Introduction
Ischemic cerebrovascular disease can lead to
hemiplegia or even death. According to epidemiological data,
ischemic cerebrovascular disease ranks third in the diseases
causing the death of the elderly, seriously affecting the life
quality and life health of patients (1,2).
Clinically, the ischemic cerebrovascular disease is often treated
with surgery supplemented with drug therapy, and a large number of
studies have shown that patients with ischemic cerebrovascular
disease will suffer from vascular cognitive dysfunction; reducing
the thrombosis via early diagnosis can effectively prevent the
occurrence of vascular cognitive dysfunction (3–5).
Stenting can be effective in the treatment of ischemic
cerebrovascular disease, which is widely used in clinic due to its
definite efficacy and small trauma (6). Stenting for patients with ischemic
cerebrovascular disease may lower the risk of vascular cognitive
dysfunction of patients. However, El Hammi et al (7) found that the cognitive function of
patients with ischemic cerebrovascular disease treated with
stenting still has a certain degree of cognitive dysfunction and
damage to attention network function compared with normal people.
Li and Liu (8) found through
detecting the levels of serum S100B, nerve growth factor (NGF) and
brain-derived neotrophic factor (BDNF) that dexmedetomidine
provides significant improvement on the recovery of cognitive
function of patients receiving epilepsy foci resection, and S100B,
NGF and BDNF are highly sensitive and can accurately predict the
patient's brain damage. The effects of dexmedetomidine on recovery
of cognitive function and attention network function of patients
with ischemic cerebrovascular disease have not been studied.
Therefore, in this study, the cognitive function and attention
network function of patients with ischemic cerebrovascular disease
after operation were studied, expecting to clarify the effects of
dexmedetomidine on the cognitive dysfunction and attention network
function of patients with ischemic cerebrovascular disease, and to
provide a theoretical basis and related guidance for the recovery
of patients with ischemic cerebrovascular disease.
Patients and methods
Patients of the study
The samples in this study were from patients
diagnosed as ischemic cerebrovascular disease by deputy director
and above in Guizhou Provincial People's Hospital from March 2015
to September 2016. Inclusion criteria: 1) patients diagnosed as
ischemic cerebrovascular disease via head CT or MRI; 2) patients
without obvious hemiplegia, with normal language skills; 3)
patients who could complete the cognitive and attention network
function tests; 4) patients who agreed to receive stenting, signed
the informed consent and were willing to participate in this
experimental study. Fifty-eight patients meeting inclusion criteria
were selected, and they were aged 48–73 years, including 31 males
and 27 females. Other wasting diseases were excluded from patients
enrolled, and the clinical and pathological data and complete
treatment program of the above patients in the treatment process
were retained. The study was approved by the Ethics Committee of
Guizhou Provincial People's Hospital.
Experimental grouping
The above patients selected were randomly divided
into control group (n=29) and dexmedetomidine group (n=29). The
dexmedetomidine group was treated with intravenous administration
of 1 µg/kg dexmedetomidine using a micro-injection pump before
induced anesthesia, while the control group was given the same dose
of normal saline. Other operative procedures and treatment regimens
were the same, and the normal volunteers at the same age were
selected in the same period as the normal group; the cognitive
function and attention network function of patients were
analyzed.
Detection of serum S100B and NGF
levels via enzyme-linked immunosorbent assay (ELISA)
The peripheral venous blood was drawn at 3, 7 and 30
days after operation, and 2 ml blood was collected after separation
of serum to detect the levels of serum S100B and NGF strictly
according to the S100B and NGF ELISA kit (Boster Biological
Technology Co. Ltd., Wuhan, China). The remaining serum was stored
at −80°C for subsequent experiments.
