Introduction
Laryngeal squamous cell carcinoma (LSCC), which is
the second most prevalent cancer of the head and neck region
worldwide (1), predominantly affects
individuals aged 60–70 years old, which has a high morbidity and
mortality, with ~650,000 new cases and ~350,000 instances of
mortality every year (2). Patients
with LSCC typically have poor survival, which has not been improved
in the last 30 years (3). Invasion
and metastasis remain the factors which typically lead to
mortality. Management of LSCC depends primarily on early detection
and appropriate surgical resection, and adjunctive treatment of
radiotherapy and chemotherapy; however the benefit and optimal
timing of the combined chemotherapy and radiotherapy remain
uncertain (4). Therefore, in order
to improve its detection and treatment, an improved understanding
of the pathogenesis and biological features of LSCC is required.
Although several diagnostic and prognostic biomarkers for LSCC have
been described previously (5,6), there
is still a lack of optimal biomarkers that may be widely used in
the clinical setting.
DNA-dependent protein kinase catalytic subunit
(DNA-PKcs), a 460 kDa serine/threonine protein kinase, the gene of
which is primarily located in 8q11, is an essential component of
the non-homologous end-joining (NHEJ) DNA repair pathway (7–11). In
DNA double-strand breaks (DSBs) repair, at least two major repair
mechanisms, homologous recombination (HR) and NHEJ, have been
reported (7). In NHEJ pathway, DNA
DSBs are rejoined directly at an appropriate chromosomal end or
following processing of the DNA ends, and DNA-PKcs serve an
important role in DNA DSBs repair by NHEJ throughout the cell cycle
(8). DNA-PKcs is the catalytic
subunit of DNA-dependent protein kinase (DNA-PK) complex and a
member of the phosphatidylinositol 3-kinase family (12). Previous studies have identified the
products of the X-ray repair cross-complementing protein 5
(XRCC5) and XRCC7 DSB repair genes as subunits of the
DNA-PK complex, and the XRCC7 gene appears to encode
DNA-PKcs (9,10). A unique feature of DNA-PKcs is that
its enzymatic function is only active when in the presence of DNA
ends, and it is suggested that catalytic activity is enhanced when
substrate and enzyme are colocalized to the same DNA molecule
(13). Furthermore, DNA-PKcs is able
to maintain normal immune function, regulate DNA repair, and
prevent further malignant transformation of cells (14). It is well known that DNA-PKcs is
required for the NHEJ pathway of DSBs (15). Recently, the overexpressed DNA-PKcs
was detected in various human tumors (16–19), and
its expression level was correlated with the differentiation and
proliferation of some cell types or the development of productive
tissues (20–22). In contrast, DNA-PKcs deficiency
results in severe combined immunodeficiency in mammals (23). However, in gastric and ovarian
cancer, low expression of DNA-PKcs is associated with adverse
outcome in patients (24). The role
of DNA-PKcs in carcinogenesis, however, remains to be
characterized.
At present, the clinicopathological importance of
the expression of DNA-PKcs in LSCC is unknown. In the present
study, reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), western blot analysis and immunohistochemical analyses
were performed to explore the expression of DNA-PKcs mRNA and
protein in LSCC tissues and in paired adjacent normal mucosa
tissues, and to detect the subcellular localization of DNA-PKcs in
LSCC cells. The aim of the study was to elucidate whether DNA-PKcs
served a role in development of LSCC and a novel diagnostic
biomarker for LSCC.
Materials and methods
Patients and tissue specimens
All patients recruited in the present study were of
Chinese ethnicity. Tumor tissue and corresponding adjacent normal
mucosa specimens were obtained from 96 consecutive patients with
LSCC who underwent total or partial laryngeal resection at the
Department of Otolaryngology-Head and Neck Surgery, People's
Hospital of Guizhou Province (Guiyang, China) between 2009 and
2011. These tumor tissue samples and corresponding adjacent normal
mucosa tissues were randomly harvested from LSCC tissues stored at
−80°C (for qPCR and western blot assays) or in 4% paraformaldehyde
(for immunohistochemistry analyses) in 2012. Adjacent normal mucosa
samples, which were used as a control group, were harvested from 1
cm within the border of the tumor and were pathologically excluded
from cancer cell infiltration. Patients who received any
anti-cancer treatment before operation were excluded from the
study. The specimens were divided into four groups according
pathological diagnosis following the World Health Organization
tumor classification system (25):
Normal mucosa tissue group (96 cases); well-differentiated LSCC
group (32 cases); moderately-differentiated LSCC group (28 cases);
and poorly-differentiated LSCC group (36 cases). Follow-up duration
was defined as the interval (months) from the diagnosis of LSCC to
the final visit. The present study was approved by the Ethics
Committee of the People's Hospital of Guizhou Province, and written
informed consent was obtained from all patients prior to surgery. A
summary of the clinicopathological characteristics of the patients
is presented in Table I.
 | Table I.The clinical/pathological
characteristics of patients with LSCC. |
Table I.
