Introduction
Vitiligo is characterized by depigmented patches of
skin and is considered to be a depigmentary disorder. It affects
1–2% of the world's population and its incidence is increasing
(1). The precise cause of vitiligo
and its underlying mechanism of action remain unknown, thus the
effective treatment of vitiligo remains challenging. Phototherapy
is widely used as a second-line treatment to treat patients that
fail local or systemic immunosuppressive therapy (2). Although the exact mechanism of action
of vitiligo is poorly understood, continuous therapeutic trials
have indicated that ultraviolet (UV) irradiation is able to promote
the proliferation and availability of melanocytes and therefore
weaken autoimmunity (3). Clinical
trials have indicated that narrow band (NB)-UVB is a therapeutic
option for vitiligo, as it increases the growth and migration of
melanocytes and induces the expression of keratinocytic and
melanocytic cytokines associated with repigmentation (4,5).
However, a previous study by El Mofty et al (6) demonstrated that broad band (BB)-UVA may
be an alternative therapeutic approach to treat vitiligo, as it
results in a marked clinical improvement and induces few side
effects.
Phototherapy irradiation is commonly used to treat
vitiligo; however, UV radiation is considered to be the predominant
factor that causes mutations in the skin. UVA and UVB affect the
skin in different ways. Research into UVA has suggested that it is
predominantly absorbed by cells in the basal layer of the epidermis
(7). UVA frequently induces lesions
via the accumulation of reactive oxygen species (ROS). Following
UVA exposure, intracellular chromophores may generate ROS (8). UVB radiation also increases the
generation of cellular ROS (9). ROS
have a paradoxical effect on vitiligo as they promote
depigmentation and increase pigmentation of the skin (10). The skin of patients with vitiligo
contains high levels of superoxide dismutase (SOD) and low levels
of catalase; this induces the transfer of
H2O2 from keratinocytes to melanocytes. This
transfer of H2O2 is considered to be one of
the mechanisms by which vitiligo is induced (11,12).
Nuclear factor E2-related factor 2 (Nrf2) serves an
essential role in coordinating the transcriptional induction of
common antioxidant enzymes, including SOD, glutathione
S-transferase (GST) and catalase. Nrf2 is a nuclear transcriptional
activator that belongs to the nuclear factor E2 family of typical
leucine zipper proteins (13). Under
normal conditions, Nrf2 binds to kelch-like ECH-associated protein
1 (Keap1) in the cytoplasm and has a high dissociation rate
(14). Following stimulation by ROS,
the Nrf2-Keap1 complex is disrupted and Nrf2 is rapidly
translocated to the nucleus. In the nucleus, Nrf2 is combined with
antioxidant response element (ARE) in a heterodimer that induces
the phase 2 detoxification enzymes and antioxidant proteins
(15). It has been demonstrated that
Nrf2 is important in protecting against ROS and the cellular
expression of Nrf2 may be the primary target in evaluating the
intracellular antioxidant level.
Protease-activated receptor-2 (PAR-2), which belongs
to the PAR family of G protein-coupled receptors (16), is also associated with vitiligo
(17). PAR-2 is activated by
trypsin-like serine proteases and is expressed by almost all cell
types, particularly by keratinocytes (18). It has been demonstrated that PAR-2 is
expressed predominantly in the granular layer of epidermis,
suggesting that PAR-2 may be associated with epidermal mutations
(19). PAR-2 is also associated with
skin inflammation and cellular ROS generation. Increased levels of
PAR-2 expression and distribution have been detected in the
epidermal layers of lesions in atopic dermatitis and rosacea
(20–22). Additionally, the regulation of PAR-2
expression by solar UV irradiation and its role in melanosome
transfer has been determined (23).
However, the association between PAR-2 and UVA/UVB remains to be
elucidated.
During vitiligo treatment, keratinocytes adjacent to
melanocytes contribute to UV-induced skin pigmentation (24,25);
however, the precise functional effects of phototherapy and
melanocytes on keratinocytes remain unknown. Therefore, the aim of
the present study was to investigate the effects induced by
clinical doses of BB-UVA, NB-UVB and melanocytes on human
keratinocytes in vitro. The proliferation and expression of
PAR-2 and Nrf2, and the lipid peroxidation and intracellular
antioxidant levels in HaCaT cells were analyzed to evaluate these
effects.
Materials and methods
Cell culture
HaCaT human immortalized keratinocyte cells and A375
human melanoma cells used in the present study were obtained from
the American Type Culture Collection (Manassas, VA, USA). HaCaT and
A375 cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Hyclone, Logan, UT, USA) and minimum essential medium (MEM;
Hyclone), respectively, supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
U/ml penicillin and 100 µg/ml streptomycin. In addition, a
co-culture of HaCaT and A375 cells was also established, with an
initial seeding ratio of 3:1. Co-cultured cells were maintained in
culture dish with 3:1 DMEM to MEM (supplemented with 10% FBS, 100
U/ml penicillin and 100 µg/ml streptomycin). Cultures were
maintained at 37°C in a humidified atmosphere containing 5%
CO2.
BB-UVA and NB-UVB irradiation
Prior to irradiation, HaCaT cells were rinsed with
PBS to avoid toxicity induced by UV exposure of the culture medium
compounds. BB-UVA/NB-UVB irradiation was subsequently performed.
The lid of the culture dish was replaced by a quartz plate and
HaCaT cells were exposed to BB-UVA radiation at doses of 1, 5 or 10
J/cm2 with an emission centered at 365 nm or NB-UVB
radiation at doses of 100, 200 or 400 mJ/cm2 with an
emission centered at 311 nm. Emissions were based on the results of
a previous study (26). Cells
without any treatment were used as a negative control. Sigma SS-02
and SS-01 fluorescent lamps (Shanghai Sigma High-Tech Co., Ltd.,
Shanghai, China) were used as sources of UVA and UVB, respectively.
