The
apoptosis rate of MCF-7 cells was significantly suppressed in cells
transfected with pβig-h3 compared with the control (Fig. 3A). By contrast, knockdown of βig-h3
by si-Rβig-h3 significantly promoted the apoptosis of MCF-7 cells
induced by cisplatin compared with the control (Fig. 3B). These results indicate that βig-h3
expression is associated with the apoptosis of breast carcinoma
cells induced by cisplatin.
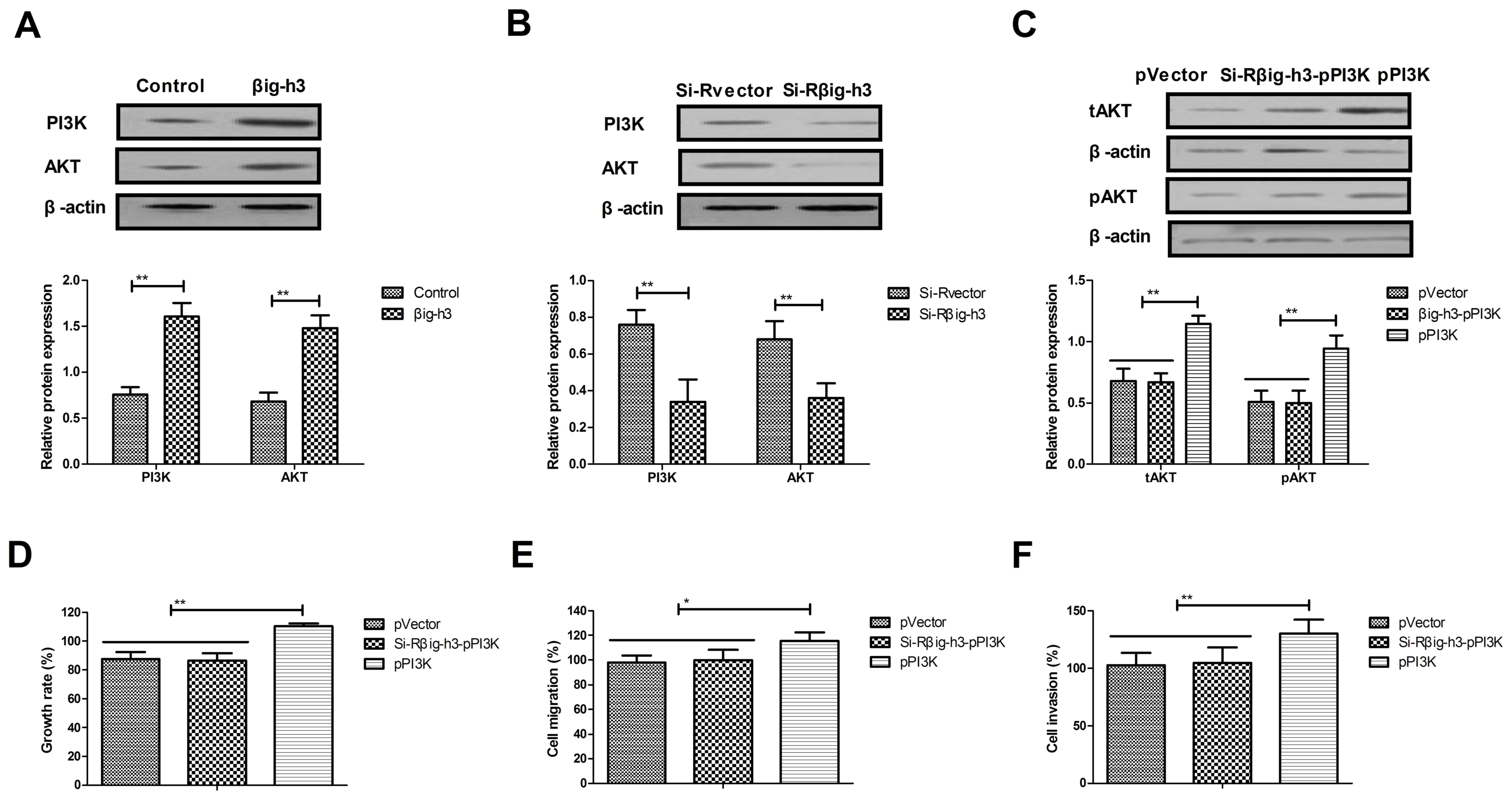
Knockdown of βig-h3 inhibits the
growth and aggressiveness of breast carcinoma cells via the
PI3K/Akt signaling pathway
It was subsequently determined whether βig-h3
promotes the growth, migration and invasion of breast carcinoma
cells via the PI3K/Akt signaling pathway. The results indicated
that the expression of PI3K and Akt was significantly increased in
MCF-7 cells transfected with pβig-h3 compared with controls
(Fig. 4A). By contrast, the
expression of PI3K and Akt was significantly decreased in MCF-7
cells transfected with si-Rβig-h3 compared with the control
(Fig. 4B). The results also
demonstrated that PI3K upregulation reversed the effects of
si-Rβig-h3 on the expression of Akt in MCF-7 cells (Fig. 4C). In addition, PI3K upregulation
reversed the effects of si-Rβig-h3 on the growth, migration and
invasion of MCF-7 cells (Fig. 4D-F).