Serum BDNF level
Serum specimens stored at −80°C and collected from
each group of patients were thawed and the total protein was
extracted after addition of RIPA lysate (1:1; Beyotime
Biotechnology Co., Ltd., Shanghai, China), the protein content of
each group was detected using the BCA protein quantification kit
(Thermo Fisher Scientific, Waltham, MA, USA), and the same
concentration of loading sample was prepared, followed by
preparation of 10% sodium dodecyl sulfate-polyacrylamide separation
gel and spacer gel and electrophoresis separation. Then the protein
was transferred onto the PVD membrane and sealed in 5% skimmed milk
powder for 1 h. The target bands were cut and incubated with BDNF
and GAPDH antibodies (diluted at 1:1,000; cat. nos. SAB1405514 and
SAB1405848; Sigma) at 4°C overnight. The bands were washed with
Tris-buffered saline Tween (TBST) 3 times (10 min each time), and
the rabbit anti-mouse secondary polyclonal antibody (diluted at
1:5,000; cat. no. SAB3701038; Sigma) were incubated at room
temperature for 2 h. After that, bands were washed with TBST again
3 times (10 min each time). The DAB coloring solution was added in
the dark room for development and fixation, and the gray value of
band was analyzed using the gel imaging system. The BDNF/GAPDH
ratio indicated the expression level of BDNF protein in serum.
Montreal cognitive assessment
(MoCA)
The cognitive function was evaluated via MoCA in an
independent and quiet environment, including visual space and
executive function: 1 point, the patients can describe the profile
of clock, record the number order and position, and identify the
distinction between the hour hand and the minute hand; 0 point, the
patients cannot identify as above. Attention score: 1 point, the
patient can repeat a series of figures said by the experimental
staff; 0 point, the patient cannot repeat; 1 point, the patients
can quickly find the difference of figures between the first and
second time; 0 point, the patients cannot recognize that.
Continuous subtraction (100 minus 6) is performed for a total of 5
times; 3 points, one error or normal; 2 points, 2 or 3 errors; 1
point, 4 errors; 0 point, all errors. Delayed memory score: 1 point
for each of 5 words recalled by the patients.
Attention network test (ANT)
The attention network function was evaluated via ANT
in an independent and quiet environment, including the targeted
network efficiency and executive control network efficiency. The
patients were trained and tested in strict accordance with the
training and testing process of ANT. The patients were stimulated
using the E-Prime 2.0 program; the thumb was placed on the number
key 1 and 3 to determine the direction of target arrow. The
patient's attention network function was evaluated based on the
targeted network efficiency and executive control network
efficiency of patients.
Statistical analysis
The data in this study are presented as mean ±
standard deviation, and SPSS 19.0 software (SPSS Inc., Chicago, IL,
USA) was used for data processing. The t-test was used for
intergroup comparison, while the analysis of variance was used for
comparison among groups. The homogeneity test of variance was
performed; Bonferroni method was used for pairwise comparison if
the variance was homogeneous; otherwise, Welch method was used for
analysis. Dunnett's T3 method was used for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General data analysis
The general data of patients in control group and
dexmedetomidine group are recorded in detail and analyzed (Table I). The age, sex, percentage of
hypertension history, percentage of diabetes mellitus history,
percentage of drinking history and percentage of smoking history
had no statistically significant differences between control group
and dexmedetomidine group (P>0.05).
 | Table I.General data analysis. |
Table I.
General data analysis.