Following irradiation, PBS was removed and HaCaT cells were
maintained in DMEM culture medium at 37°C and in 5% CO2.
Cell proliferation was analyzed at 0, 12, 24 and 48 h following UV
irradiation. mRNA expression, lipid peroxidation and antioxidant
levels were assessed at 48 h following UV irradiation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total mRNA from HaCaT and co-cultured cells was
isolated using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. cDNA was synthesized from 1 µg RNA using a RevertAid
First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc., Pittsburgh, PA, USA). To evaluate the expression
of PAR-2 and Nrf-2 in cells, qPCR was performed in an ABI-7300
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using a SYBR-Green PCR kit (Fermentas; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were 10 min at
95°C, 40 cycles of 15 sec at 95°C and 45 sec at 60°C, followed by 1
min at 60°C, 15 sec at 95°C and 15 sec at 60°C. The primer
sequences used were as follows: PAR-2, forward
5′-TGGCACCATCCAAGGAAC-3′ and reverse 5′-GGCAAACCCACCACAAAC-3′;
Nrf-2, forward 5′-CAAGTCCAGAAGCCAAAC-3′ and reverse
5′-GATGCTGCTGAAGGAATC-3′; and GAPDH, forward
5′-AATCCCATCACCATCTTC-3′ and reverse 5′-AGGCTGTTGTCATACTTC-3′.
Experiments were repeated three times and GAPDH expression was used
as an internal control. Gene expression was calculated using the
2−∆∆Cq method (27).
Cell proliferation
HaCaT and co-cultured cells were seeded into 96-well
plates to evaluate cell proliferation. Following UVB irradiation,
the effect of UVB exposure on cell proliferation was examined using
the Cell Counting Kit-8 (CCK-8; 7 Sea Biotech, Shanghai, China) at
0, 12, 24 and 48 h according to the manufacturer's protocol.
Briefly, 10 µl CCK-8 was added to each well and cells were
maintained in the dark at 37°C and 5% CO2 for 2 h.
Absorbance was measured at a wavelength of 450 nm to calculate
relative proliferation.
Lipid peroxidation assay
Lipid peroxidation was assessed using a
thiobarbituric acid (TBA) reactive substances assay (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) (28). Malondialdehyde (MDA), which is a
product of lipid oxidative degradation, reacts with TBA, yielding
red complexes that are absorbent at 532 nm. HaCaT cells and
co-cultured cells were washed twice with PBS, incubated with TBA
(2.8% w/v) at 95°C for 40 min and centrifuged at 4°C, 2,500 × g for
10 min. The relative total protein was determined by bicinchoninic
acid (BCA) assay following the manufacturer's protocol (cat. no.
A045-4; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). The amount of reactive complexes was measured using a
spectrophotometer at 532 nm.
Antioxidant level assay
SOD, total antioxidant capacity (TOAC), and protein
content were measured in cells using SOD, TOAC and BCA kits (cat.
nos. A003-1, A015 and A045-4, respectively; Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's
protocols. At 24 h following UVB/UVA irradiation and A375
co-culture, HaCaT cells were homogenized in PBS (pH 7.4) and
centrifuged at 4°C and 2,500 × g for 10 min. The resulting
supernatant was used for the subsequent assays. A xanthine oxidase
assay was used to measure SOD activity detected at 450 nm using a
microplate reader, which monitors the inhibition of reducing the
nitro blue tetrazolium in samples. TOAC was analyzed using the
ferric reducing-antioxidant power method and measured at 520 nm
with the spectrophotometer. Protein content was analyzed using a
BCA assay; absorbance was monitored at 562 nm using a microplate
reader.
Statistical analysis
At least three independent duplicates were performed
for each experiment. Statistical analysis was performed by GraphPad
Prism software, version 5 (GraphPad Software, Inc., La Jolla, CA,
USA) using one-way analysis of variance followed by Tukey's
post-hoc test. Data are presented as the mean ± standard deviation
and P<0.05 was considered to indicate a statistically
significant difference.
Results
Proliferation analysis
To determine the effect of UVA and UVB radiation,
and melanocytes on keratinocytes, HaCaT cells were exposed to 1, 5
or 10 J/cm2 BB-UVA, or 100, 200 or 400 mJ/cm2
NB-UVB, and co-cultured with A375 cells. Cell proliferation was
measured at 0, 12, 24 and 48 h following UV irradiation and
co-culture, according to the aforementioned procedure. As presented
in Fig. 1, a significant inhibition
in HaCaT cell proliferation compared with control cells was induced
by UV irradiation (P<0.01), apart from by low dose UVA.
Furthermore, the inhibition of cellular proliferation took place
progressively over a 48 h period. The most significant decrease in
the proliferation index was observed 24 h following UVA exposure
(P<0.01; Fig. 1A), whereas, the
decrease in the proliferation index following UVB radiation was
greatest at 48 h (P<0.01; Fig.
1B). Co-culture with A375 cells induced a significant increase
in cell proliferation at 48 h (P<0.05; Fig. 1C).