These data suggest that βig-h3 promotes the growth and metastasis
of breast carcinoma cells via the PI3K/Akt signaling pathway.
Knockdown of βig-h3 suppresses the
formation of breast tumor masses in xenografted mice
The role of βig-h3 in the formation of breast
carcinoma was investigated by implanting βig-h3-upregulated or
βig-h3-knockdown MCF-7 cells into experimental mice. The results
demonstrated that tumor weight in the si-Rβig-h3 group was
significantly decreased compared with the control and pβig-h3
groups, suggesting that βig-h3 knockdown inhibits the formation of
breast tumor masses. By contrast, tumor weight in the pβig-h3 group
was significantly increased compared with the control, indicating
that βig-h3 upregulation promotes the growth of breast tumors
(Fig. 5A). It was also determined
that the metastasis rate was significantly decreased in the
si-Rβig-h3 group compared with the control and pβig-h3 groups,
suggesting that βig-h3 knockdown inhibits breast carcinoma
metastasis compared with the control (Fig. 5B). By contrast, βig-h3-upregulation
significantly promoted breast carcinoma metastasis in the
subcutaneous tissue of experimental mice, suggesting that βig-h3
upregulation stimulates carcinoma metastasis. Immunohistochemistry
analysis indicated that the expression of βig-h3, PI3K and Akt were
markedly increased in βig-h3-overexpressed breast tumors, while
PI3K and Akt expression were markedly decreased in MCF-7 breast
tumors following βig-h3 knockdown (Fig.
5C). The expression of phosphorylated Akt was also
