Introduction
Allergic rhinitis (AR), the most common form of
noninfectious rhinitis, is characterized by chronic inflammation of
the nasal mucosa and hypersensitivity (1). AR is a common disease that serves as a
risk factor for the development of other diseases, such as asthma
(2). The symptoms of AR, including
nasal congestion, rhinorrhea and sneezing, considerably affect the
daily activities, quality of sleep and work productivity of
patients (3). Due to the effects of
AR on quality of life, as well as its increasing prevalence
(4), it is necessary to identify an
effective diagnostic and treatment strategy for AR patients.
The mechanism underlying the occurrence of AR is
complicated. Currently, it is generally considered that the
emergence of AR is closely associated with the activation and
infiltration of mast cells, eosinophils, basophils and
CD4+ T-helper type 2 cells, as well as the generation
and release of various inflammatory mediators (5). MicroRNAs (miRNAs) are small noncoding
RNAs, which bind to the 3′-untranslated region (3′-UTR) of target
genes and function mainly as suppressors of gene expression at the
post-transcriptional level (6,7). In
addition, miRNAs regulate the activity of immune cells within the
innate and adaptive immune systems (8,9), and
their aberrant expression may result in immune disorders. Previous
studies have emphasized the important role of miRNAs in controlling
allergic airway inflammation (10,11).
Furthermore, Teng et al (12)
observed that that miRNA-143 involved in the pathologic process of
AR, which was significantly down-regulated in nasal mucosal tissues
of AR patients compared with healthy control subjects. Recently,
Suojalehto et al (1) detected
the abnormally expression of miR-let-7e in the nasal mucosa tissues
of AR patients; however, the underling mechanism remains
unclear.
The present study aimed to investigate the
expression of miR-let-7e in AR and its effects on the AR occurrence
at the molecular level. In addition, the study further explored the
underlying molecular mechanism of miR-let-7e involved in AR
progression in order to provide the necessary theoretical basis for
understanding the etiology of AR and a novel insight for the
diagnosis and treatment of this disease.
Materials and methods
Experimental animals
A total of 20 specific pathogen-free male BALB/c
mice (age, 6–7 weeks; weight, 16–18 g) were purchased from the
Experimental Animal Center of The Second Affiliated Hospital of
Nanchang University (Nanchang, China). All mice were maintained
under standard conventional conditions, including a 12 h light/dark
cycle, a temperature of 18–22°C and humidity of 50–60%, with access
to food and water ad libitum. All animal experiments were
approved by the Animal Ethics Committee of The Second Affiliated
Hospital of Nanchang University.
Preparation of the AR mouse model
Initially, mice were intraperitoneally injected for
primary sensitization, followed by intranasal treatment for local
stimulation and secondary immunization. The mice were randomly
divided into the AR and control groups (n=10 per group). Mice in
the AR group were intraperitoneally injected with 400 µl saline
containing 10 µg ovalbumin (OVA) and 2 mg aluminum hydroxide
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) on days 1 and 8 to
promote drug sensitization. Between days 15 and 21, the mice were
administered intranasal drops of 20 µl saline containing 200 µg OVA
once daily to continuously challenge AR. Mice in the control group
were injected intraperitoneally or treated intranasally with an
identical dose of saline at the same time points. The mice were
sacrificed 24 h following the final intranasal challenge (day 22).
Nasal mucosa samples were then collected by swabbing the anterior
nares from all mice.
Serological analysis
Blood samples were collected from the heart of each
mouse 24 h following the final intranasal challenge to determine
the histamine content of the serum. The blood was clotted at room
temperature for 2 h and then centrifuged at 2,000 × g for 20 min at
4°C to obtain the serum. Subsequently, the serum levels of
histamine (ab213975; dilution 1:4,000; Abcam, Cambridge, UK),
anti-OVA specific Immunoglobulin (Ig) E (3010; dilution 1:4,000;
Chondrex, Inc., Redmond, WA, USA) and tumor necrosis factor-α
(TNF-α; MTA00B; dilution 1:4,000; R&D Systems Inc.,
Minneapolis, MN, USA) in each mouse were measured using ELISA. The
concentrations of histamine, anti-OVA specific IgE and TNF-α were
calculated from the equations obtained from the standard curve
plots drawn against the standard solutions provided in the
kits.