RT-qPCR assessing PAR-2 expression in
UVA/UVB/A375-treated HaCaT cells
The keratinocyte receptor PAR-2 is a key molecule
associated with inflammation and pigment transfer. In the present
study, PAR-2 mRNA expression in UVA/UVB/A375-treated keratinocytes
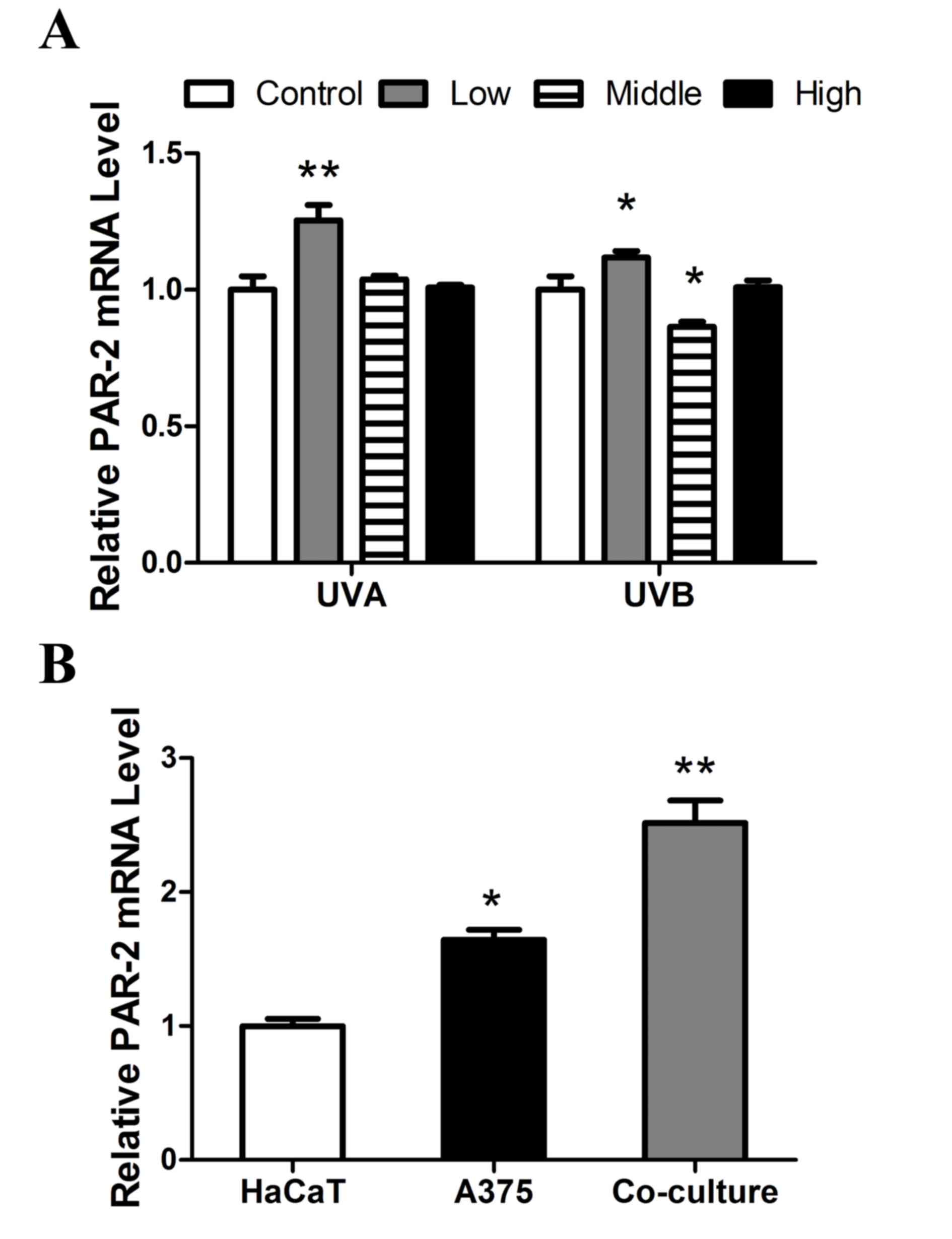
was determined using RT-qPCR. As presented in Fig. 2A, PAR-2 expression was significantly
upregulated following low dose UVA (P<0.01) and UVB irradiation
(P<0.05). A marked decrease in PAR-2 mRNA expression was
observed following exposure to medium dose UVA and UVB radiation
compared with low dose treatment and the expression of PAR-2 was
significantly decreased following exposure to medium dose UVB
compared with the control (P<0.05). High dose UVA and UVB
radiation treatment had no significant impact on PAR-2 mRNA
expression. PAR-2 mRNA expression in co-cultured HaCaT and A375
cells was significantly increased compared with HaCaT cells
cultured alone (P<0.01; Fig.
2B).
Expression of Nrf2 in HaCaT cells
treated with UVA/UVB/A375
Nrf2 serves a key role in anti-inflammatory and
antioxidant response of cells to UV irradiation. To determine the
effect of UVA/UVB/A375 treatment on the expression of Nrf2 mRNA,
RT-qPCR was performed. The expression of Nrf2 mRNA was
significantly decreased compared with the control following low
dose UVB (P<0.05), whereas no significant change was observed
following low dose UVA exposure (Fig.
3A). However, following medium dose irradiation, UVA and UVB
significantly elevated Nrf2 expression compared with the controls
(each, P<0.01). Furthermore, a significant increase in Nrf2
expression was observed in cells following high dose UVA
irradiation, whereas there was no difference in Nrf2 expression
treatment following high dose UVB treatment compared with the
control. As presented in Fig. 3B,
the expression of Nrf2 mRNA in co-culture cells was significantly
inhibited, compared with HaCaT cells. This indicates that A375 may
inhibit Nrf2 expression in HaCaT.