| Characteristics | Control group | Dexmedetomidine
group | P-value |
|---|
| Age (years) | 56.27±8.32 | 59.76±7.57 | >0.05 |
| Sex
(male/female) | 15/14 | 16/13 | >0.05 |
| Hypertension (%) | 68.96 | 72.41 | >0.05 |
| Diabetes mellitus
(%) | 34.48 | 31.03 | >0.05 |
| Drinking (%) | 41.37 | 44.82 | >0.05 |
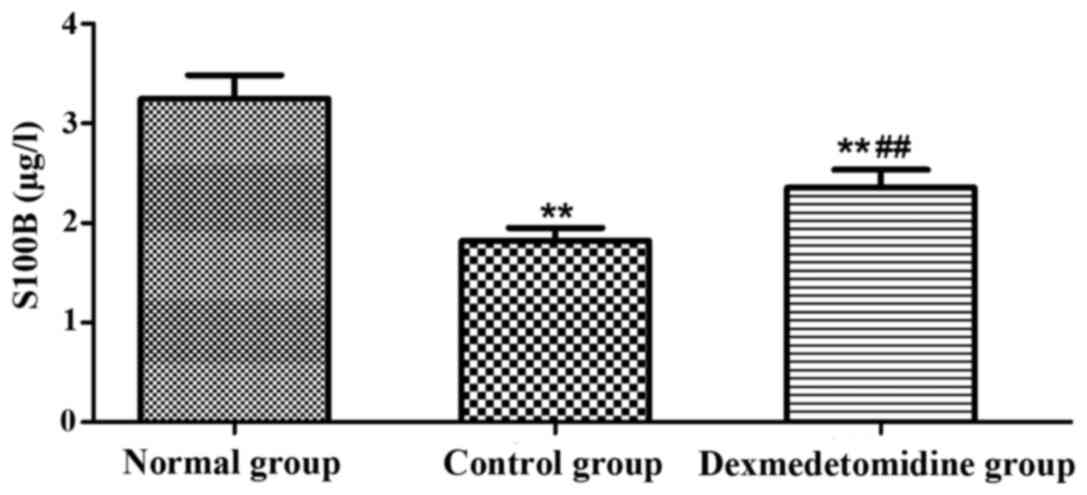
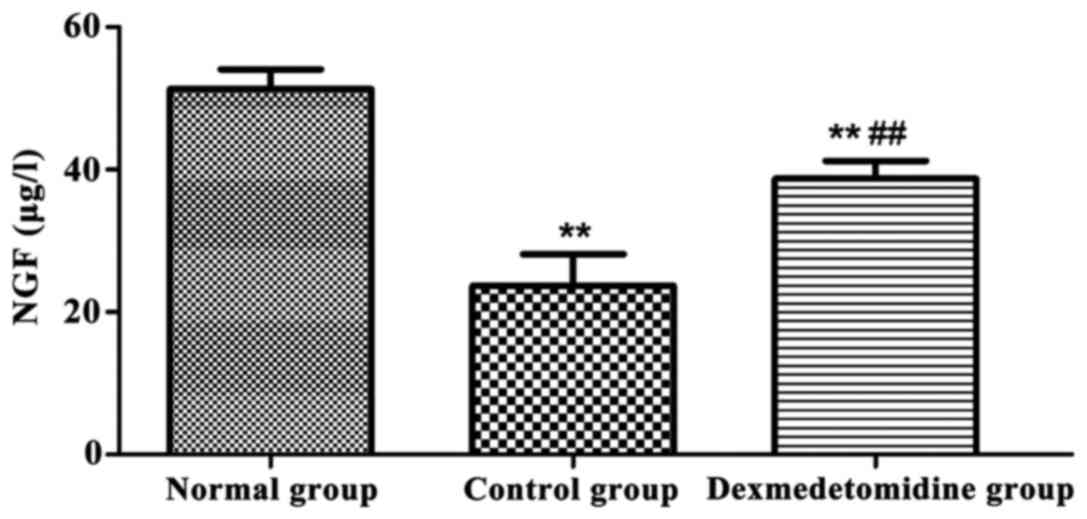
Serum S100B and NGF levels
The levels of serum S100B and NGF in each group were
detected using the ELISA kit (Figs.
1 and 2). Compared with those in
normal group, the levels of serum S100B and NGF in control group
and dexmedetomidine group were significantly decreased (P<0.01),
and they were significantly higher in dexmedetomidine group than
those in control group (P<0.01).