The clinical/pathological
characteristics of patients with LSCC.
|
|
| DNA-PKcs |
|---|
|
|
|
|
|---|
| Clinical/pathological
indexes | Total (n) | Positive n (%) | Chi-square value | P-value |
|---|
| Age |
|
|
|
|
| ≤60 | 46 | 41 (89.13) | 2.482 | 0.116 |
|
>60 | 50 | 37 (74.00) |
|
|
| Sex |
|
|
|
|
| Male | 91 | 74 (81.32) | 0.323 | 0.570 |
|
Female | 5 | 4 (80.00) |
|
|
| Differentiation
degree |
|
|
|
|
| Well | 32 | 22 (68.75) |
0.940 | 0.332 |
|
Moderately | 28 | 21 (75.00) |
4.012 | 0.028 |
|
Poorly | 36 | 35 (97.22) | 4.978 | 0.026 |
| TNM stage |
|
|
|
|
|
I+II | 43 | 33 (76.74) | 2.450 | 0.118 |
|
III+IV | 53 | 45 (84.91) |
|
|
| Lymph node
metastasis |
|
|
|
|
| No | 39 | 29 (74.36) | 2.852 | 0.262 |
|
Yes | 57 | 49 (85.96) |
|
|
| LSCC and ANM |
|
|
|
|
|
LSCC | 96 | 78 (81.25) | 9.724 | 0.008 |
|
ANM | 96 | 11 (11.45) |
|
|
qPCR
The expression levels of the DNA-PKcs of the four
groups were analyzed by qPCR assay. Total RNA of tumor and adjacent
normal mucosa tissue specimens were extracted using total RNA
extraction kit (BioFlux Corporation, Tokyo, Japan) according to the
manufacturer's protocol. cDNA was synthesized using an Access
RT-PCR system (Promega Corporation, Madison, WI, America). qPCR was
performed using SYBR® Green ER qPCR SuperMix-UDG with
ROX (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to
the manufacturer's instructions. The following thermocycling
conditions were used: 50°C for 2 min; 95°C for 2 min; 95°C for 15
sec; and 60°C for 30 sec for 40 cycles. The primer sequences used
for qPCR were as follows: DNA-PKcs, forward
5′-AGCATCATGGTACACGCACT-3′ and reverse 5′-TCCATCAGGCAC1TrCACTFG-3′;
and β-actin, forward 5′-CCTCGCCTTTGCCGATCC-3′ and reverse
5′-GGATCTTCATGAGGTAGTCAGTC-3′. The length of the PCR products of
DNA-PKcs and β-actin were 383 and 626 bp, respectively. Relative
mRNA levels were calculated based on 2−∆∆Ct method
(26). All experiments were
performed in triplicate.
Western blot analysis
The protein levels of DNA-PKcs expression in tissue
samples were detected by western blotting. Nuclear proteins were
extracted using the nuclear protein extraction kit (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) and concentrations were analyzed
using the bicinchoninic acid protein assay kit (Beyotime Institute
of Biotechnology, Shanghai, China) according to the manufacturer's
protocol. Proteins (20 µg per lane) underwent electrophoresis on 6%
SDS-PAGE. Following electrophoresis, the separated proteins were
transferred to polyvinylidene fluoride membranes (Bio-Rad
Laboratories, Inc.). The membranes were blocked for 2 h at room
temperature in 5% milk dissolved in Tris-buffered saline with 0.1%
Tween, and were subsequently incubated overnight at 4°C with
primary antibodies against DNA-PKcs (ab32566, 1:2,000; Abcam,
Cambridge, UK) and β-actin (ab8224, 1:1,000; Abcam) as the internal
control. Antigen and the first antibody complexes were detected by
the horseradish peroxidase (HRP)-conjugated anti-mouse
immunoglobulin G (no. 7076, 1:1,000) and anti-rabbit secondary
antibody (no. 7074, 1:1,000) Cell Signaling Technology, Inc.,
Danvers, MA, USA), and enhanced chemiluminescence reagent was used
for band detection (GE Healthcare, Chicago, IL, USA). The signal
was measured using Image Lab software 4.1 (Bio-Rad Laboratories,
Inc.). Western blot analyses were performed in triplicate.