Lipid peroxidation assay in
UVA/UVB/A375-treated HaCaT cells
It has been demonstrated that lipid peroxidation may
induce the breakdown of cell membranes and cell death (29). MDA is a key indicator of lipid
peroxidation; therefore the concentration of intracellular and
supernatant MDA was examined 24 h after the exposure of HaCaT cells
to different doses of UVA or UVB irradiation, or following
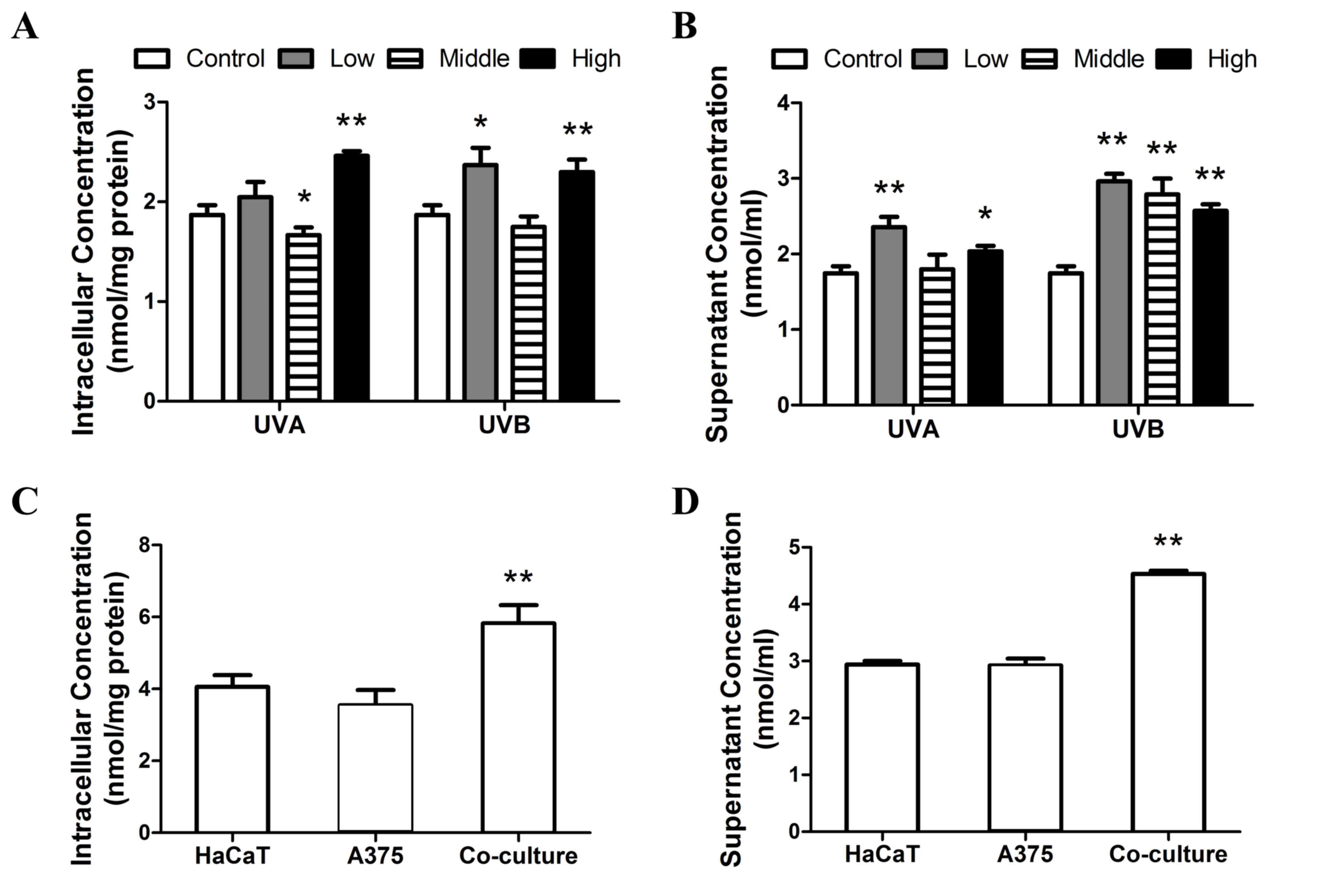
co-culture with A375 cells. As presented in Fig. 4A, similar responses in intracellular
MDA were detected following exposure to UVA and UVB. Following
treatment with high doses of UVA and UVB, the concentration of
cellular MDA increased significantly (P<0.01). However, MDA
levels were significantly inhibited compared with controls
following treatment with medium doses of UVA (P<0.05). MDA
levels were significantly increased following low dose UVB
treatment (P<0.05). As presented in Fig. 4B, all doses of UVB irradiation, as
well as low and high doses of UVA irradiation significant increased
the concentration of supernatant MDA compared with controls
(P<0.05). However, medium doses of UVA radiation did not
increase supernatant MDA levels (Fig.
4B). Furthermore, the concentration of intracellular and
supernatant MDA in HaCaT cells co-cultured with A375 cells was
significantly higher than in HaCaT cells (P<0.01; Fig. 4C and D). These results indicate that
medium doses of UVA or UVB treatment did not affect lipid
peroxidation, whereas low and high dose irradiation and A375
co-culture increased lipid peroxidation levels.
