Introduction
Fatigue is defined as a difficulty in the initiation
or sustainment of voluntary activity and is caused by serious
stress and/or intensive physical or mental activity (1). Fatigue can therefore be divided into
two categories: Physical and mental fatigue. Physical fatigue is
the inability of a muscle to maintain normal movement (2), which may induce a heightened stress
response, endocrine dyscrasia, immune dysfunction and organ injury
(3).
It has been demonstrated that metabolic dysfunction,
oxidative stress and lipid peroxidation resulting from excessive
exercise can lead to physical fatigue (4). Additionally, the accumulation of
metabolic by-products, such as lactic acid (LA) and the depletion
of energy resources, including adenosine triphosphate (ATP) and
glycogen, may cause metabolic dysfunction (5). The accumulation of harmful metabolites
is eliminated and the repair of damaged cells occurs following the
consumption of proteins, saccharides and fatty acids post-exercise
(6). However, an imbalance between
levels of reactive oxygen species (ROS) and antioxidants may induce
oxidative stress (7), leading to
lipid peroxidation (8).
Malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione
peroxidase (GSH-Px) levels reflect antioxidant capacity (9,10). It
has been demonstrated that reversing the depletion of energy
resources and inhibiting the generation of free radicals induces
positive effects on anti-fatigue and physical abilities,
respectively (11).
Due to the high prevalence of fatigue, screening for
agents that may prevent it and ensure a rapid recovery has become
an attractive prospect (12).
Various species of fungi and certain herbs may postpone the onset
of fatigue, improve athletic ability and enhance the elimination of
fatigue-related metabolites (13).
The anti-fatigue effects of Tricholoma matsutake, Cordyceps
militaris and Antrodia cinnamomea in murine models have
been examined in previous studies by assessing their effect on
oxidative stress (3,14,15).
Tuber melanosporum (TM), a fungus containing unique
bioactive metabolites, contains important nutrients including
proteins, unsaturated fatty acids, amino acids, sphingolipids,
cerebrosides and polysaccharides (16). It has been demonstrated that TM
exhibits antioxidant and anti-tumor activity in A549, HepG2 and
HL-60 cells (17). The anti-fatigue
effects of TM have not yet been examined; thus it was hypothesized
that its function as an antioxidant may prevent the symptoms of
fatigue. Therefore, the present study assessed the anti-fatigue
effects of TM using a BALB/c mouse model. Energy metabolism, the
balance between pro- and antioxidants and hormone levels in the
mice were assessed to reveal the mechanisms by which TM may improve
endurance during exercise.
Materials and methods
TM preparation
TM fungus was purchased from Senzhong Co., Ltd.
(Yunnan, China) and broken apart using a crushing machine.
Preliminary experiments identified the constituent components of TM
using the phenol-sulfate method (18), bicinchoninic acid protein assay
(19), rutin standard colorimetry
(20) and high performance liquid
chromatography (21). The amount of
polysaccharides, proteins, flavone and adenosine in TM were
determined to be 10.8, 14.2, 0.4 and 0.1%, respectively.
Animals
A total of 96 BALB/c mice (age, 8 weeks; weight,
20–22 g; sex ratio, 48 male and 48 female) were purchased from the
Changchun Institute of Biological Products Co., Ltd. (Jilin, China)
and were maintained under a 12 h light/dark cycle (lights on
between 7:00 a.m. and 7:00 p.m.) at 23±1°C with 50±5% humidity.
Food and water was available ad libitum. All experiments
were performed in a quiet room. The experimental animal protocol
used in the present study was approved by the Animal Ethics
Committee of Jilin University (Jilin, China; approval no. 2017
nsfc0005).
Measurement of anti-fatigue
activity
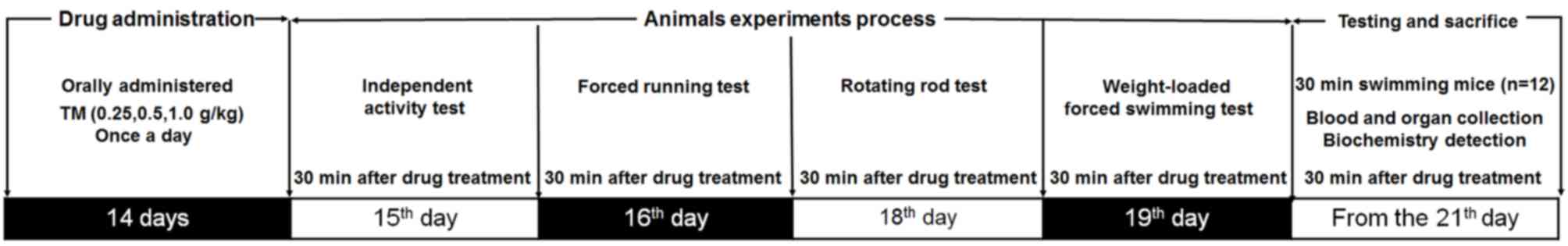
Following 1 week adaptation, mice were randomly
divided into 4 equal groups (n=24; consisting of equal numbers of
males and females). Mice underwent treatment for a total of 21 days
and were either orally treated with physiological saline (control,
0.01 ml/g) or TM at doses of 0.25, 0.5 or 1.0 g/kg once a day.
Doses and administration routes of TM were selected based on
previous studies (14). The
experimental protocol and drug administrations are presented in
Fig. 1.
Independent activity test
On day 15 of treatment, 30 min following TM
administration, the mice were placed in an autonomous chamber
(ZZ-6; Chengdu Techman Software Co., Ltd., Chengdu, China).
Following 2 min adaption time the number of horizontal movements
and vertical movements made by the mice were recorded for 5 min.
The test was repeated three times.
Forced running test
On day 16 of treatment, 30 min following TM
administration, mice were placed on a treadmill (FT-200; Chengdu
Techman Software Co., Ltd.) to assess their endurance capabilities.