Immunohistochemistry analyses
The specimens of the four groups were immersed in 4%
paraformaldehyde solution for >24 h at room temperature,
embedded in paraffin and cut into 5-µm-thick sections. The paraffin
embedded sections were dried at 65°C for 30 min, deparaffinized
with xylene, and dehydrated in a graded series of ethanol. The
sections were treated with 3% H2O2 for 10
min, followed by treatment with normal goat serum (Beyotime
Biological Technology Co., Ltd., Shanghai, China) for 10 min at
room temperature. The slides were incubated with monoclonal mouse
anti-human DNA-PKcs (1:100), diluted in antibody diluent buffer
(Boster Biological Technology Co., Ltd., Wuhan, China) at 4°C for
8–12 h or overnight. Following washing three times with PBS (2 min
each), the samples were incubated with the secondary antibody
(859643, Invitrogen; Thermo Fisher Scientific, Inc.) for 15–30 min
at room temperature. Following washing three times with PBS (2 min
each), the samples were stained with 0.01% DAB hydrogen peroxide
for 3–10 min at room temperature, washed with tap water, and then
counterstained with hematoxylin (AR0005, Boster Biological
Technological Co., Ltd., Wuhan, China) for 1 min at room
temperature. For immunohistochemistry analysis under light
microscopy.
Immunohistochemical staining was assessed by two
senior pathologists who were blinded to the patients'
characteristics. Any discrepancies were resolved by consensus.
DNA-PKcs was expressed in the nucleus, and brown or tan particles
were defined as positive. The following scoring system was used to
evaluate DNA-PKcs expression according to the staining intensity of
positive cells (27): Strong
staining (scored as 3), dark brown staining which obscured the
nucleus of tumor cells; yellow staining (scored as 2); primrose
yellow staining (scored as 1); and absent (scored as 0), no marked
staining in tumor cells. DNA-PKcs was also scored according to the
percentage of positive cells as follows: Negative, 0% positive
cells (scored as 0); ≤10% positive cells (scored as 1); 11–50%
positive cells (scored as 2); 51–75% positive cells (scored as 3);
and ≥75% positive cells (scored as 4). The staining intensity score
and percentage score were added together, and the final score was
obtained. For the final score, 0–1 indicated negative staining (−);
2–3 indicated weak positive staining (+); 4–6 indicated moderate
positive staining (++); >6 indicated strong positive staining
(+++).
Statistical analysis
Statistical analyses and creation of graphs were
performed using GraphPad Prism 7.0 software (GraphPad Software,
Inc., La Jolla, CA, USA). The expression of DNA-PKcs between the
four groups were presented as the mean ± standard deviation. Mean
values were compared using the Student's t-test. The association
between DNA-PKcs expression and clinicopathological features was
analyzed using the χ2 test or Fisher's exact test.
Survival curves were plotted using the Kaplan-Meier method and
compared using the log-rank test. Cox regression models were used
for multiple factor analysis. All prognostic factors were evaluated
individually using univariate analyses and then analyzed in
combination with multivariate models. The primary end point in the
present study was percent survival. The significant association
between DNA-PKcs expression and percent survival in univariate
analyses was used as the criterion for including DNA-PKcs
expression in the multivariate backward stepwise elimination
procedure. The final multivariate model retained some prognostic
factors. P<0.05 was considered to indicate a statistically
significant difference.