Antioxidant levels in
UVA/UVB/A375-treated HaCaT cells
Antioxidants serve an essential role in balancing
the production of ROS by mitochondria. To detect the effect of UVA
or UVB radiation, or A375 co-culture on HaCaT cellular antioxidant
level, intracellular SOD activity and TOAC levels were measured
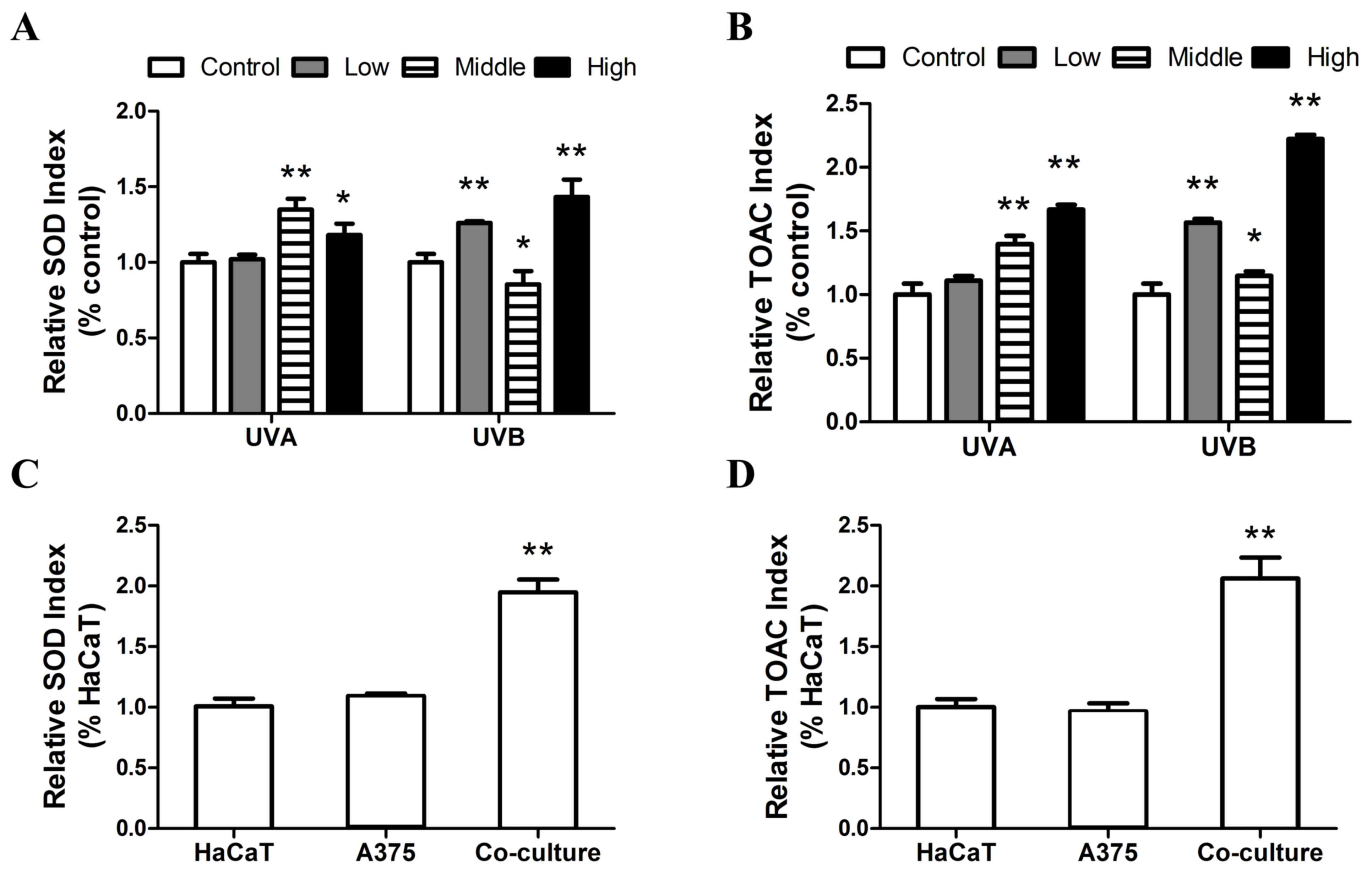
following treatment. Notably, a significant increase in SOD
activity was observed following exposure to medium and high doses
of UVA radiation (P<0.05), with a non-significant increase
detected following treatment with low doses of UVA. A significant
increase in SOD activity was observed following treatment with low
and high doses of UVB, however, there was a significant decrease in
SOD activity following treatment with medium doses of SOD (Fig. 5A). Additionally, the data presented
in Fig. 5B indicate that there was a
dose-dependent increase in TOAC levels following UVA irradiation,
with significant differences compared with controls at medium and
high doses (P<0.05). Furthermore, the change in TOAC levels
following UVB exposure exhibited an analogous trend to that of SOD
activity.
Following co-culture with A375 cells, cellular SOD
activity and TOAC levels in HaCaT cells significantly increased
(P<0.01; Fig. 5C and D). A 93.5
and 106.15% increase were observed in SOD activity and TOAC levels
compared with sole HaCaT cells, respectively (Fig. 5C and D). The results of the present
study are summarized in Table I.
 | Table I.Effects of UVA and UVB irradiation,
and A375 co-culture on keratinocytes. |
Table I.
Effects of UVA and UVB irradiation,
and A375 co-culture on keratinocytes.
|
| UVA | UVB |
|
|---|
|
|
|
|
|
|---|
| Relative
factors | Low | Med | High | Low | Med | High | Co-culture
A375 |
|---|
| Proliferation | 0 | − | − | − | − | − | + |
| PAR-2 | + | 0 | 0 | + | − | 0 | + |
| Nrf2 | 0 | + | + | − | + | 0 | − |
| Lipid peroxidation
(supernatant) | + | 0 | + | + | + | + | + |
| SOD | 0 | + | + | + | − | + | + |
| TOAC | 0 | + | + | + | − | + | + |
Discussion
UV irradiation is the ‘gold standard’ of therapies
to treat patients with vitiligo. Phototherapy has been used to
treat vitiligo since the 1800s and NB-UVB is the most frequently
used method (30). It has been
demonstrated that BB-UVA may be an alternative treatment method
(6). Melanocytes are important in
therapy for vitiligo and may be affected by various factors,
including UV light, oxidation and keratinocytes (31,32). The
association between melanocytes and keratinocytes is essential
during the pathogenesis of vitiligo; however, few studies have
focused on the effects of melanocytes on healthy keratinocytes. In
the present study, keratinocyte HaCaT cells were treated with
different clinical doses of BB-UVA or NB-UVB radiation and
co-cultured with melanocyte A375 cells. The expression of PAR-2,
Nrf2 and cellular antioxidant levels were examined to evaluate the
effects of UV light and melanocytes on HaCaT. The present results
demonstrated that UV radiation was able to inhibit cell
proliferation, apart from low doses of UVA radiation. Medium doses
(5 J/cm2) of UVA radiation increased intracellular
antioxidant levels in HaCaT cells and did not affect lipid
peroxidation. However, medium or high dose UVB radiation promoted
lipid peroxidation. Furthermore, treatment with A375 co-culture
induced a similar effect on lipid peroxidation in HaCaT cells as
low dose UVB radiation.