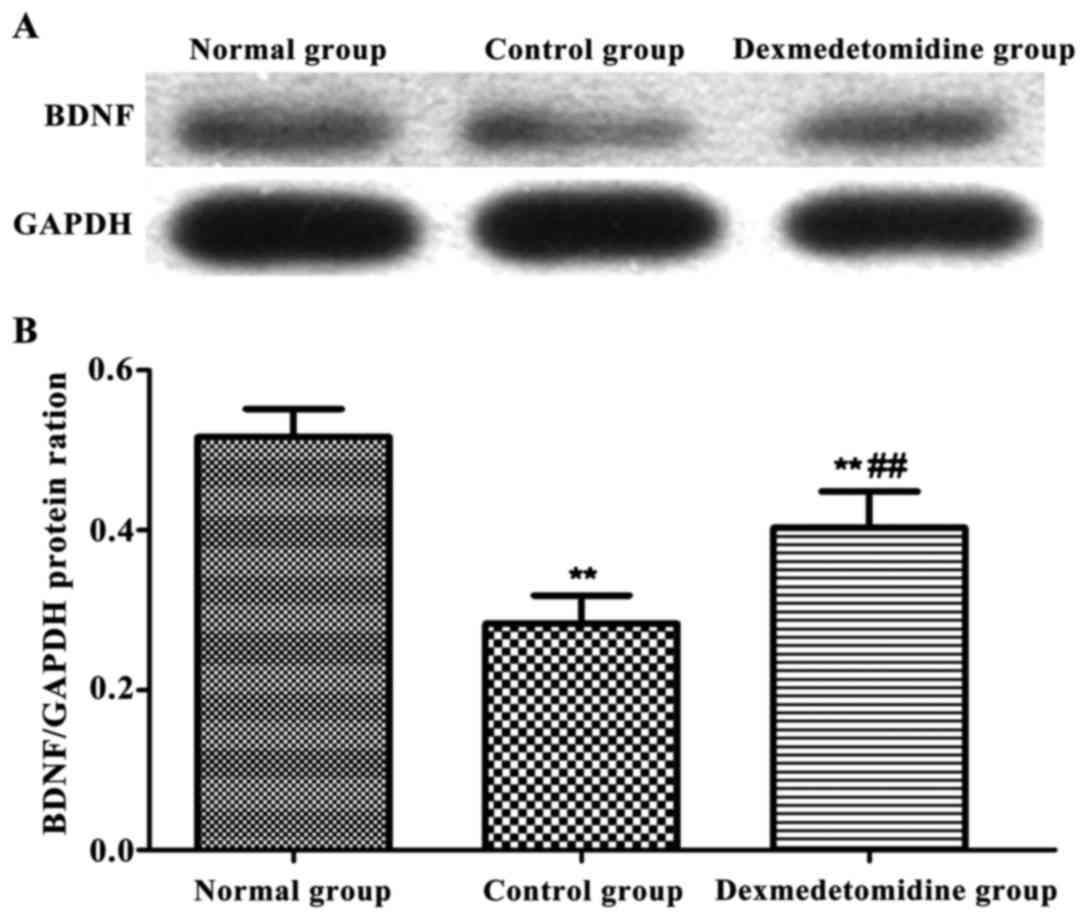
Serum BDNF level
The level of serum BDNF in each group was detected
via western blotting (Fig. 3). The
levels of serum BDNF in control group and dexmedetomidine group
were significantly decreased compared with that in normal group
(P<0.01), and it was higher in dexmedetomidine group than that
in control group (P<0.01).