Collection of human nasal mucosal
specimens from rhinitis patients
Human nasal mucosal samples were obtained from the
inferior turbinate sections of 23 patients with perennial AR and 18
patients with nonallergic rhinitis (NAR), serving as the controls.
The AR patients included 13 males and 10 females with a mean age of
34.1 years (range, 20–62 years), while the NAR patients included 11
males and 7 females with a mean age of 32.3 years (range, 22–60
years). All patients were diagnosed according to the Clinical
Practice Guidelines (13), including
their medical history, nasal endoscopy examination, an allergen
skin-prick test and a specific IgE assay (ImmunoCAP; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Partial inferior turbinectomy
was conducted for relief of nasal obstruction in the AR and NAR
patients. Nasal tissues were obtained from nasal polyps and
inferior turbinates in patients receiving endoscopic sinus surgery.
These samples were used for measuring the expression of miR-let-7e
by RT-qPCR or for the isolation of primary nasal epithelial cells
(NECs). No patient received topical or systemic corticosteroid
therapy 4 weeks prior to participation into the present study. The
study protocol was approved by the Ethics Committee of the Second
Affiliated Hospital of Nanchang University, and written informed
consents were signed by all patients.
Isolation and culture of primary NECs
from rhinitis patients
Human primary NECs were isolated from the inferior
turbinate tissues of 23 AR patients, as previously described
(14). Following isolation, the
cells were cultured in bronchial epithelial growth medium (Lonza
Group, Ltd., Walkersville, MD, USA) at 37°C in humidified air with
5% CO2. The NECs were passaged when a 70–80% confluent
monolayer appeared, and passage 2 cells were used for subsequent
experiments. In addition, 293T cells (American Type Culture
Collection, Manassas, VA, USA) were cultured in Dulbecco's modified
Eagle's medium (Sigma-Aldrich, Merck KGaA) supplemented with 10%
fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA), 100 units/ml
penicillin, 100 mg/ml streptomycin (Invitrogen, Thermo Fisher
Scientific, Inc.) and maintained in 5% CO2 at 37°C.
Lentivirus production and infection in
NECs
A recombinant lentiviral expression plasmid
(pLVX-IRES-ZsGreen+miR-let-7e) with green fluorescent protein (GFP)
was constructed and confirmed by DNA sequencing. In order to
generate lentiviral particles, the recombinant expression plasmid
was co-transfected into 293T cells with a packaging plasmid system
(psPAX2 and pMD2G). The resultant viral particles were harvested
after 48 h of transfection. Next, NECs were infected with the
miR-let-7e lentiviral vector or with a negative-control vector
without miR-let-7e at a multiplicity of infection of 60 pfu per
cell using 8 µg/ml polybrene infection reagent (Sigma-Aldrich,
Merck KGaA) and were incubated for 2–3 days at 37°C. The infection
efficiency was then detected under a fluorescence microscope
according to the GFP expression, which was detected by fluorescence
microscopy (TE300; Nikon Corporation, Tokyo, Japan).
To examine the effect of enhanced suppressor of
cytokine signaling 4 (SOCS4) expression, a SOCS4 overexpressing
vector (pcDNA3.1-SOCS4) was constructed by sub-cloning the
full-length wild-type (WT) SOCS4 coding sequence into pcDNA3.1
[forward (+)], which was also confirmed by sequencing. The pcDNA
plasmids were designed and produced by GenePharma (Shanghai, China)
and transfected into NECs according to the manufacturer's protocol.