UV radiation may induce intracellular mutations,
which may in turn induce malignant transformation. The results of
previous studies have suggested that environmentally relevant doses
of UVA (>20 J/cm2) irradiation may induce highly
pernicious transformation, including anchorage-independent growth,
the hypersecretion or overexpression of carcinogenic factors, and
alterations in the morphology and apoptosis of keratinocytes
(33,34). However, in the present study,
clinical doses of UVA irradiation negative influences in HaCaT
cells; they promoted the expression of Nrf2 and cellular
antioxidant levels. Lehmann et al (35) previously identified that a 5
J/cm2 dose of UVA did not impair cellular viability or
DNA mutations. It has also been demonstrated that UVA irradiation
promotes the expression of various cytoprotective genes, including
HO-1 and Nrf2. Nrf2 mRNA expression and the intracellular
antioxidant level exhibited a dose-dependent increase following UVA
irradiation. This may be responsible for the generation of ROS
following UVA radiation, which may mediate Nrf2 activation and its
accumulation in the nucleus (36).
Previous studies have demonstrated that increased Nrf2 mRNA
expression occurs due to UVA-induced oxidative stress (37,38).
Additionally, Marrot et al (39) elucidated that UVA radiation promotes
the expression of phase2 enzymes, particularly heme oxygenase 1, in
keratinocytes. These phase 2 enzymes are the main method by which
cells inhibit ROS generation.
NB-UVB phototherapy is frequently used to treat
vitiligo and is considered to be an effective method of treatment
(40). However, previous studies
have demonstrated that environmental doses of UVB radiation
exposure may induce various cutaneous disorders (41,42). In
the present study, various effects on keratinocytes were observed
following exposure to UVB radiation. For example, the expression of
Nrf2 mRNA was inhibited by low doses of UVB, but was significantly
promoted by medium doses of UVB. However, levels of cellular
antioxidants exhibited an opposing trend. Increased antioxidant
levels may be a result of ROS generation induced by UVB radiation,
whereas the antioxidant inhibition following exposure to medium
doses of UVB may be associated with DNA damage. UVB has been
demonstrated to cause the irreversible damage of DNA due to the
formation of cyclobutane pyrimidine dimers, pyrimidine (6–4)
pyrimidine photodimers and 8-hydroxy-2′-deoxyguanosine, which
efficiently activates the p53 pathway (43). Faraonio et al (44) demonstrated that p53 is able to
compete with Nrf2 on ARE-containing promoters, which inhibits the
transcription of antioxidant response genes. Furthermore, the high
expression of Nrf2 may be regulated by nuclear factor-κB, which may
be activated by UVB irradiation (45).
Following co-culture with A375 cells, marked
mutation was detected in HaCaT cells, which may be induced by the
association between the two cell lines. The expression of PAR-2
mRNA was significantly elevated and Nrf2 expression was decreased,
whereas the intracellular antioxidant levels were significantly
increased. These results suggest that melanocytes may affect the
regulation of oxidative stress in keratinocytes, and that the high
expression of PAR-2 may promote the regulation of pigmentation and
the phagocytosis of melanosomes. Joshi et al (46) previously demonstrated that a
‘ligand-receptor’ type interaction exists between melanocytes and
keratinocytes and that this interaction may regulate pigment
transfer by triggering intracellular calcium signaling in
keratinocytes. Melanin is considered to be a key factor in
protecting cells against the oxidative stress caused by UV
irradiation. The accordant phenomena have also been identified
following low dose UVA and UVB treatment in keratinocytes. Previous
studies have demonstrated that low dose UV radiation is able to
upregulate the expression of PAR-2 (47,48),
which may be relevant to the overexpression of cytokines including
interleukin-1 and tumor necrosis factor-α, caused by exposure to UV
radiation (47). Therefore this
pigment may affect the cellular secretion of cytokines or
chemokines.
It was also demonstrated in the present study that
the expression of PAR-2 mRNA was positively associated with MDA
levels in the supernatant. This result illustrates that PAR-2 may
regulate the permeability of the cellular membrane. Previous
studies on PAR-2 have determined that PAR2 activation increases
intracellular Ca+ concentrations (49–51).
Furthermore, melanocytes increase pigment transfer in keratinocytes
by triggering intracellular calcium signaling (37). Therefore, PAR-2 may upregulate the
absorption of melanin in keratinocytes via a previously unknown
method, which differs from Rho-dependent mediating phagocytosis in
keratinocytes (52). Further studies
are therefore required to further elucidate the association between
PAR-2 and melanin.
In conclusion, the results of the present study
demonstrate that clinical doses of BB-UVA and NB-UVB radiation
induce varying effects on the proliferation of HaCaT cells and the
expression of Nrf2 and PAR2. Co-culture with A375 induced similar
effects as those of low dose UVA and UVB radiation. Therefore, the
present study may provide novel therapeutic targets for the
treatment of vitiligo; however further in vitro and in
vivo studies are required.
References
|
1
|
Njoo MD, Spuls PI, Bos JD, Westerhof W and
Bossuyt PM: Nonsurgical repigmentation therapies in
vitiligo-Meta-analysis of the literature. Arch Dermatol.