Following three preliminary test sessions at a speed of 20 rpm/min
for 1 min, the formal test was performed at the same speed and the
time period until the mice fell off due to muscle fatigue was
measured. Exhaustion time was identified as failure to run for 10
sec following an electric shock (1 mA), which motivates the mice to
run, as previously described (22).
Rotating rod test
On day 18 of treatment, 30 min following TM
administration, mice were placed on a turning device (ZB-200,
Chengdu Techman Software Co., Ltd.) and performed rotating rod
training exercises at a speed of 15 rpm for 1 min, which were
repeated three times. During the formal experiment, the speed was
set up to 20 rpm and the time taken for mice to succumb to muscle
fatigue and fall off the equipment was recorded (3).
Weight loaded forced swimming
test
On day 19 of treatment, 30 min following TM
administration, weights weighing 5% of the mouse's total body
weight were added to each mouse, which were placed into water at a
temperature and depth of 22±1°C and 30 cm, respectively. Exhaustion
time was identified as the failure of mice to return to the water's
surface within 8 sec (23).
Sample collection and parameter
determination
On day 21, mice were forced to swim in water at a
temperature and depth of 22±1°C and 30 cm respectively, for 30 min.
Following a 10 min recess, blood samples were collected from the
caudal veins and the mice were subsequently sacrificed. Following
10 min at room temperature the blood samples were centrifuged at
604 × g for 15 min at room temperature and the upper layer of serum
was collected. Liver and muscle tissue was quickly dissected,
washed in ice-cold physiological saline solution and homogenized in
double distilled water.
The serum levels of LA (cat. no. CK-E93905), lactic
dehydrogenase (LDH; cat. no. CK-E20034), progesterone (PROG; cat.
no. CK-E20376), follicle stimulating hormone (FSH; cat. no.
CK-E20419), estradiol (E2; cat. no. CK-E20381),
testosterone (T; cat. no. CK-E20375) and luteinizing hormone (LH;
cat. no. CK-E20343), alongside the serum, liver and muscular levels
of SOD (cat. no. CK-E20348), GSH-Px (cat. no. CK-E92669) and MDA
(cat. no. CK-E20347) were detected using ELISA according to the
protocols provided by the relevant assay kits (Shanghai Yuanye
Bio-Technology Co. Ltd, Shanghai, China). ATP levels (cat. no.
CK-E93365) in the liver and muscles of mice was also assessed via
ELISA. The concentration of muscle glycogen (MG) and hepatic
glycogen (HG; cat. no. A043) as well as ROS levels (cat. no. E004)
in the liver and muscles of mice were detected using the relevant
assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China) following the manufacturer's protocol.
Statistical analysis
All data were expressed as the mean ± standard error
of the mean. One-way analysis of variance followed by Dunn's
multiple comparisons was used to evaluate differences among groups.
Data were analyzed using SPSS 16.0 software (SPSS, Inc., Chicago,
IL, USA) and P<0.05 was considered to indicate a statistically
significant difference.
Results
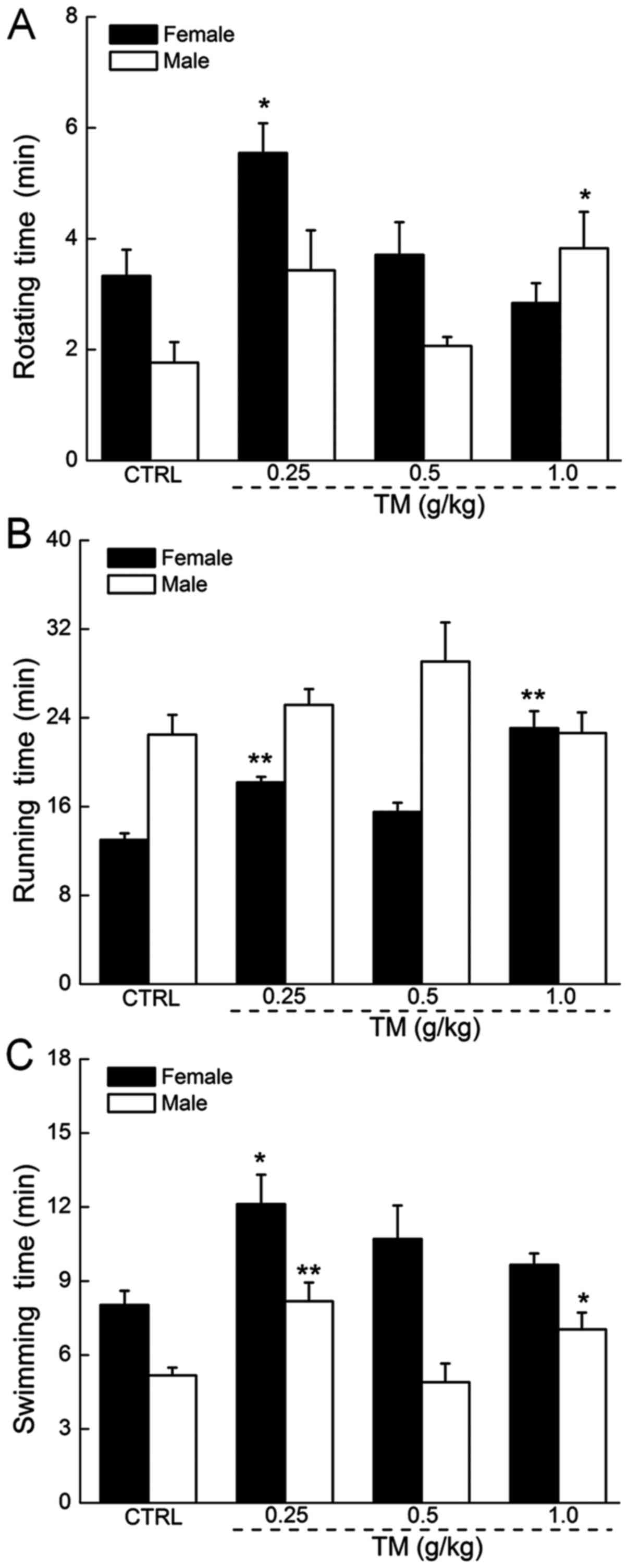
The anti-fatigue effects of TM
TM administration did not significantly affect the
body weights of mice (Table I). In
the rotating rod test, TM at 0.25 g/kg caused a 67% increase in the
rotating time in female mice and TM at 1.0 g/kg caused a 117%
increase in the rotating time in male mice, which were each
significantly increased compared with the control group (P<0.05;
Fig. 2A). In the forced running
test, no significant increase in the exercise times of male mice
was observed following TM treatment. In contrast, TM at a dose of
0.25 and 1.0 g/kg resulted in a 40 and 78% increase in the running
times of female mice, which was significantly increased compared
with the control group (P<0.01; Fig.