Briefly, the wild-type version was PCR-amplified and inserted
between the BamHI and NotI restriction sites of the pcDNA3.1+
vector. Then cDNA was generated from the cells with the
oligonucleotides UpBam and DownNot1. GFP was detected using an
anti-GFP rabbit IgG. Transfection with an empty construct pcDNA3.1
was performed in the control group.
Interleukin-13 (IL-13) stimulation of
NECs
Following lentiviral infection, NECs were stimulated
with 10 or 50 ng/ml IL-13 (R&D Systems, Inc., Minneapolis, MN,
USA) for 24 h or 14 days in bronchial epithelial growth medium. In
the control group the NECs were cultured in bronchial epithelial
growth medium supplemented with 10% FBS without test substances.
The medium was changed twice a week. Cell supernatants and pellets
were collected and centrifuged for 3 min at 100 × g at 4°C for
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis to determine the mRNA and
protein levels, respectively, of the target genes.
Prediction of miR-let-7e target
genes
The target genes of miR-let-7e were predicted based
on three public bioinformatics databases, including miRanda
(www.microrna.org/) and TargetScan Human Release
6.2 (www.targetscan.org/) (15). The target genes were selected by
miRanda if the mirSVR score ≤-0.1 and by TargetScan if the total
context score was ≤-0.1 as previously descried (16). As predicted by miRanda and
TargetScan, there was a complementary sequence between miR-let-7e
and SOCS4 3′UTR therefore the levels of these were further
examined.
Dual-luciferase reporter assay
The 3′-UTR of SOCS4, with WT or mutant (Mut) binding
sites for miR-let-7e, was amplified and cloned into the XbaI
site of the pGL3 vector (Promega Corp., Madison, WI, USA) to
generate the plasmid pGL3-WT-SOCS4-3′-UTR or pGL3-Mut-SOCS4-3′-UTR,
respectively. The mutant 3′-UTR was constructed by inserting five
mismatch mutations into the putative seed regions of SOCS4. For the
luciferase reporter assay, NECs were co-transfected with luciferase
reporter vectors and with miR-let-7e mimic or the corresponding
negative control (GenePharma Co., Ltd., Shanghai, China). The
pRL-TK plasmid (Promega Corp.) was used as a normalizing control.
The luciferase activity was analyzed using a Dual-Luciferase
Reporter Assay System (Promega Corp.) after 48 h of incubation.
RNA extraction and RT-qPCR
Total RNA was extracted from tissues isolated from
mice and humans and NECs using an RNAiso Plus reagent (Takara Bio,
Inc., Dalian, China). RNA concentration was assessed by a
spectrophotometer (DU800, Beckman Coulter, Inc., Brea, CA, USA) at
260 and 280 nm. RNA purity was measured by spectrophotometer
(MD2000D; Civic BioScience Ltd., Beloeil, QC, Canada). Subsequent
to measuring the RNA concentration and purity, total RNA was
reverse transcribed into cDNA using PrimeScript RT Reagent kit
(Promega Corp.). Primers were designed with Primer 5.0 software
(Premier Biosoft International, Palo Alto, CA, USA) and the
sequences are shown in Table I. The
reverse primer used for miR-let-7e and U6 (internal control) was
the Uni-miR qPCR primer, which was provided by the SYBR PrimeScript
miRNA RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China).
The RT reaction and qPCR were performed with an ABI PRISM 7300 Fast
Real-Time PCR system (Thermo Fisher Scientific, Inc.). PCR was
performed under the following parameters: 1 predenaturation cycle
of 1 min at 94°C, 36 cycles of 95°C for 30 sec, 60°C for 30 sec,
72°C for 2 min, and a final extension at 72°C for 5 min. The PCR
mixture (50 µl) contained 10 ng of linearized plasmid DNA,
1xAmpliTaq Gold PCR buffer (Applied Biosystems, Foster City, CA,
USA), 1.5 mM MgCl2 (Applied Biosystems; Thermo Fisher
Scientific, Inc.), 0.2 mM deoxynucleoside triphosphates (Thermo
Fisher Scientific, Inc.), 0.3 µM forward primer, 0.3 µM reverse
primer, and 5 U/µl DyNAzyme II polymerase. The products were
separated by electrophoresis on 0.2% agarose gels, and the
expression levels were calculated using the 2−ΔΔCq
method (17).
 | Table I.Primers used for target
amplification. |
Table I.