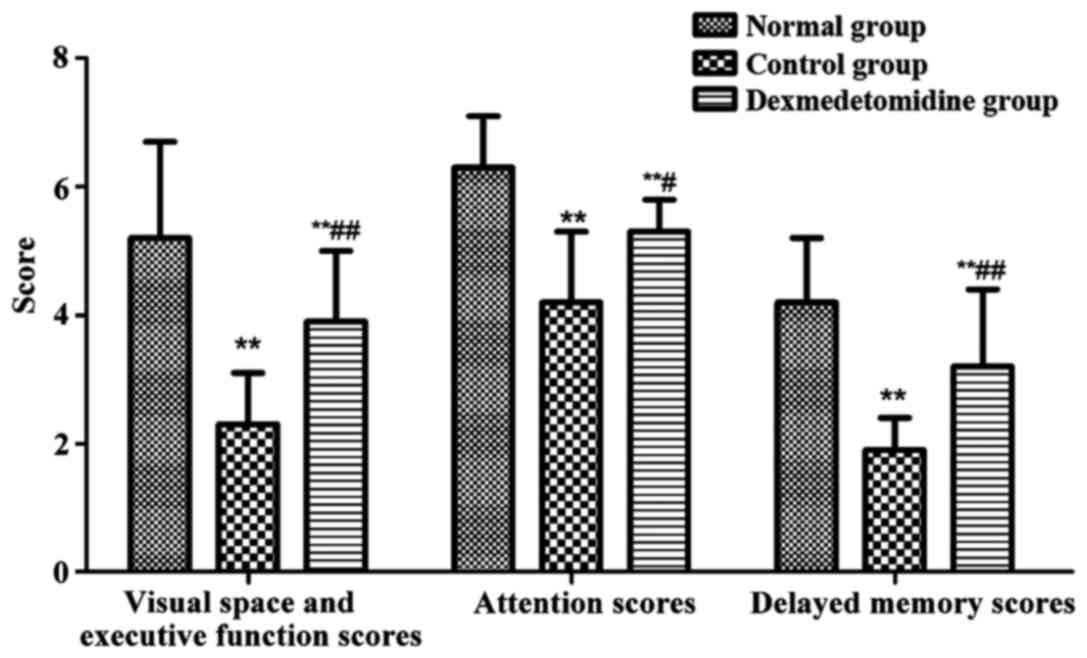
MoCA scores of patients in each
group
MoCA was used to record the visual space and
executive function scores, attention score and delayed memory score
of each group in detail in the experiment, and the cognitive
function of patients was evaluated (Fig.
4). The visual space and executive function scores, attention
scores and delayed memory scores in control group and
dexmedetomidine group were obviously decreased compared with those
in normal group (P<0.01), and they were obviously higher in
dexmedetomidine group than those in control group (P<0.01,
P<0.05).
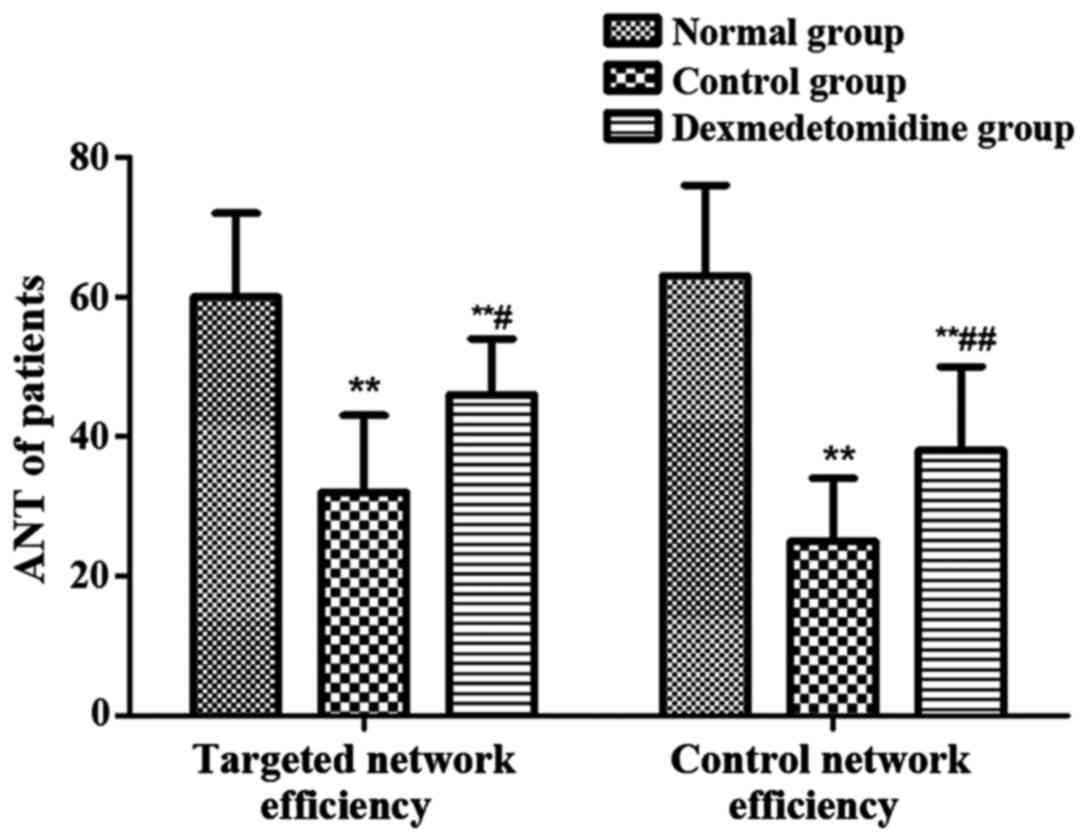
ANT of patients in each group
ANT was used to record the targeted network
efficiency and control network efficiency of each group (Fig. 5). The targeted network efficiency and
control network efficiency in control group and dexmedetomidine
group were obviously decreased compared with those in normal group
(P<0.01), and they were obviously higher in dexmedetomidine
group than those in control group (P<0.01, P<0.05).
Discussion
Patients with ischemic cerebrovascular disease will
suffer from dysfunction or deletions in memory, attention,
perception and feeling, making it impossible for patients to
participate in the normal social activities and daily work and
life, seriously affecting the life quality of patients; the above
symptoms are collectively referred to as cognitive dysfunction
(9). Researchers proposed the
attention network theory by analyzing a large number of research
results of cognitive psychology and brain function imaging, and
evaluated the network function with the targeted network efficiency
and control network efficiency (10,11).