2B). Treatment with 0.25 g/kg TM improved the swimming times of
female and male mice by 51 and 58%, respectively, which were
significantly increased compared with the control group (P<0.05
and P<0.01; Fig. 2C).
Additionally, treatment with TM did not influence the horizontal
and vertical movements of female or male mice included in the
independent activity tests (data not shown).
 | Table I.Effects of TM on the weights of
female and male mice. |
Table I.
Effects of TM on the weights of
female and male mice.
|
|
| 14-day drug
treatment |
|---|
|
|
|
|
|---|
| Group | TM dose (g/kg) | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 |
|---|
| Female |
|
|
|
|
|
|
|
|
|
|
CTRL | 0 |
20.2±0.3 |
20.2±0.4 |
19.9±0.3 |
20.1±0.3 |
20.2±0.3 |
20.3±0.3 |
19.9±0.3 |
20.3±0.3 |
| TM
1 | 0.25 |
19.5±0.5 |
20.1±0.4 |
20.2±0.4 |
20.0±0.4 |
19.5±0.4 |
20.3±0.4 |
19.9±0.5 |
20.1±0.4 |
| TM
2 | 0.5 |
20.3±0.5 |
19.7±0.5 |
20.6±0.4 |
20.3±0.4 |
19.9±0.4 |
20.0±0.5 |
19.9±0.5 |
20.2±0.5 |
| TM
3 | 1.0 |
20.5±0.3 |
20.7±0.4 |
20.4±0.4 |
20.3±0.4 |
20.3±0.4 |
20.7±0.4 |
20.3±0.3 |
20.6±0.4 |
| Male |
|
|
|
|
|
|
|
|
|
|
CTRL | 0 |
20.7±0.5 |
20.1±0.5 |
20.2±0.4 |
20.3±0.4 |
20.2±0.3 |
20.3±0.3 |
20.4±0.4 |
20.7±0.4 |
| TM
1 | 0.25 |
20.6±0.5 |
19.3±0.5 |
19.8±0.5 |
19.1±0.5 |
19.3±0.6 |
19.6±0.5 |
19.4±0.5 |
19.9±0.5 |
| TM
2 | 0.5 |
20.0±0.5 |
19.6±0.5 |
20.3±0.5 |
20.1±0.4 |
20.1±0.4 |
19.7±0.6 |
19.8±0.4 |
20.1±0.5 |
| TM
3 | 1.0 |
20.7±0.5 |
20.6±0.4 |
20.8±0.3 |
20.3±0.3 |
20.1±0.4 |
20.6±0.3 |
20.3±0.4 |
20.7±0.3 |
The antioxidant effects of TM in mice
following 30 min exercise
It has been demonstrated that the overproduction of
ROS in vivo causes oxidative damage and deleterious effects
(7). Additionally, excessive
quantities of MDA lead to free radical generation and lipid
peroxidation, which may dysregulate cell function and induce
fatigue (9). Antioxidant enzymes,
including GSH-Px and SOD, serve important roles in preventing
excessive exercise-induced oxidative injury in animals (10). Following 30 min swimming exercise,
female mice treated with TM exhibited reduced serum MDA levels
compared with the control. Male and female mice treated with 0.25
g/kg TM exhibited significantly decreased serum MDA compared with
the control group (P<0.05 and P<0.01; Table II); Treatment with 0.5 g/kg TM
significantly increased the serum levels of SOD and GSH-Px in male
mice (P<0.01 and P<0.001; Table
II), but not female mice (Table
II).
 | Table II.Effects of TM on oxidative
stress-related factors in the serum, liver and muscle of female and
male mice. |
Table II.
Effects of TM on oxidative
stress-related factors in the serum, liver and muscle of female and
male mice.