134:1532–1540. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ezzedine K, Eleftheriadou V, Whitton M and
van Geel N: Vitiligo. Lancet. 386:74–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chu ECY: Treatment of vitiligo: Medical
treatment, phototherapy and surgical treatment. Hong Kong J
Dermatol Venereol. 23:113–117. 2015.
|
|
4
|
Kanwar AJ, Dogra S, Parsad D and Kumar B:
Narrow-band UVB for the treatment of vitiligo: An emerging
effective and well-tolerated therapy. Int J Dermatol. 44:57–60.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu CS, Yu CL, Wu CS, Lan CCE and Yu HS:
Narrow-band ultraviolet-B stimulates proliferation and migration of
cultured melanocytes. Exp Dermatol. 13:755–763. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El Mofty M, Bosseila M, Mashaly HM, Gawdat
H and Makaly H: Broadband ultraviolet A vs. psoralen ultraviolet A
in the treatment of vitiligo: A randomized controlled trial. Clin
Exp Dermatol. 38:830–835. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang XX, Bernerd F and Halliday GM:
Ultraviolet a within sunlight induces mutations in the epidermal
basal layer of engineered human skin. Am J Pathol. 174:1534–1543.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yasui H and Sakurai H: Chemiluminescent
detection and imaging of reactive oxygen species in live mouse skin
exposed to UVA. Biochem Biophys Res Commun. 269:131–136. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He YY and Häder DP: UV-B-induced formation
of reactive oxygen species and oxidative damage of the
cyanobacterium Anabaena sp.: Protective effects of ascorbic acid
and N-acetyl-L-cysteine. J Photochem Photobiol B. 66:115–124. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swalwell H, Latimer J, Haywood RM and
Birch-Machin MA: Investigating the role of melanin in UVA/UVB-and
hydrogen peroxide-induced cellular and mitochondrial ROS production
and mitochondrial DNA damage in human melanoma cells. Free Radic
Biol Med. 52:626–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pelle E, Mammone T, Maes D and Frenkel K:
Keratinocytes act as a source of reactive oxygen species by
transferring hydrogen peroxide to melanocytes. J Invest Dermatol.
124:793–797. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sravani PV, Babu NK, Gopal KV, Rao GR, Rao
AR, Moorthy B and Rao TR: Determination of oxidative stress in
vitiligo by measuring superoxide dismutase and catalase levels in
vitiliginous and non-vitiliginous skin. Indian J Dermatol Venereol
Leprol. 75:268–271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chui DH, Tang W and Orkin SH: cDNA cloning
of murine Nrf 2 gene, coding for a p45 NF-E2 related transcription
factor. Biochem Biophys Res Commun. 209:40–46. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McMahon M, Itoh K, Yamamoto M and Hayes
JD: Keap1-dependent proteasomal degradation of transcription factor
Nrf2 contributes to the negative regulation of antioxidant response
element-driven gene expression. J Biol Chem. 278:21592–21600. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Itoh K, Wakabayashi N, Katoh Y, Ishii T,
Igarashi K, Engel JD and Yamamoto M: Keap1 represses nuclear
activation of antioxidant responsive elements by Nrf2 through
binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Déry O, Corvera CU, Steinhoff M and
Bunnett NW: Proteinase-activated receptors: Novel mechanisms of
signaling by serine proteases. Am J Physiol. 274:C1429–C1452. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moretti S, Nassini R, Prignano F, Pacini
A, Materazzi S, Naldini A, Simoni A, Baroni G, Pellerito S, Filippi
I, et al: Protease-activated receptor-2 downregulation is
associated to vitiligo lesions. Pigment Cell Melanoma Res.
22:335–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rattenholl A and Steinhoff M:
Proteinase-activated receptor-2 in the skin: Receptor expression,
activation and function during health and disease. Drug News
Perspect. 21:369–381. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hachem JP, Houben E, Crumrine D, Man MQ,
Schurer N, Roelandt T, Choi EH, Uchida Y, Brown BE, Feingold KR and
Elias PM: Serine protease signaling of epidermal permeability
barrier homeostasis. J Invest Dermatol. 126:2074–2086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hachem JP, Man MQ, Crumrine D, Uchida Y,
Brown BE, Rogiers V, Roseeuw D, Feingold KR and Elias PM: Sustained
serine proteases activity by prolonged increase in pH leads to
degradation of lipid processing enzymes and profound alterations of
barrier function and stratum corneum integrity. J Invest Dermatol.
125:510–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steinhoff M, Corvera CU, Thoma MS, Kong W,
McAlpine BE, Caughey GH, Ansel JC and Bunnett NW:
Proteinase-activated receptor-2 in human skin: Tissue distribution
and activation of keratinocytes by mast cell tryptase. Exp
Dermatol. 8:282–294. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steinhoff M, Neisius U, Ikoma A, Fartasch
M, Heyer G, Skov PS, Luger TA and Schmelz M: Proteinase-activated
receptor-2 mediates itch: A novel pathway for pruritus in human
skin. J Neurosci. 23:6176–6180. 2003.PubMed/NCBI
|
|
23
|
Scott G, Deng A, Rodriguez-Burford C,
Seiberg M, Han R, Babiarz L, Grizzle W, Bell W and Pentland A:
Protease-activated receptor 2, a receptor involved in melanosome
transfer, is upregulated in human skin by ultraviolet irradiation.