Results
qPCR analysis
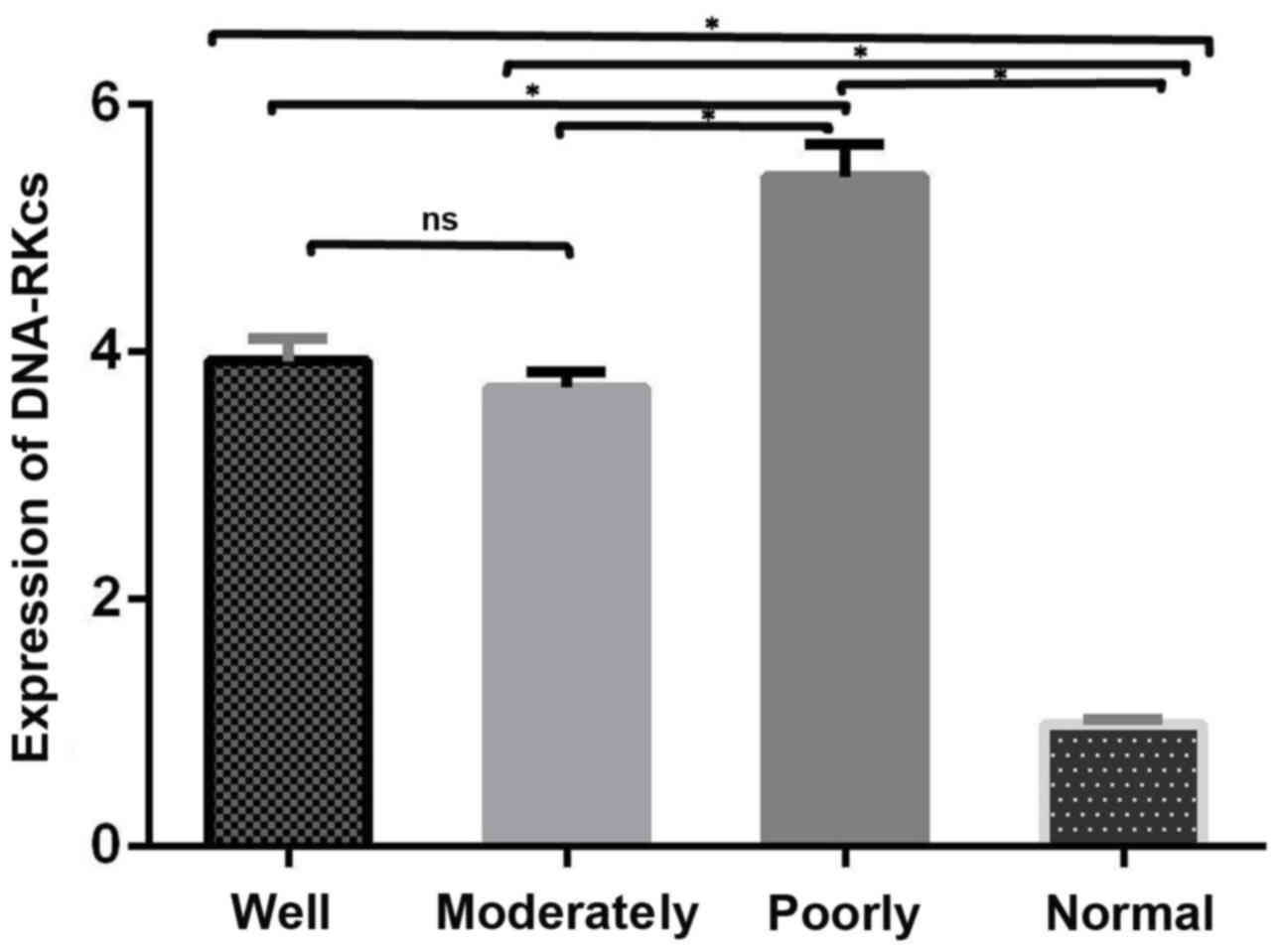
The expression of DNA-PKcs between the four groups
by qPCR assay were presented in Fig.
1. DNA-PKcs mRNA expression levels were significantly higher in
tumor tissues compared with the corresponding adjacent normal
mucosa tissues (P<0.05) and significantly correlated with tumor
differentiation (P=0.025). The mRNA expression levels of DNA-PKcs
between the well- and moderately-differentiated LSCC groups were
similar, however, the mRNA expression levels in the
poorly-differentiated LSCC were markedly higher compared with well-
and moderately-differentiated groups (P<0.05).
Western blotting analysis
Similar to the observations of qPCR, the protein
expression levels of DNA-PKcs were also higher in tumor tissues
compared with the corresponding adjacent normal mucosa tissues and
correlated with tumor differentiation (Fig. 2). The protein expression levels of
DNA-PKcs between the well- and moderately-differentiated LSCC
groups were similar, however, the protein expression levels in the
poorly-differentiated LSCC group were significantly higher compared
with well- and moderately-differentiated groups (P<0.05;
Fig. 2).
Immunohistochemistry analysis
As shown in Fig. 3,
immunohistochemistry analysis was performed to evaluate DNA-PKcs
expression in tumor tissue samples and corresponding adjacent
normal mucosa tissue samples. Regarding DNA-PKcs staining, brown or
tan particles in tissues indicated the presence of DNA-PKcs, which
was predominantly localized to nuclei. Strong positive DNA-PKcs
staining was observed in tumor tissues, whereas weak positive or
negative staining was observed in adjacent normal mucosa tissue
samples. DNA-PKcs expression was significantly higher in LSCC
tissue compared with adjacent normal mucosa (P=0.008; Table I).