|
| Female | Male |
|---|
|
|
|
|
|---|
|
|
| TM (g/kg) |
| TM (g/kg) |
|---|
|
|
|
|
|
|
|---|
| Factor | CTRL | 0.25 | 0.5 | 1.0 | CTRL | 0.25 | 0.5 | 1.0 |
|---|
| Serum |
|
|
|
|
|
|
|
|
| MDA
(nmol/ml) |
13.2±0.3 |
11.8±0.2a |
10.8±0.2b |
11.6±0.4a |
11.0±0.3 |
9.6±0.2b |
11.5±0.1 |
10.7±0.1 |
| SOD
(U/ml) |
126.8±4.8 |
129.5±7.8 |
123.9±1.2 |
108.6±3.1 |
156.1±6.3 |
163.5±1.8 |
190.5±6.4b |
191.8±6.5b |
| GSH-Px
(µmol/ml) |
87.5±10.6 |
78.3±6.8 |
75.5±10.2 |
74.2±4.9 |
375.2±4.4 |
374.1±5.9 |
409.7±1.0c |
383.9±3.8 |
| Liver |
|
|
|
|
|
|
|
|
| MDA
(nmol/mgprot) |
3.9±0.1 |
4.2±0.1 |
4.1±0.2 |
3.3±0.1a |
3.7±0.1 |
2.8±0.2b |
3.5±0.1 |
3.9±0.2 |
| ROS
(FI/mgprot) |
21,847±2,465 |
11,175±2,076a |
14,179±912a |
8,758±1,146b |
31,630±1,316 |
21,810±1,072b |
8,523±2,562c |
10,976±972c |
| SOD
(U/mgprot) |
36.8±2.1 |
47.1±1.8a |
41.1±1.4 |
44.1±3.7 |
39.4±0.9 |
40.9±1.1 |
40.1±0.9 |
36.3±1.2 |
| GSH-Px
(µmol/gprot) |
54.2±2.9 |
74.1±3.8b |
80.4±2.7c |
51.2±1.4 |
45.6±1.3 |
43.1±2.1 |
44.3±1.8 |
47.8±1.4 |
| Muscle |
|
|
|
|
|
|
|
|
| MDA
(nmol/mgprot) |
7.6±0.1 |
7.1±0.5 |
6.8±0.5 |
2.8±0.1c |
10.2±0.8 |
6.6±0.5a |
7.8±0.4 |
7.2±0.7a |
| ROS
(FI/gprot) |
444,351±11,198 |
346,146±3,3050a |
417,217±12,991 |
196,190±56,406b |
544,315±33,451 |
402,723±2,9494a |
418,932±4,461 |
324,673±11,594b |
| SOD
(U/mgprot) |
61.7±1.6 |
58.0±3.2 |
57.9±2.6 |
58.9±5.9 |
64.6±2.9 |
68.5±5.3 |
80.5±2.6a |
74.6±6.3a |
| GSH-Px
(µmol/gprot) |
68.9±2.6 |
74.7±2.6 |
71.6±3.8 |
74.9±4.6 |
82.9±5.6 |
98.0±4.8a |
123.5±2.8b |
108.3±5.8a |
The effect of TM treatment on levels of oxidation
factors in the livers of female and male mice following 30 min
exercise was assessed. In female mice, 1.0 g/kg TM significantly
reduced MDA levels in the serum of the liver by 15% compared with
the control mice (P<0.05; Table
II). All doses of TM exhibited significantly reductive effects
on ROS levels in the liver, particularly at 1.0 g/kg, which
resulted in >60% reduction (P<0.05; Table II). Treatment with 0.25 g/kg TM
significantly enhanced SOD and GSH-Px levels in female mice in the
liver (P<0.05 and P<0.01; Table
II). Conversely, in male mice TM 0.25 g/kg demonstrated no
significant effects on the levels of SOD and GSH-Px in the liver
(Table II). Treatment with 0.25
g/kg TM in male mice caused significantly reduced hepatic MDA and
ROS levels (P<0.01; Table II)
compared with the control.
The effect of TM treatment on levels of oxidation
factors in the muscle of male and female mice 30 min after
administration was also examined. Following TM treatment, levels of
MDA (1.0 g/kg TM) and ROS (0.25 and 1.0 g/kg TM) were significantly
reduced in the muscle of female mice compared with control
(P<0.001 and P<0.01; Table
II) SOD and GSH-Px were unaffected in the muscles of female
mice following treatment with all doses of TM. However, male mice
treated with TM, at 1.0 g/kg, exhibited significantly decreased
muscular MDA (P<0.05) and ROS (P<0.01) and significantly
increased SOD and GSH-Px levels, compared with the control
(P<0.05; Table II).
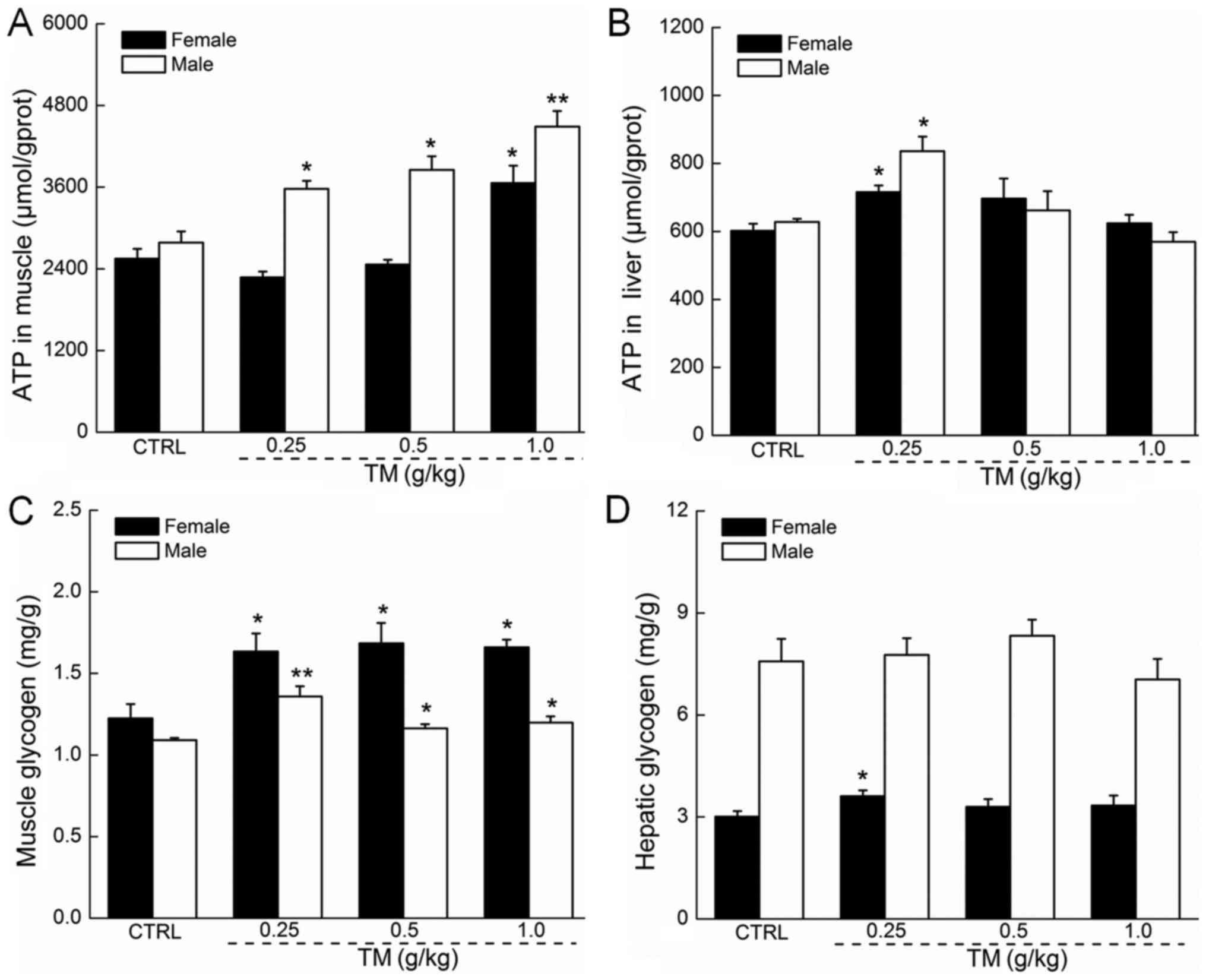
The regulatory effects of TM on ATP
and glycogen in the muscle and liver tissue of mice
The absence of ATP in hepatic and muscular tissue
may cause membrane damage (3).