Cognitive function evaluation and attention network function
evaluation are applied in the research on patients with stroke,
Parkinson's disease, epilepsy and Alzheimer's disease (12–14). The
mechanism of cognitive dysfunction and attention network damage in
patients with cerebrovascular disease is not clear, and some
studies have reported that the possible mechanism may be the
vascular inflammation, cerebrovascular injury, environmental
factors and genetic factors (15,16). In
recent years, clinicians and researchers have paid increased
attention to the cognitive dysfunction and attention network
deletion in patients with central nervous system diseases, which is
helpful to recover the patients' cognitive function and attention
network function.
In this study, the cognitive function and attention
network function of patients with ischemic cerebrovascular disease
after stenting were evaluated using the classical MoCA and ANT. The
results showed that patients with ischemic cerebrovascular disease
will suffer from severe cognitive dysfunction and attention network
damage after operation. Yang and Rosenberg (17) found that the cognitive dysfunction
and attention network function damage will be recovered in patients
with carotid atherosclerosis after stenting, but it was found in
this study that the cognitive function and attention network
function of patients with ischemic cerebrovascular disease still
have obvious abnormality compared with normal people even after
stenting.
In this study, patients with ischemic
cerebrovascular disease received intravenous administration of
dexmedetomidine before treatment, and the surgical process and
treatment protocol were the same as those of the control group.