downregulated following βig-h3-knockdown in MCF-7 breast tumors
(Fig. 5D). These results therefore
suggest that βig-h3 knockdown suppresses the formation of breast
tumor masses in vivo.
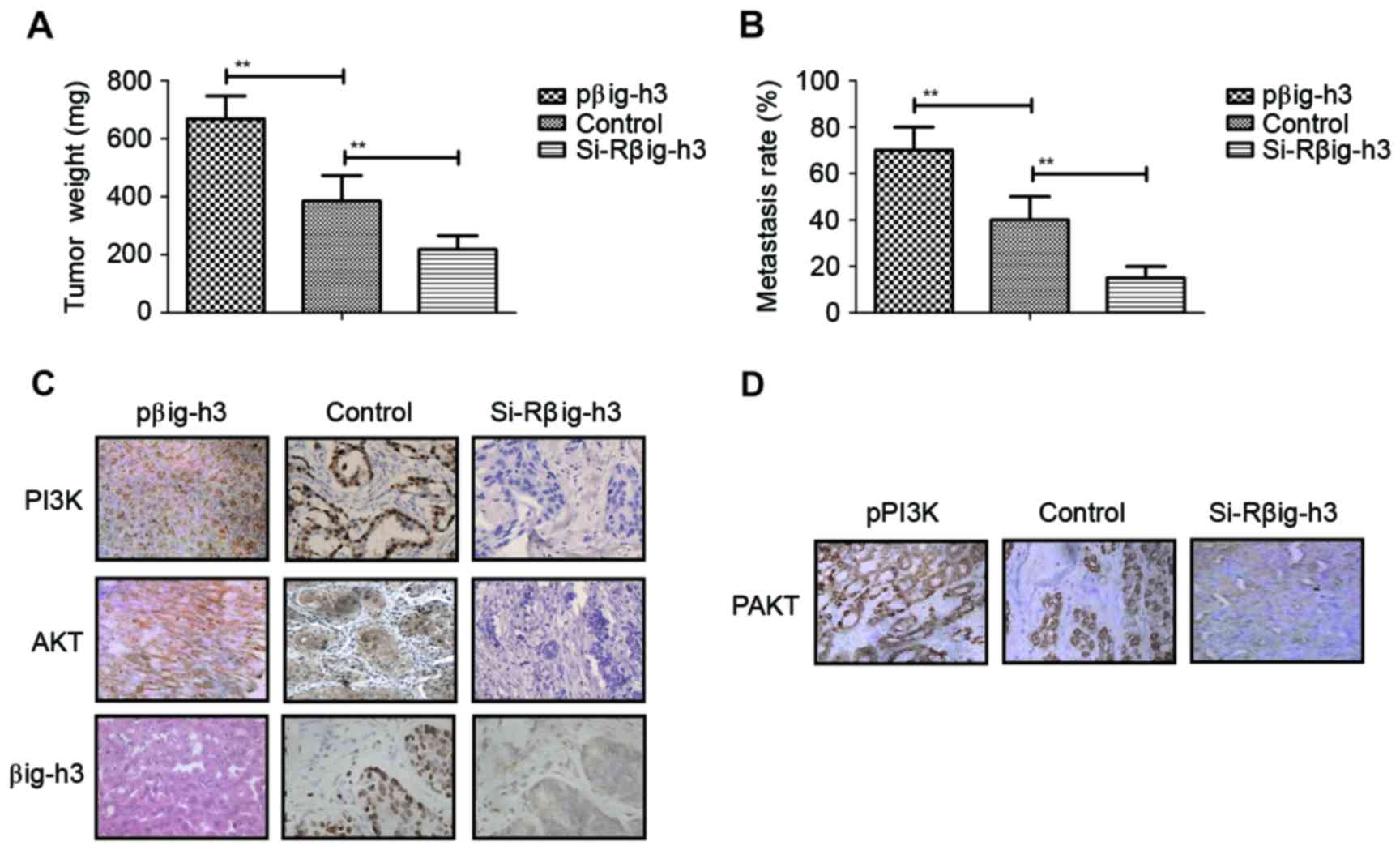 | Figure 5.βig-h3 knockdown suppresses the
formation of breast tumor mass and carcinoma metastasis in
xenografted mice. (A) Tumor weight in the pβig-h3, control and
si-Rβig-h3 groups. (B) The metastasis rate in the pβig-h3, control
and si-Rβig-h3 groups. (C) Expression of βig-h3, PI3K and Akt in
tumor tissues from the pβig-h3, control and si-Rβig-h3 groups, as
determined by immunohistochemistry. (D) Expression of pAkt in tumor
tissues from the pβig-h3, control and si-Rβig-h3 groups, as
determined by immunohistochemistry. Magnification, ×40. Values are
presented as the mean ± standard error of the mean of three
independent experiments performed in triplicate. **P<0.01.
βig-h3, β-inducible gene-h3; PI3K, phosphatidylinositol 3-kinase;
Akt, protein kinase B; si-R, small interfering RNA; p,
phosphorylated. |
Discussion
The number of women that are diagnosed with breast
cancer had increased since 2010, and the majority receive
breast-conserving surgery followed by adjuvant radiotherapy,
chemotherapy and/or comprehensive treatment (20). The results of previous studies have
suggested that increased βig-h3 expression promotes migration and
invasion in different types of human cancer cells, including
intraductal carcinoma cells, breast mucinous adenocarcinoma cells
and medullary breast carcinoma cells (8,12,13).