Association between DNA-PKcs
expression and clinicopathological parameters
As shown in Table I,
78 of the 96 (81.25%) LSCC patients had positive scores for
DNA-PKcs staining in tumor tissues. The expression of DNA-PKcs
demonstrated significant correlation with differentiation of LSCC.
There was no significant difference between well- and
moderately-differentiated cancer (P=0.332); however, there was a
significant difference between well- or moderately-differentiated
cancer and poorly-differentiated cancer (P=0.028 and P=0.026,
respectively). The expression of DNA-PKcs showed no significant
correlation with TMN stage or lymph node metastasis (P=0.118 and
P=0.262, respectively). There was no statistically significant
correlation between DNA-PKcs expression and age or sex (P=0.116 and
P=0.570, respectively).
Association between DNA-PKcs positive
expression and the survival rate of patients with LSCC
Patients were followed up for 12 to 60 months. Of
the 96 subjects involved with the present study, 35 succumbed to
mortality, 23 of which were due to tumor recurrence or metastasis.
The remaining 13 cases succumbed to other unrelated factors.
To evaluate whether DNA-PKcs expression in LSCC was
associated with patient survival, survival curves were plotted via
the Kaplan-Meier method and compared using the log-rank test. The
results suggested that patients with high DNA-PKcs expression had
lower survival rates than those with low expression. The 3- and
5-year survival rates in patients with low DNA-PKcs expression were
82.5 and 72.1%, respectively, compared with 61.1 and 49.0% in
patients with high DNA-PKcs expression. Furthermore, the survival
rate of patients with high DNA-PKcs expression was lower compared
with those with lower expression, and a significant difference was
observed in the percent survival curves between the high and low
DNA-PKcs expression groups (Fig. 4A;
log-rank test, x2=3.998; P=0.045).
Fig. 4B demonstrates
that the percentage survival of patients with poorly-differentiated
LSCC was the most sever out of all groups as the survival rate was
the lowest. Furthermore, the survival rate was markedly higher in
the patients with well- and moderately-differentiated LSCC.
Kaplan-Meier analysis of well-, moderately- and
poorly-differentiated LSCC patients revealed the 5-year percent
survival rates were 83.1, 73.2 and 30.8%, respectively, which
varied with the degree of differentiation and decreased gradually.
Univariate Cox regression analysis revealed that DNA-PKcs
expression and tumor differentiation were significantly associated
with survival (Table II; P=0.035).
Furthermore, multivariate Cox regression analyses demonstrated that
DNA-PKcs expression remained an independent prognostic factor for
disease progression. Notably, subgroup analyses based on tumor
differentiation suggested that a significant difference was
observed between high and low DNA-PKcs expression groups in well-
or moderately-differentiated and poorly-differentiated LSCC
patients (Table II; P=0.023).
 | Table II.Univariate and multivariate analysis
of survival in patients with laryngeal squamous cell carcinoma. |
Table II.
Univariate and multivariate analysis
of survival in patients with laryngeal squamous cell carcinoma.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| 1.188
(0.068–2.016) | 0.558 | 1.203
(0.655–2.249) | 0.559 |
| 1.025
(0.616–1.699) | 0.918 | 1.248
(0.598–3.613) | 0.556 |
| 1.505
(1.012–2.241) | 0.044 | 1.500
(1.091–2.236) | 0.020 |
| 1.723
(0.698–2.834) | 0.078 | 1.312
(0.718–2.395) | 0.378 |
| 1.103
(0.912–1.332) | 0.319 | 0.692
(0.321–1.511) | 0.354 |
| 2.025
(1.321–3.106) | 0.001 | 1.633
(1.128–2.661) | 0.001 |
Discussion
In mammalian cells, DNA DSBs are widely considered
to be the most lethal form of DNA damage (28,29).
NHEJ is a major repair mechanism for DSBs. The capture of both
broken DNA ends and presenting them together to the DNA-protein
complex is an initial and important step of NHEJ (30), and DNA-PKcs is the main component of
NHEJ. The DNA repair mechanism serves a key role in the maintenance
of genomic integrity, and the DNA-PKcs is predominantly associated
with the repair of DNA DSBs in mammalian Cells (28,29). In
the present study, tumor tissues from 96 LSCC patients and their
corresponding adjacent normal tissues were enrolled, and qPCR,
western blot analysis and immunohistochemical analyses were
performed to investigate DNA-PKcs protein and mRNA expression in
LSCC tissues and detect the subcellular localization of DNA-PKcs in
LSCC cells. The aim of the present study was to elucidate whether
DNA-PKcs served a role in disease development of LSCC.