Following 30 min forced swimming, TM dose-dependently, enhanced the
muscular ATP levels in male mice, which were all significantly
increased compared with the control (P<0.05 and P<0.01;
Fig. 3A). In contrast, only TM at
1.0 g/kg significantly increased the muscular ATP levels in female
mice (P<0.05; Fig. 3A). Compared
with the controls, 0.25 g/kg TM caused a significant 19 and 33%
increase in hepatic ATP levels in female and male mice,
respectively (P<0.05; Fig.
3B).
Glycogen is the predominant source of energy during
exercise, and its depletion may be a primary element for the
development of physical fatigue (24). A shortage of hepatic glycogen may
severely impair nervous function (25). Male and female mice treated with TM
following 30 min forced swimming exhibited significantly increased
MG concentration in all doses, compared with the control (P<0.05
and P<0.01; Fig. 3C). TM
administration did not significantly affect hepatic glycogen levels
in male and female mice. However, female mice treated with TM
exhibited increased hepatic glycogen concentration of ~20% compared
with controls (P<0.05; Fig.
3D).
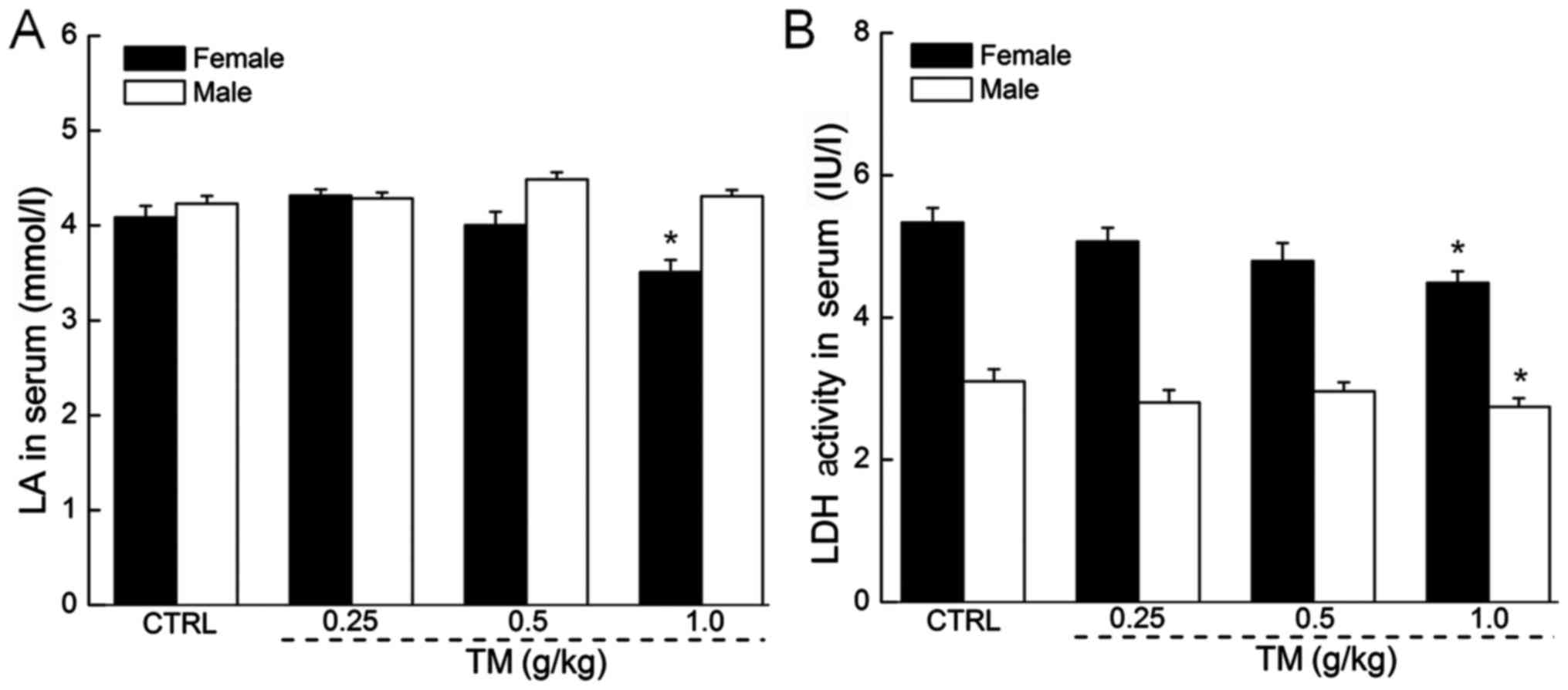
The regulatory effects of TM on LA and
LDH levels in the serum
In anaerobic conditions, LA accumulates and affects
the ability of an individual to maintain intense exercise (26). Only female mice treated with 1.0 g/kg
TM exhibited significantly reduced LA serum levels compared with
the control (3.5 mmol/l vs. 4.1 mmol/l; P<0.05; Fig. 4A). Other doses of TM did not
significantly affect LA serum levels in females. LA serum levels in
males remained unchanged following treatment with all doses of TM.