J Invest Dermatol. 117:1412–1420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sasaki M, Horikoshi T, Uchiwa H and
Miyachi Y: Up-regulation of tyrosinase gene by nitric oxide in
human melanocytes. Pigment Cell Res. 13:248–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roméro-Graillet C, Aberdam E, Clément M,
Ortonne JP and Ballotti R: Nitric oxide produced by
ultraviolet-irradiated keratinocytes stimulates melanogenesis. J
Clin Invest. 99:635–642. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
El-Mofty M, Mostafa W, Youssef R,
El-Fangary M, El-Ramly A, Mahgoub D, Fawzy M and El-Hawary M:
BB-UVA vs. NB-UVB in the treatment of vitiligo: A randomized
controlled clinical study (single blinded). Photodermatol
Photoimmunol Photomed. 29:239–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Plaa GL and Witschi H: Chemicals, drugs,
and lipid peroxidation. Annu Rev Pharmacol Toxicol. 16:125–142.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roelandts R: Photo (chemo) therapy for
vitiligo. Photodermatol Photoimmunol Photomed. 19:1–4. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang HT, Choi B and Tang MS: Melanocytes
are deficient in repair of oxidative DNA damage and UV-induced
photoproducts. Proc Natl Acad Sci USA. 107:pp. 12180–12185. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roméro-Graillet C, Aberdam E, Clément M,
Ortonne JP and Ballotti R: Nitric oxide produced by
ultraviolet-irradiated keratinocytes stimulates melanogenesis. J
Clin Invest. 99:635–642. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He YY, Pi J, Huang JL, Diwan BA, Waalkes
MP and Chignell CF: Chronic UVA irradiation of human HaCaT
keratinocytes induces malignant transformation associated with
acquired apoptotic resistance. Oncogene. 25:3680–3688. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He YY, Huang JL and Chignell CF: Delayed
and sustained activation of extracellular signal-regulated kinase
in human keratinocytes by UVA: Implications in carcinogenesis. J
Biol Chem. 279:53867–53874. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lehmann J, Pollet D, Peker S, Steinkraus V
and Hoppe U: Kinetics of DNA strand breaks and protection by
antioxidants in UVA- or UVB-irradiated HaCaT keratinocytes using
the single cell gel electrophoresis assay. Mutat Res. 407:97–108.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hirota A, Kawachi Y, Itoh K, Nakamura Y,
Xu X, Banno T, Takahashi T, Yamamoto M and Otsuka F: Ultraviolet A
irradiation induces NF-E2-related factor 2 activation in dermal
fibroblasts: Protective role in UVA-induced apoptosis. J Invest
Dermatol. 124:825–832. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hseu YC, Chou CW, Senthil Kumar KJ, Fu KT,
Wang HM, Hsu LS, Kuo YH, Wu CR, Chen SC and Yang HL: Ellagic acid
protects human keratinocyte (HaCaT) cells against UVA-induced
oxidative stress and apoptosis through the upregulation of the HO-1
and Nrf-2 antioxidant genes. Food Chem Toxicol. 50:1245–1255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kimura S, Warabi E, Yanagawa T, Ma D, Itoh
K, Ishii Y, Kawachi Y and Ishii T: Essential role of Nrf2 in
keratinocyte protection from UVA by quercetin. Biochem Biophys Res
Commun. 387:109–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marrot L, Jones C, Perez P and Meunier JR:
The significance of Nrf2 pathway in (photo)-oxidative stress
response in melanocytes and keratinocytes of the human epidermis.
Pigment Cell Melanoma Res. 21:79–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bhatnagar A, Kanwar A, Parsad D and De D:
Comparison of systemic PUVA and NB-UVB in the treatment of
vitiligo: An open prospective study. J Eur Acad Dermatol Venereol.
21:638–642. 2007.PubMed/NCBI
|
|
41
|
Armstrong BK and Kricker A: The
epidemiology of UV induced skin cancer. J Photochem Photobiol B.
63:8–18. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rhodes LE, Belgi G, Parslew R, McLoughlin
L, Clough GF and Friedmann PS: Ultraviolet-B-induced erythema is
mediated by nitric oxide and prostaglandin E 2 in combination. J
Invest Dermatol. 117:880–885. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brash DE, Ziegler A, Jonason AS, Simon JA,
Kunala S and Leffell DJ: Sunlight and sunburn in human skin cancer:
p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc.
1:136–142. 1996.PubMed/NCBI
|
|
44
|
Faraonio R, Vergara P, Di Marzo D,
Pierantoni MG, Napolitano M, Russo T and Cimino F: p53 suppresses
the Nrf2-dependent transcription of antioxidant response genes. J
Biol Chem. 281:39776–39784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rushworth SA, Zaitseva L, Murray MY, Shah
NM, Bowles KM and MacEwan DJ: The high Nrf2 expression in human
acute myeloid leukemia is driven by NF-κB and underlies its
chemo-resistance. Blood. 120:5188–5198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Joshi PG, Nair N, Begum G, Joshi NB,
Sinkar VP and Vora S: Melanocyte-keratinocyte interaction induces
calcium signalling and melanin transfer to keratinocytes. Pigment
Cell Res. 20:380–384. 2007.PubMed/NCBI
|
|
47
|
Scott G, Deng A, Rodriguez-Burford C,
Seiberg M, Han R, Babiarz L, Grizzle W, Bell W and Pentland A:
Protease-activated receptor 2, a receptor involved in melanosome
transfer, is upregulated in human skin by ultraviolet irradiation.
J Invest Dermatol. 117:1412–1420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Paine C, Sharlow E, Liebel F, Eisinger M,
Shapiro S and Seiberg M: An alternative approach to depigmentation
by soybean extracts via inhibition of the PAR-2 pathway. J Invest
Dermatol. 116:587–595. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lerner DJ, Chen M, Tram T and Coughlin SR:
Agonist recognition by proteinase-activated receptor 2 and thrombin
receptor. Importance of extracellular loop interactions for
receptor function. J Biol Chem. 271:13943–13947. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Molino M, Barnathan ES, Numerof R, Clark
J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M and
Brass LF: Interactions of mast cell tryptase with thrombin
receptors and PAR-2. J Biol Chem. 272:4043–4049. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Molino M, Woolkalis MJ, Reavey-Cantwell J,
Praticó D, Andrade-Gordon P, Barnathan ES and Brass LF: Endothelial
cell thrombin receptors and PAR-2. Two protease-activated receptors
located in a single cellular environment. J Biol Chem.
272:11133–11141. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Scott G, Leopardi S, Parker L, Babiarz L,
Seiberg M and Han R: The proteinase-activated receptor-2 mediates
phagocytosis in a Rho-dependent manner in human keratinocytes. J
Invest Dermatol. 121:529–541. 2003. View Article : Google Scholar : PubMed/NCBI
|