Primers used for target
amplification.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| SOCS4 |
TGTTCGTCCATTGAGTTGGA |
GAAGCACTGTTGGCAGTTAT |
| JAK1 |
CTTCTCTGAAGTAGCTTTGGAAAG |
AATAGTGGTGAACATCTAGGAGAG |
| STAT3 |
GCTTCCTGCAAGAGTCGAAT | ATTGGCTTCTCAA
GATACCTG |
| GAPDH |
TGGACTCCACGACGTACTCAG |
CGGGAAGCTTGTCATCAATGGAA |
Western blot analysis
Total protein was extracted using
radioimmunoprecipitation assay cell lysis buffer (BD Biosciences,
San Jose, CA, USA) and the protein concentration was determined by
a bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Next, protein was separated by 12% SDS-PAGE and
blotted on nitrocellulose membranes. The membranes were blocked
with 1% TBS-Tween-20 containing 5% non-fat milk for 1 h at room
temperature, washed with PBS three times and subsequently incubated
with monoclonal antibodies at 4°C overnight. The primary antibodies
used were as follows: Anti-SOCS4 antibodies (ab170437; dilution
1:1,000), anti-p-Janus kinase 1 (JAK1) antibodies (ab138005;
dilution 1:1,000), anti-JAK1 antibodies (ab47435; dilution
1:1,000), anti-p-signal transducers and activators of transcription
3 (STAT3) antibodies (ab76315; dilution 1:1,000), anti-STAT3
antibodies (ab68153; dilution 1:1,000). GAPDH (ab9485; dilution
1:2,000) was used as an internal reference. The membranes were then
incubated with horseradish peroxidase-conjugated goat anti-rabbit
secondary antibodies (ab6721; dilution 1:5,000) for 1 h at room
temperature. All antibodies were purchased from Abcam. Signals were
visualized with SuperSignal West Pico Chemiluminescent substrate
(Pierce; Thermo Fisher Scientific, Inc.). The samples in each group
were analyzed three times.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Differences were analyzed by a two-tailed t-test or
one-way analysis of variance, followed by post-hoc Bonferroni
tests. Statistical analysis was conducted using SPSS software
(version 16.0; SPSS, Inc., Chicago, IL, USA) for Windows.
Differences with a P-value of <0.05 were considered as
statistically significant.
Results
Expression of miR-let-7e in blood
samples and tissues of mice and patients
The expression levels of miR-let-7e in the blood and
nasal mucosa of AR mice were detected using RT-qPCR. The result
revealed that the expression of miR-let-7e was significantly
decreased in the serum and nasal mucosa tissue of AR mice when
compared with the control group (P<0.01; Fig. 1A and B). In addition, the nasal
mucosa tissues of AR and NAR patients were also collected, and the
expression levels of miR-let-7e in these tissues were detected. As
shown in Fig. 1C, the expression of
miR-let-7e was significantly lower in the nasal mucosa of AR
patients in comparison with that in NAR patients (P<0.01).
Expression of inflammatory factors in
mouse blood samples
Subsequent to successfully establishing an AR mouse
model with OVA administration, the protein expression levels of
inflammatory factors histamine, IgE and TNF-α in the mouse blood
were determined by ELISA. As presented in Fig. 2, the three serum inflammatory factors
presented significantly higher levels in AR mice as compared with
those in the control group (P<0.01 or P<0.001).