In addition, excessive quantities of LDH serve as an indicator of
muscle damage (27). Following 30
min exercise, a 14-day treatment of 1.0 g/kg TM reduced serum LDH
levels by 15.9 and 11.4% in female and male mice respectively
compared with the control (both P<0.05; Fig. 4B). Other doses of TM did not
significantly affect LDH levels in male or female mice.
The effects of TM treatment on serum
hormone concentrations
As TM exhibited different efficacies in male and
female mice, its effects on hormone levels were evaluated.
Treatment with 0.25 g/kg TM significantly increased serum PROG, E2
and T in female mice compared with the control group (P<0.05 and
P<0.01; Table III). Treatment
with 0.25 g/kg TM significantly increased serum T (P<0.01) and
FSH (P<0.05; Table III) and 1.0
g/kg TM significantly increased serum PROG, FSH and E2 (P<0.05;
Table III) in male mice compared
with the control group. Female mice treated with 0.5 g/kg of TM
exhibited a 12.2% decrease in serum FSH, while male mice undergoing
the same dose treatment exhibited an increase of 33% (P<0.05;
Table III). Additionally, female
mice treated with 1.0 g/kg of TM exhibited a decrease of 20% of
serum LH (P<0.05; Table III),
however, TM treatment did not significantly affect serum LH levels
in male mice.
 | Table III.Effects of TM on serum hormone levels
of female and male mice. |
Table III.
Effects of TM on serum hormone levels
of female and male mice.
|
| Female | Male |
|---|
|
|
|
|
|---|
|
|
| TM (g/kg) |
| TM (g/kg) |
|---|
|
|
|
|
|
|
|---|
| Hormone | CTRL | 0.25 | 0.5 | 1.0 | CTRL | 0.25 | 0.5 | 1.0 |
|---|
| PROG (ng/ml) |
3.2±0.03 |
4.1±0.2a |
3.4±0.1 |
2.9±0.1 |
9.1±0.2 |
10.1±0.3 |
10.4±0.3a |
10.7±0.3a |
| FSH (mIU/ml) |
37.8±1.5 |
35.6±1.1 |
33.2±1.2a |
34.8±0.7 |
18.1±0.9 |
21.5±0.5a |
24.1±1.3a |
22.9±0.7a |
| E2 (pmol/l) |
65.6±0.9 |
72.1±0.5a |
63.2±1.3 |
65.7±1.3 |
43.8±0.7 |
45.1±0.5 |
46.6±0.3 |
47.3±0.4a |
| T (pg/ml) |
108.5±9.0 |
154.7±3.6b |
128.6±9.6a |
104.3±6.2 |
469.6±11.1 |
531.1±7.7b |
500.4±65.4 |
445.6±18.1 |
| LH (mIU/ml) |
6.6±0.2 |
6.3±0.1 |
4.5±0.3b |
5.3±0.2a |
5.4±0.2 |
5.7±0.08 |
5.8±0.04 |
5.9±0.1 |
Discussion
Excessive energy consumption and the accumulation of
metabolic products as a result of physical and mental stress may
cause irreversible oxidative tissue damage and lead to depressed
immunity, accelerated ageing and cardio-cerebrovascular disease
(28,29). The present study identified the
anti-fatigue properties of TM in a mouse model by performing forced
swimming, rotary rod and exhausted running tests, which are
considered to be valid methods of evaluating the exercise capacity
of mice (30). It was demonstrated
that the same dose of TM had different effects in different tests,
and TM was observed to have a non-dose dependent effect in the
majority of the experiments. TM contains multi-effective
components, which may demonstrate a synergistic effect on exercise
tolerance. This may explain the non-dose dependent effects of
TM.
ATP, the primary rapid and direct energy source for
exercise, is affected by the activities of myofibrillar ATPase
(31). In the present study,
following TM administration and 30 min subsequent swimming
exercise, 0.25 g/kg TM significantly increased hepatic ATP and 1.0
g/kg TM significantly increased muscular ATP in male and female
mice. Glycogen is regarded as an immediate source of energy for the
synthesis of ATP, which is important as ATP possesses an
ultra-short half-life (3). The
quantity of glycogen within tissues may serve as an indicator for
persistent exercise-induced fatigue, as a reduction of glycogen is
observed in these conditions (32).
Following 30 min forced swimming, mice treated with TM exhibited
increased levels of muscular glycogen compared with controls.
Additionally LA, which is primarily produced during anaerobic
glucose metabolism, reduces the pH of blood and muscle, inducing
exercise-induced fatigue and tissue damage (26). Under normal conditions, muscular LDH
catalyzes the mutual transformation of LA and pyruvate (24). High serum LDH levels are also a
marker of muscle damage (27). The
present study demonstrated that TM possesses anti-fatigue
properties, as 1.0 g/kg TM treated female mice exhibited
significantly reduced serum LA and LDH levels.
Oxygen can be transformed into oxygen free radicals
(OFR), which serve important roles in signal transduction and other
physiological processes (14).
Unremitting and strenuous exercise increases energy consumption and
accelerates the accumulation of OFR derivatives, such as ROS,
leading to oxidative stress and muscle fatigue. ROS may combine
with macromolecules, including DNA and proteins, causing lipid
peroxidation and muscle fatigue (33). MDA regulates ATP synthase via the
mitochondrial respiratory chain, which is an indirect indicator of
membrane damage (34). Enzymatic and
non-enzymatic antioxidants exhibit two classic patterns of
endogenous protective mechanisms to prevent oxidative stress
(35). Antioxidant enzymes,
including SOD and GSH-Px, may clear accumulated OFR and associated
metabolites to maintain homeostasis and attenuate the effects of
ROS, thus protecting cellular structures from destruction and
prevent the onset of fatigue (36).
In the present study, TM treated female and male mice exhibited
increased serum, hepatic and muscular SOD and GSH-Px, and decreased
MDA and ROS levels to different degrees depending on the
concentration of TM administered. Antrodia cinnamomea and
Polygonatum alte-lobatum hayata reduce MDA levels and
increase the activity of antioxidant enzymes including SOD and
GSH-Px, thus protecting cellular structures by combating lipid
peroxidation and fatigue (10,15). The
antioxidant properties of TM may therefore be responsible for its
anti-fatigue effects.