The overexpression of DNA-PKcs has previously been
detected in various types of human cancer (16–19). For
instance, Tonotsuka et al (19) detected the expression of DNA-PKcs in
esophageal cancer tissue and adjacent normal mucosa in 13 patients
with primary esophageal cancer, and examined for quantitative
differences in expression levels of DNA-PKcs proteins by western
blotting and immunohistochemistry. It was revealed that the
expression levels of DNA-PKcs were higher in tumor tissues than
those in normal mucosa. Furthermore, another previous study
conducted extensive profiling of DNA-PKcs mRNA and protein levels
across normal and cancerous tissues (31). In contrast to the results of the
present study, previous studies have suggested that the expression
of DNA-PKcs in gastric cancer and breast cancer was significantly
downregulated compared with benign lesions or peritumoral tissues
(32,33). However, to the best of our knowledge,
the present study was the first to demonstrate that DNA-PKcs
expression of tumor tissues in patients with LSCC was significantly
higher than that in adjacent normal mucosa, whereas the gene level
and the protein level of DNA-PKcs expression were correlated with
each other. Western blot analysis showed that the protein
expression of DNA-PKcs was very strong, which indicated that
DNA-PKcs had high expression in LSCC cells. The results of integral
optical density revealed that the expression of DNA-PKcs was
significantly different between LSCC and adjacent normal mucosa.
The correlation between DNA-PKcs expression at the mRNA level and
at the protein level was demonstrated, which indicated that the
protein expression may be determined by the transcription of these
genes. Someya et al (34)
previously examined the DNA-PKcs expression of patients with
uterine, cervix or breast cancer using qPCR and western blot
analysis, and found that they were significantly lower than those
in normal volunteers. The present results disagreed with these
findings. The present study also demonstrated that the expression
of DNA-PKcs was significantly higher in LSCC than in adjacent
normal mucosa.
A previous study by Tonotsuka et al (19) indicated that DNA-PKcs was
predominantly located in the nuclei of tumor tissue and normal
mucosa, which was further confirmed by immunofluorescence on whole
cells (35). In the present study,
InVision immunohistochemistry was performed to detect the
subcellular localization and the expression of DNA-PKcs in LSCC
tissue and adjacent normal mucosa. The present study demonstrated
that DNA-PKcs expression was predominantly located in the nuclei in
LSCC cells, but was reduced in normal laryngeal mucosa, which was
consistent with the results of Ren et al (36).
In the present study, the clinicopathological
characteristics of DNA-PKcs in 96 patients with LSCC were also
evaluated using immunohistochemical analysis. Immunohistochemical
analysis results indicated that 81.25% of LSCC samples (78 of 96
patients) were positive for DNA-PKcs expression. The results
suggested that the different proteins in the differently
differentiated tumor showed different expression levels of DNA-PKcs
proteins, and even each tumor cell exhibited different expression
levels. No significant difference was observed in DNA-PKcs
expression between the well- and moderately-differentiated LSCC.
Furthermore, it was observed that there was a significant
difference in DNA-PKcs expression between the well- or
moderately-differentiated LSCC and the poorly-differentiated LSCC.
Therefore, the present study indicated that DNA-PKcs expression was
significantly correlated with the differentiation degree of LSCC,
and changes of DNA-PKcs expression gradually increased with the
decrease of the degree of differentiation. The well- and
moderately-differentiated LSCC had similar clinicopathological
characteristics. Therefore, the clinicopathological correlation
indicated that DNA-PKcs expression was associated with tumor
differentiation. However, a study recently demonstrated that low
DNA-PKcs expression was associated with tumor differentiation
(37). As such, the present results
disagreed with these findings. Results of the present study
indicated that DNA-PKcs expression had no significant association
with age and sex, and no significant difference was observed in
lymph node metastasis and TNM stage.
Of particular importance, patients with
highly-expressed DNA-PKcs had lower survival rates in
poorly-differentiated LSCC. Univariate analysis revealed that
elevated DNA-PKcs was closely associated with the survival rate in
LSCC patients. Multivariate analysis suggested that DNA-PKcs
expression was also an independent prognostic factor for poor
clinical outcomes of LSCC patients. Taken together, these results
suggested that DNA-PKcs may be used as a novel prognostic marker
for patients with LSCC.
In conclusion, the present results suggested that
the expression levels of DNA-PKcs were significantly increased in
tumor tissues of patients with LSCC compared with adjacent normal
mucosa, and DNA-PKcs expression was correlated with differentiation
of LSCC. It was also demonstrated that DNA-PKcs overexpression
occurred in LSCC cells and patients with LSCC. Therefore, DNA-PKcs
expression was associated with carcinogenesis, tumor progression
and the risk of cancer. This suggests that DNA-PKcs expression in
LSCC may be used to identify individuals with increased
susceptibility to cancer. Furthermore, these results suggested that
DNA-PKcs may become a novel prognostic marker for patients with
LSCC.