However, the role of βig-h3 in the progression of human breast
cancer remains unknown. The present study investigated the effects
of βig-h3 on the growth, migration, invasion and apoptosis of human
breast cancer cells. The association between the βig-h3-mediated
PI3K/Akt signaling pathway, and cell growth, invasion and migration
in human breast cancer cells was also assessed. The results
suggested that βig-h3 upregulation promotes the growth and invasion
of breast cancer cells, whereas βig-h3 knockdown inhibits the
growth and invasion of breast cancer cells via downregulation of
the PI3K/Akt signaling pathway, which also decreases the resistance
of breast carcinoma cells to apoptosis following its induction by
cisplatin.
βig-h3 is a protein that serves a role in the
proliferation, migration, apoptosis and differentiation of tumor
cells (14). It has been suggested
that βig-h3 promotes carcinogenesis in different types of cancer
(21). In addition, Kim et al
(22) demonstrated that βig-h3
interacts with α3β1 integrin to promote the adhesion and migration
of human hepatoma cells by activating focal adhesion
kinase-paxillin signaling. Furthermore, βig-h3 promotes the
adhesion, migration and proliferation of gastric cancer cells in
peritoneal carcinomatosis (8,22). Jeong
and Kim (23) demonstrated that
transforming growth factor-β1 enhances βig-h3-mediated
gastrointestinal tract tumorigenesis migration via the
FAK/Akt/Akt1S1/PRS6/EIF4EBP pathways. The results of the present
study indicated that βig-h3 knockdown inhibited the growth,
migration and invasion of breast carcinoma cells via the PI3K/Akt
signaling pathway. In addition, it was indicated that the
upregulation of βig-h3 promotes the growth, migration and invasion
of breast cancer cells and also increases apoptotic resistance in
breast cancer cells.
In conclusion, the results of the current study
indicate that βig-h3 is associated with the growth and metastasis
of breast cancer cells. The data suggest that the inhibition of
PI3K and Akt expression induced by βig-h3 is associated with the
growth, apoptosis and metastasis of breast cancer cells.
Additionally, βig-h3 knockdown inhibits the proliferation and
increases the apoptosis of breast carcinoma cells. These results
imply that the decreased expression of βig-h3 decreases the risk of
metastasis in patients with breast cancer, whereas the increased
expression of βig-h3 may require the administration of intensive
adjuvant chemotherapy to patients. It may therefore be advantageous
to tailor therapy to individual patients, according to levels of
βig-h3 expression, as higher βig-h3 expression seems to increase
the risk of metastasis. The results indicate that βig-h3 is a
potential molecular target for breast carcinoma treatment, which
may contribute to understanding of the mechanism of human breast
carcinoma metastasis.
References
|
1
|
Benson R, Madan R, Julka PK and Rath GK:
Metaplastic carcinoma of breast: A case series of seven patients
from a tertiary care center and review of literature. Gulf J
Oncolog. 1:74–76. 2016.PubMed/NCBI
|
|
2
|
Conlon N, Sadri N, Corben AD and Tan LK:
Acinic cell carcinoma of breast: Morphologic and
immunohistochemical review of a rare breast cancer subtype. Hum
Pathol. 51:16–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Conlon N, Howard J, Catalano J, Gallagher
M, Tan LK and Corben AD: Breast carcinoma in young women: No
evidence of increasing rates of metastatic breast carcinoma in a
single tertiary center review. Breast J. 22:287–292. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ziyadi M, Boujoual M, Raiteb H, Babahabib
MA, Kouach J, Moussaoui DR and Dehayni M: Squamous cell carcinoma
of the breast: Report of a case and review of the literature. Pan
Afr Med J. 24:2132016.PubMed/NCBI
|
|
5
|
Zagelbaum NK, Ward MF II, Okby N and
Karpoff H: Invasive ductal carcinoma of the breast with
osteoclast-like giant cells and clear cell features: A case report
of a novel finding and review of the literature. World J Surg
Oncol. 14:2272016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Modesto A, Gandy C, Mery E, Filleron T,
Massabeau C, Izar F, Charitansky H, Roché H and de Lafontan B:
Breast ductal carcinoma in situ with microinvasion: Pathological
review and clinical implications. Cancer Radiother. 18:107–110.
2014.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Falco G, Buggi F, Sanna PA, Dubini A and
Folli S: Breast metastases from a renal cell carcinoma. A case
report and review of the literature. Int J Surg Case Rep.