In the present study, the serum hormone levels of
mice were assessed, as TM treatment exhibited different degrees of
anti-fatigue activity in males compared with females. The results
indicated that TM regulates FSH and LH levels differently in male
and female mice. It has been demonstrated that TM induces
estrogen-like effects and increases the indices of the ovaries and
uterus [ovaries or uterus indice=ovaries or uterus weight (mg)/body
weight (g)] (37). Long-term and
high-intensity exercise inhibits the secretion of
gonadotropin-releasing hormone from the hypothalamus which, in
turn, inhibits the secretion of LH and FSH from the pituitary
(38). LH and FSH have synergistic
effects on testicular stromal cells and may alleviate the
dysfunction caused by the hypothalamus-pituitary-gonadal axis
(38). However, the
hypothalamic-pituitary-testicular axis can be stimulated by
continuous exercise, resulting in T secretion, which increases
exercise capacity (39). PROG and E2
also reduce muscular fatigue, which may increase performance of
certain physical activities (40).
Based on the aforementioned studies, TM-mediated hormone regulation
may contribute to its anti-fatigue effects, which may also serve to
explain the differences in its effects on male and female mice in
the present study.
The present study did have limitations. TM exhibited
different effects on excise performance in male and female mice.
Although it was observed that TM effectively regulated hormone
levels in male and female mice, the underlying mechanisms for how
it does this are still unclear and this requires further
investigation to explore in more detail.
In conclusion, the results of the present study
indicate that the anti-fatigue effects observed in mice following
TM treatment are primarily caused by the regulation of oxidative
stress, energy metabolism and hormone levels. This suggests that TM
may increase endurance capabilities during exercise, which provides
experimental evidence to support the clinical use of TM as a
functional natural product against fatigue.
Acknowledgements
The present study was supported by The Science and
Technology Key Project in Jilin, China (grant nos. 20150203002NY,
20160520036JH and 20160204029YY) and The Postdoctoral Science
Foundation of China (grant no. 2016M591495).
References
|
1
|
Lamou B, Taiwe GS, Hamadou A, Abene,
Houlray J, Atour MM and Tan P: Antioxidant and antifatigue
properties of the aqueous extract of moringa oleifera in rats
subjected to forced swimming endurance test. Oxid Med Cell Longev.
2016:35178242016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun S, Niu H, Yang T, Lin Q, Luo F and Ma
M: Antioxidant and anti-fatigue activities of egg white peptides
prepared by pepsin digestion. J Sci Food Agric. 94:3195–3200. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Q, Wang Y, Cai G, Kong F, Wang X, Liu
Y, Yang C, Wang D and Teng L: Antifatigue activity of liquid
cultured tricholoma matsutake mycelium partially via regulation of
antioxidant pathway in mouse. Biomed Res Int. 2015:5623452015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chi A, Li H, Kang C, Guo H, Wang Y, Guo F
and Tang L: Anti-fatigue activity of a novel polysaccharide
conjugates from Ziyang green tea. Int J Biol Macromol. 80:566–572.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Q, Jin W, Lv X, Dai P, Ao Y, Wu M,
Deng W and Yu L: Effects of macamides on endurance capacity and
anti-fatigue property in prolonged swimming mice. Pharm Biol.
54:827–834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao YQ, Zeng L, Yang ZS, Huang FF, Ding
GF and Wang B: Anti-fatigue effect by peptide fraction from protein
hydrolysate of croceine croaker (Pseudosciaena crocea) swim bladder
through inhibiting the oxidative reactions including DNA damage.
Mar Drugs. 14:pii: E2212016. View Article : Google Scholar
|
|
7
|
Nam SY, Kim HM and Jeong HJ: Anti-fatigue
effect by active dipeptides of fermented porcine placenta through
inhibiting the inflammatory and oxidative reactions. Biomed
Pharmacother. 84:51–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bao L, Cai X, Wang J, Zhang Y, Sun B and
Li Y: Anti-fatigue effects of small molecule oligopeptides isolated
from panax ginseng C. A. meyer in mice. Nutrients. 8:pii: E8072016.
View Article : Google Scholar
|
|
9
|
Huang S, Lin H and Deng SG: Study of
Anti-fatigue effect in rats of ferrous chelates including hairtail
protein hydrolysates. Nutrients. 7:9860–9871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Horng CT, Huang JK, Wang HY, Huang CC and
Chen FA: Antioxidant and antifatigue activities of Polygonatum
Alte-lobatum Hayata rhizomes in rats. Nutrients. 6:5327–5337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shao JT, Wang MY and Zheng LB: Antifatigue
effect of Gracilaria eucheumoides in mice. Exp Ther Med.
6:1512–1516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miao F, Wu D and Ni G: Evaluation of
Anti-Fatigue Activity of Flavonoids from Tartary Buckwheat in Mice.
African J Trad Complement Alterna Med. 13:522016. View Article : Google Scholar
|
|
13
|
Liu J, Du C, Wang Y and Yu Z: Anti-fatigue
activities of polysaccharides extracted from hericium erinaceus.
Exp Ther Med. 9:483–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song J, Wang Y, Teng M, Cai G, Xu H, Guo
H, Liu Y, Wang D and Teng L: Studies on the antifatigue activities
of cordyceps militaris fruit body extract in mouse model. Evid
Based Complement Alternat Med. 2015:1746162015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Li L, An S, Zhang Y, Feng S, Zhao
L, Teng L and Wang D: Antifatigue effects of antrodia cinnamomea
cultured mycelium via modulation of oxidative stress signaling in a
mouse model. Biomed Res Int. 2017:93740262017.PubMed/NCBI
|
|
16
|
Liu RS and Tang YJ: Tuber melanosporum
fermentation medium optimization by Plackett-Burman design coupled
with Draper-Lin small composite design and desirability function.