Acknowledgements
The present study was supported by the Science and
Technology Research Program of Guizhou Province [grant no. (2011)
015].
References
|
1
|
Mojica-Manosa P, Reidy J, Wilson K and
Douglas W: Larynx squamous cell carcinoma: Concepts and future
directions. Surg Oncol Clin N Am. 13:99–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Almadori G, Bussu F, Cadoni G, Galli J,
Paludetti G and Maurizi M: Molecular markers in laryngeal squamous
cell carcinoma: Towards an integrated clinicobiological approach.
Eur J Cancer. 41:683–693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ou G, Itasaka S, Zeng L, Shibuya K, Yi J,
Harada H and Hiraoka M: Usefulness of HIF-1 imaging for determining
optimal timing of combining bevacizumab and radiotherapy. Int J
Radiat Oncol Biol Phys. 75:463–467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
García-Fernández E, De Diego JI,
Collantes-Bellido E, Mendiola M, Prim MP, Pérez-Fernández E,
Miguel-Martín M, Nistal M and Hardisson D: Aurora B kinase
expression in laryngeal squamous cell carcinoma and its prognostic
implications. Histopathology. 58:368–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Y, Gong HL, Zhou L, Tian J and Wang Y:
CD24: A novel cancer biomarker in laryngeal squamous cell
carcinoma. ORL J Otorhinolaryngol Relat Spec. 74:78–85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong X, Shen Y, Jiang N, Fei X and Mi J:
Emerging roles of DNA-PK besides DNA repair. Cell Signal.
23:1273–1280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neal JA and Meek K: Choosing the right
path: Does DNA-PK help make the decision. Mutat Res. 711:73–86.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hill R and Lee PW: The DNA-dependent
protein kinase (DNA-PK): More than just a case of making ends meet.
Cell Cycle. 9:3460–3469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meek K, Dang V and Lees-Miller SP: DNA-PK:
The means to justify the ends. Adv Immunol. 99:33–58. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salles B, Calsou P, Frit P and Muller C:
The DNA repair complex DNA-PK, a pharmacological target in cancer
chemotherapy and radiotherapy. Pathol Biol (Paris). 54:185–193.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Xu X, Hao Y, Chen J, Lu H, Qin J,
Peng L and Chen B: Expression of DNA-PKcs and BRCA1 as prognostic
indicators in nasopharyngeal carcinoma following
intensity-modulated radiation therapy. Oncol Lett. 5:1199–1204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gottlieb TM and Jackson SP: The
DNA-dependent protein kinase: Requirement for DNA ends and
association with Ku antigen. Cell. 72:131–142. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren JH, Dai XF, Yan GL, Jin M, Liu CW,
Yang KY, Wu G and Ma CM: Acute oral mucositis in nasopharyngeal
carcinoma patients treated with radiotherapy: Association with
genetic polymorphism in DNA DSB repair genes. Int J Radiat Biol.
90:256–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jackson SP: Sensing and repairing DNA
double-strand breaks. Carcinogenesis. 23:687–696. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosoi Y, Watanabe T, Nakagawa K, Matsumoto
Y, Enomoto A, Morita A, Nagawa H and Suzuki N: Up-regulation of
DNA-dependent protein kinase activity and Sp1 in colorectal cancer.
Int J Oncol. 25:461–468. 2004.PubMed/NCBI
|
|
17
|
Shintani S, Mihara M, Li C, Nakahara Y,
Hino S, Nakashiro K and Hamakawa H: Up-regulation of DNA-dependent
protein kinase correlates with radiation resistance in oral
squamous cell carcinoma. Cancer Sci. 94:894–900. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Um JH, Kwon JK, Kang CD, Kim MJ, Ju DS,
Bae JH, Kim DW, Chung BS and Kim SH: Relationship between
antiapoptotic molecules and metastatic potency and the involvement
of DNA-dependent protein kinase in the chemosensitization of
metastatic human cancer cells by epidermal growth factor receptor
blockade. J Pharmacol Exp Ther. 311:1062–1070. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tonotsuka N, Hosoi Y, Miyazaki S, Miyata
G, Sugawara K, Mori T, Ouchi N, Satomi S, Matsumoto Y, Nakagawa K,
et al: Heterogeneous expression of DNA-dependent protein kinase in
esophageal cancer and normal epithelium. Int J Mol Med. 18:441–447.