After operation, the serum S100B, NGF and BDNF expression levels in
patients were detected, and the cognitive function and attention
network function were evaluated. The results showed that the levels
of serum S100B, NGF and BDNF in dexmedetomidine group were
significantly higher than those in control group, and the cognitive
function and attention network function were also significantly
superior to those in control group. The above results indicated
that dexmedetomidine has a significant protective effect on
patients with ischemic cerebrovascular disease and contributes to
the recovery of cognitive function and attention network function
of patients with of ischemic cerebrovascular disease. Moreover, the
cognitive function and attention network function are closely
related to the brain tissue structure and brain blood flow
distribution; if the brain tissues are in an ischemia and hypoxia
state for a long time, there will be severe cognitive dysfunction
and attention network function damage (18,19).
Dexmedetomidine is a highly-selective excitatory
center and peripheral α2 receptor drug, which can make the blood
pressure change stable under the stimulation and the blood flow
more stable. Substantial research evidence reveals that
dexmedetomidine has a significant protective effect on brain, which
can significantly reduce the coma time and promote the
postoperative cognitive function recovery of patients with brain
injury (20). Dexmedetomidine can
contract the vessels and produce sedative and analgesic effects.
The latest research data show that dexmedetomidine can affect the
expression levels of inflammatory factors and reduce the
inflammatory response in blood vessels through activating the
corresponding signal pathway (21).
In this study, the protective effect of dexmedetomidine on cerebral
blood vessels may be also manifested as reducing the level of
inflammatory factors in cerebral blood vessels, reducing the level
of inflammation in brain tissues of patients with ischemic
cerebrovascular disease and reducing the damage of inflammation to
the brain tissues, thus improving the cognitive function and
attention network function.
In conclusion, patients with ischemic
cerebrovascular disease will suffer from obvious cognitive
dysfunction and attention network function damage after stenting,
and the preoperative administration of dexmedetomidine can
effectively protect the cerebral blood vessels, improve the
cognitive dysfunction and attention network function damage, and
increase the postoperative life quality of patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ was mainly responsible for the study design and
manuscript writing. QZ contributed to the conception and design of
the study. GW collected and assembled the data. FZ mainly
participated in the collection of the data and the follow-up
management of the patients. QZ reviewed and finalized the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Guizhou Provincial People's Hospital. Signed written informed
consents were obtained from the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wong TS, Liao KF, Lin CM, Lin CL, Chen WC
and Lai SW: Chronic pancreatitis correlates with increased risk of
cerebrovascular disease: A retrospective population-based cohort
study in Taiwan. Medicine (Baltimore). 95:e32662016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang R, Hu Z, Feng Y, Yu L and Li X: The
transcription factor IRF6 co-represses PPARγ-mediated
cytoprotection in ischemic cerebrovascular endothelial cells. Sci
Rep. 7:21502017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Napoli M and McLaughlin B: The
ubiquitin-proteasome system as a drug target in cerebrovascular
disease: Therapeutic potential of proteasome inhibitors. Curr Opin
Investig Drugs. 6:686–699. 2005.PubMed/NCBI
|
|
4
|
Fancellu L, Borsini W, Romani I, Pirisi A,
Deiana GA, Sechi E, Doneddu PE, Rassu AL, Demurtas R, Scarabotto A,
et al: Exploratory screening for Fabry's disease in young adults
with cerebrovascular disorders in northern Sardinia. BMC Neurol.
15:2562015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu X, Ma H, Xu J, Huang H, Wu X, Xiong Y,
Zhan H and Huang F: Elevation in circulating YKL-40 concentration
in patients with cerebrovascular disease. Bosn J Basic Med Sci.
14:120–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Russo C, Jin Z, Liu R, Iwata S, Tugcu A,
Yoshita M, Homma S, Elkind MS, Rundek T, Decarli C, et al: LA
volumes and reservoir function are associated with subclinical
cerebrovascular disease: The CABL (Cardiovascular Abnormalities and
Brain Lesions) study. JACC Cardiovasc Imaging. 6:313–323. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
El Hammi E, Samp J, Rémuzat C, Auray JP,
Lamure M, Aballéa S, Kooli A, Akhras K and Toumi M: Difference of
perceptions and evaluation of cognitive dysfunction in major
depressive disorder patients across psychiatrists internationally.