Bioresour Technol. 101:3139–3146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao W, Wang XH, Li HM, Wang SH, Chen T,
Yuan ZP and Tang YJ: Isolation and characterization of
polysaccharides with the antitumor activity from tuber fruiting
bodies and fermentation system. Appl Microbiol Biotechnol.
98:1991–2002. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chow PS and Landhausser SM: A method for
routine measurements of total sugar and starch content in woody
plant tissues. Tree Physiol. 24:1129–1136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang SH, Liou JS, Huang BY, Chan WJ and
Tsao YT: Surface characteristics of the 316L stainless steel
modified by ethylene vinyl acetate/chitosan composite films.
Surface Coatings Technol. 320:635–639. 2017. View Article : Google Scholar
|
|
20
|
Zhao C, Zhao X, Zhang J, Zou W, Zhang Y,
Li L and Liu J: Screening of bacillus strains from sun vinegar for
efficient production of flavonoid and phenol. Indian J Microbiol.
56:498–503. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue XF, Zhou JH, Wu LM, Fu LH and Zhao J:
HPLC determination of adenosine in royal jelly. Food Chem.
115:715–719. 2009. View Article : Google Scholar
|
|
22
|
Zhu M, Zhu H, Tan N, Wang H, Chu H and
Zhang C: Central anti-fatigue activity of verbascoside. Neurosci
Lett. 616:75–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen H, He X, Liu Y, Li J, He Q, Zhang C,
Wei B, Zhang Y and Wang J: Extraction, purification and
anti-fatigue activity of γ-aminobutyric acid from mulberry (Morus
alba L.) leaves. Sci Rep. 6:189332016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao HP, Zhang Y, Liu Z, Chen JY, Zhang
SY, Yang XD and Zhou HL: Acute toxicity and anti-fatigue activity
of polysaccharide-rich extract from corn silk. Biomed Pharmacother.
90:686–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cho HD, Lee JH, Jeong JH, Kim JY, Yee ST,
Park SK, Lee MK and Seo KI: Production of novel vinegar having
antioxidant and anti-fatigue activities from Salicornia herbacea L.
J Sci Food Agric. 96:1085–1092. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oh HA, Kim DE, Choi HJ, Kim NJ and Kim DH:
Anti-fatigue effects of 20(S)-protopanaxadiol and
20(S)-protopanaxatriol in mice. Biol Pharm Bull. 38:1415–1419.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong L, Zhao L, Yang F, Yang W, Sun Y and
Hu Q: Evaluation of anti-fatigue property of the extruded product
of cereal grains mixed with Cordyceps militaris on mice. J Int Soc
Sports Nutr. 14:152017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ni W, Gao T, Wang H, Du Y, Li J, Li C, Wei
L and Bi H: Anti-fatigue activity of polysaccharides from the
fruits of four Tibetan plateau indigenous medicinal plants. J
Ethnopharmacol. 150:529–535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye J, Shen C, Huang Y, Zhang X and Xiao M:
Anti-fatigue activity of sea cucumber peptides prepared from
Stichopus japonicus in an endurance swimming rat model. J Sci Food
Agric. 97:4548–4556. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang Q, Miao X, Ju X, Zhu L, Huang C,
Huang T, Zuo X and Gao C: Effects of pulse current on endurance
exercise and its anti-fatigue properties in the hepatic tissue of
trained rats. PLoS One. 8:e750932013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rahman M, Yang DK, Kim GB, Lee SJ and Kim
SJ: Nigella sativa seed extract attenuates the fatigue induced by
exhaustive swimming in rats. Biomed Rep. 6:468–474. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo Z, Lin D, Guo J, Zhang Y and Zheng B:
In vitro antioxidant activity and in vivo anti-fatigue effect of
sea horse (Hippocampus) peptides. Molecules. 22:pii: E4822017.
View Article : Google Scholar
|
|
33
|
Li Y, Liu B, Yang F, Yu Y, Zeng A, Ye T,
Yin W, Xie Y, Fu Z and Zhao C: Lobaplatin induces BGC-823 human
gastric carcinoma cell apoptosis via ROS-mitochondrial apoptotic
pathway and impairs cell migration and invasion. Biomed
Pharmacother. 83:1239–1246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee HI, McGregor RA, Choi MS, Seo KI, Jung
UJ, Yeo J, Kim MJ and Lee MK: Low doses of curcumin protect
alcohol-induced liver damage by modulation of the alcohol metabolic
pathway, CYP2E1 and AMPK. Life Sci. 93:693–699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jing C, Li X, Zhang J and Wang J:
Responses of the antioxidant system in QGY-7701 cells to the
cytotoxicity and apoptosis induced by 1-octyl-3-methylimidazolium
chloride. J Biochem Mol Toxicol. 27:330–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Verma K, Mehta SK and Shekhawat GS: Nitric
oxide (NO) counteracts cadmium induced cytotoxic processes mediated
by reactive oxygen species (ROS) in Brassica juncea: Cross-talk
between ROS, NO and antioxidant responses. Biometals. 26:255–269.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong Y, Wang HY and Zhao ZT: Primary study
on the health value of Tuber indicum. Heibeisheng Kexueyuan Xuebao.
29:68–73. 2012.(In Chinese).
|
|
38
|
Zhao Y, Cao J, Guo X and Zhou H: Effect of
lepidium meyennii on testosterone content' correlated hormones
content and exercise capacity in rats receiving exercise training.
Chin J Exp Trad Med Formul. 20:164–168. 2014.
|
|
39
|
Cavallini G, Caracciolo S, Vitali G,
Modenini F and Biagiotti G: Carnitine versus androgen
administration in the treatment of sexual dysfunction, depressed
mood, and fatigue associated with male aging. Urology. 63:641–646.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schneider BS, Fine JP, Nadolski T and
Tiidus PM: The effects of estradiol and progesterone on
plantarflexor muscle fatigue in ovariectomized mice. Biol Res Nurs.
5:265–275. 2004. View Article : Google Scholar : PubMed/NCBI
|