2006.PubMed/NCBI
|
|
20
|
Moll U, Lau R, Sypes MA, Gupta MM and
Anderson CW: DNA-PK, the DNA-activated protein kinase, is
differentially expressed in normal and malignant human tissues.
Oncogene. 18:3114–3126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sallmyr A, Miller A, Gabdoulkhakova A,
Safronova V, Henriksson G and Bredberg A: Expression of
DNA-dependent protein kinase in human granulocytes. Cell Res.
14:331–340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holgersson A, Erdal H, Nilsson A,
Lewensohn R and Kanter L: Expression of DNA-PKcs and Ku86, but not
Ku70, differs between lymphoid malignancies. Exp Mol Pathol.
77:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van der Burg M, van Dongen JJ and van Gent
DC: DNA-PKcs deficiency in human: Long predicted, finally found.
Curr Opin Allergy Clin Immunol. 9:503–509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsu FM, Zhang S and Chen BP: Role of
DNA-dependent protein kinase catalytic subunit in cancer
development and treatment. Transl Cancer Res. 1:22–34.
2012.PubMed/NCBI
|
|
25
|
Razek AA and Huang BY: Soft tissue tumors
of the head and neck: Imaging-based review of the WHO
classification. Radiographics. 31:1923–1954. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Xu LZ and Yang WT: The criteria for
judging the results of immunohistochemical reaction. Zhong Guo Ai
Zheng Za Zhi. 6:229–231. 1996.(In Chinese).
|
|
28
|
Takata M, Sasaki MA, Sonoda E, Morrison C,
Hashimoto M, Utsumi H, Yamaguchi-Iwai Y, Shinohara A and Takeda S:
Homologous recombination and non-homologous end-joining pathways of
DNA double-strand bread repair have overlapping roles in the
maintenance of chromosomal integrity in vertebrate cells. EMBO J.
17:5497–5508. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rothkamm K, Kühne M, Jeggo PA and Löbrich
M: Radiation-induced genomic rearrangement formed nonhomologous end
-joining of DNA double-strand breaks. Cancer Res. 61:3886–2893.
2001.PubMed/NCBI
|
|
30
|
Bouchaert P, Guerif S, Debiais C, Irani J
and Fromont G: DNA-PKcs expression predicts response to
radiotherapy in prostate cancer. Int J Radiat Oncol Biol Phys.
84:1179–1185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee HS, Choe G, Park KU, Park DJ, Yang HK,
Lee BL and Kim WH: Altered expression of DNA-dependent protein
kinase catalytic subunit (DNA-PKcs) during gastric carcinogenesis
and its clinical implications on gastric cancer. Int J Oncol.
31:859–866. 2007.PubMed/NCBI
|
|
32
|
Nacht M, Strasser A, Chan YR, Harris AW,
Schlissel M, Bronson RT and Jacks T: Mutations in the p53 and SCID
genes cooperate in tumorigenesis. Genes Dev. 15:2055–2066. 1996.
View Article : Google Scholar
|
|
33
|
Treilleux I, Chapot B, Goddard S, Pisani
P, Angèle S and Hall J: The molecular causes of low ATM protein
expression in breast carcinoma; promoter methylation and levels of
the catalytic subunit of DNA-dependent protein kinase.
Histopathology. 51:63–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Someya M, Sakata K, Matsumoto Y, Yamamoto
H, Monobe M, Ikeda H, Ando K, Hosoi Y, Suzuki N and Hareyama M: The
association of DNA-dependent protein kinase activity with
chromosomal instability and risk cancer. Carcinohenesis.
27:117–122. 2006. View Article : Google Scholar
|
|
35
|
Carter T, Vancurová I, Sun I, Lou W and
DeLeon S: A DNA-activated protein kinase from HeLa cell nuclei. Mol
Cell Boil. 10:6460–6471. 1990. View Article : Google Scholar
|
|
36
|
Ren F, Yang ZL, Tan X, Liu D, Zou Q, Yuan
Y, Li J, Liang L, Zeng G and Chen S: DNA-PKcs and Ku70 are
predictive markers for poor prognosis of patients with gall bladder
malignancies. Appl Immunohistochem Mol Morphol. 22:741–747. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abdel-Fatah T, Arora A, Agarwal D, Moseley
P, Perry C, Thompson N, Green AR, Rakha E, Chan S, Ball G, et al:
Adverse prognostic and predictive significance of low DNA-PKcs
expression in early-stage breast cancers. Breast Cancer Res Treat.
146:309–320. 2014. View Article : Google Scholar : PubMed/NCBI
|