Ther Adv Psychopharmacol. 4:22–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li Y and Liu S: The effect of
dexmedetomidine on oxidative stress response following cerebral
ischemia-reperfusion in rats and the expression of intracellular
adhesion molecule-1 (ICAM-1) and S100B. Med Sci Monit. 23:867–873.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santos CY, Snyder PJ, Wu WC, Zhang M,
Echeverria A and Alber J: Pathophysiologic relationship between
Alzheimer's disease, cerebrovascular disease, and cardiovascular
risk: A review and synthesis. Alzheimers Dement Amst. 7:69–87.
2017.PubMed/NCBI
|
|
10
|
Francoeur RB: Symptom profiles of
subsyndromal depression in disease clusters of diabetes, excess
weight, and progressive cerebrovascular conditions: A promising new
type of finding from a reliable innovation to estimate exhaustively
specified multiple indicators-multiple causes (MIMIC) models.
Diabetes Metab Syndr Obes. 9:391–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee K, Kim H, Heo JH, Bae HJ, Koh IS and
Chang S: Application of magnetic resonance imaging and magnetic
resonance angiography as diagnostic measures for the first attack
of suspected cerebrovascular diseases in Korea. Yonsei Med J.
52:727–733. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perneczky R, Tene O, Attems J,
Giannakopoulos P, Ikram MA, Federico A, Sarazin M and Middleton LT:
Is the time ripe for new diagnostic criteria of cognitive
impairment due to cerebrovascular disease? Consensus report of the
International Congress on Vascular Dementia working group. BMC Med.
14:1622016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manukhina EB, Downey HF, Shi X and Mallet
RT: Intermittent hypoxia training protects cerebrovascular function
in Alzheimer's disease. Exp Biol Med (Maywood). 241:1351–1363.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daulatzai MA: Pathogenesis of cognitive
dysfunction in patients with obstructive sleep apnea: A hypothesis
with emphasis on the nucleus tractus solitarius. Sleep Disord.
2012:2510962012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Behrouz R, Malek AR and Torbey MT: Small
vessel cerebrovascular disease: The past, present, and future.
Stroke Res Treat. 2012:8391512012.PubMed/NCBI
|
|
16
|
Humpel C: Chronic mild cerebrovascular
dysfunction as a cause for Alzheimer's disease? Exp Gerontol.
46:225–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y and Rosenberg GA: Blood-brain
barrier breakdown in acute and chronic cerebrovascular disease.
Stroke. 42:3323–3328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi H, Xia P, Cui J, Talantova M,
Bodhinathan K, Li W, Saleem S, Holland EA, Tong G, Piña-Crespo J,
et al: Pharmacologically targeted NMDA receptor antagonism by
NitroMemantine for cerebrovascular disease. Sci Rep. 5:147812015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pannarale G, Moroni C, Acconcia MC,
Pannitteri G, Truscelli G, Valente L, Gentile P, Lopreiato F,
Licitra R, Tancredi M, et al: The natural history of
prehypertension. A 20-year follow-up. Eur Rev Med Pharmacol Sci.
21:1329–1334. 2017.PubMed/NCBI
|
|
20
|
Choi IY, Hwang L, Jin JJ, Ko IG, Kim SE,
Shin MS, Shin KM, Kim CJ, Park SW, Han JH, et al: Dexmedetomidine
alleviates cerebral ischemia-induced short-term memory impairment
by inhibiting the expression of apoptosis-related molecules in the
hippocampus of gerbils. Exp Ther Med. 13:107–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng X, Wang H, Xing X, Wang Q and Li W:
Dexmedetomidine protects against transient global cerebral
ischemia/reperfusion induced oxidative stress and inflammation in
diabetic rats. PLoS One. 11:e01516202016. View Article : Google Scholar : PubMed/NCBI
|