5:193–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Z, Miao Z, Jin G, Li X, Li H, Lv Z and
Xu HM: βig-h3 supports gastric cancer cell adhesion, migration and
proliferation in peritoneal carcinomatosis. Mol Med Rep. 6:558–564.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shao G, Berenguer J, Borczuk AC, Powell
CA, Hei TK and Zhao Y: Epigenetic inactivation of Betaig-h3 gene in
human cancer cells. Cancer Res. 66:4566–4573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, El-Gabry M and Hei TK: Loss of
Betaig-h3 protein is frequent in primary lung carcinoma and related
to tumorigenic phenotype in lung cancer cells. Mol Carcinog.
45:84–92. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schneider D, Kleeff J, Berberat PO, Zhu Z,
Korc M, Friess H and Büchler MW: Induction and expression of
betaig-h3 in pancreatic cancer cells. Biochim Biophys Acta.
1588:1–6. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang F, Li XW, Lu WB and Jin JH: betaig-h3
correlates with related factors of peritoneal metastasis of gastric
cancer. J Biol Regul Homeost Agents. 29:181–186. 2015.PubMed/NCBI
|
|
13
|
Ween MP, Oehler MK and Ricciardelli C:
Transforming growth factor-beta-induced protein
(TGFBI)/(betaig-H3): A matrix protein with dual functions in
ovarian cancer. Int J Mol Sci. 13:10461–10477. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma C, Rong Y, Radiloff DR, Datto MB,
Centeno B, Bao S, Cheng AW, Lin F, Jiang S, Yeatman TJ and Wang XF:
Extracellular matrix protein betaig-h3/TGFBI promotes metastasis of
colon cancer by enhancing cell extravasation. Genes Dev.
22:308–321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo L, Wu H, Zhu J, Zhang C, Ma J, Lan J
and Xie X: Genetic variations in the PI3K/AKT pathway predict
platinum-based neoadjuvant chemotherapeutic sensitivity in squamous
cervical cancer. Life Sci. 143:217–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Komeili-Movahhed T, Fouladdel S, Barzegar
E, Atashpour S, Hossein Ghahremani M, Nasser Ostad S, Madjd Z and
Azizi E: PI3K/Akt inhibition and down-regulation of BCRP
re-sensitize MCF7 breast cancer cell line to mitoxantrone
chemotherapy. Iran J Basic Med Sci. 18:472–477. 2015.PubMed/NCBI
|
|
17
|
Zhuang T, Djemil T, Qi P, Magnelli A,
Stephans K, Videtic G and Xia P: Dose calculation differences
between Monte Carlo and pencil beam depend on the tumor locations
and volumes for lung stereotactic body radiation therapy. J Appl
Clin Med Phys. 14:40112013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davey G and Wu Z: Attitudes in China
toward the use of animals in laboratory research. Altern Lab Anim.
35:313–316. 2007.PubMed/NCBI
|
|
19
|
Castillo-Pichardo L, Cubano LA and
Dharmawardhane S: Dietary grape polyphenol resveratrol increases
mammary tumor growth and metastasis in immunocompromised mice. BMC
Complement Altern Med. 13:62013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beck RE, Kim L, Yue NJ, Haffty BG, Khan AJ
and Goyal S: Treatment techniques to reduce cardiac irradiation for
breast cancer patients treated with breast-conserving surgery and
radiation therapy: A review. Front Oncol. 4:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma J, Cui W, He SM, Duan YH, Heng LJ, Wang
L and Gao GD: Human U87 astrocytoma cell invasion induced by
interaction of βig-h3 with integrin α5β1 involves calpain-2. PLoS
One. 7:e372972012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim YH, Kwon HJ and Kim DS: Matrix
metalloproteinase 9 (MMP-9)-dependent processing of βig-h3 protein
regulates cell migration, invasion, and adhesion. J Biol Chem.
287:38957–38969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeong HW and Kim IS: TGF-beta1 enhances
betaig-h3-mediated keratinocyte cell migration through the
alpha3beta1 integrin and PI3K. J Cell Biochem. 92:770–780. 2004.
View Article : Google Scholar : PubMed/NCBI
|