Effect of miR-let-7e overexpression on
inflammatory factor expression
To investigate the association between miR-let-7e
and inflammatory factors, NECs were isolated from AR patients and
transfected with miR-let-7e mimic or negative control.
Subsequently, NECs were stimulated with IL-13 to observe the
regulatory association between miR-let-7e overexpression and
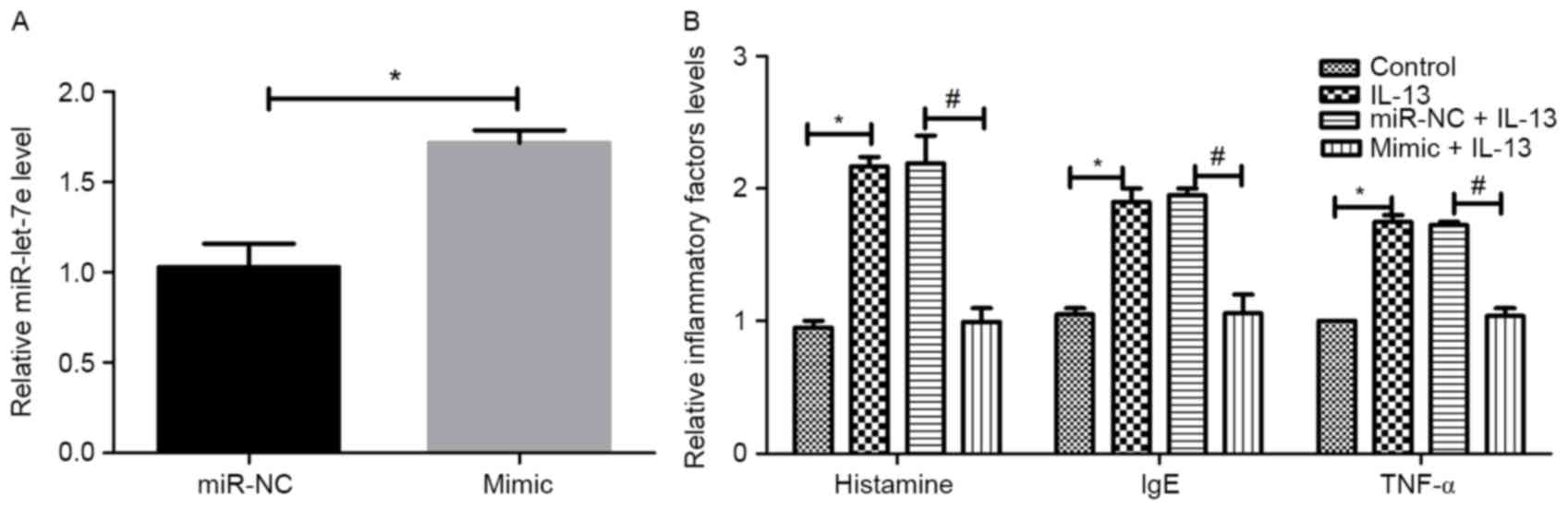
inflammatory factors. The transfection efficiency is shown in
Fig. 3A. Compared with the control,
the expression of miR-let-7e increased significantly in the
miR-let-7e mimic group (P<0.05), suggesting that miR-let-7e
overexpression was successfully performed. As revealed in Fig. 3B, the administration of IL-13
significantly increased the expression of the inflammatory factors,
histamine, IgE and TNF-α compared with the control group
(P<0.05). However, no significant differences were observed
between the IL-13 group and the miR-NC+IL-13 group. The effects of
IL-13 were reversed by miR-let-7e overexpression in the mimic+IL-13
group compared with the miR NC+IL-13 group (P<0.05).
Prediction and examination of the
targeting effect of miR-let-7e on SOCS4
The candidate target genes of miR-let-7e were
predicted based on three public miRNA databases. It was observed
that the 3′-UTR of SOCS4 was a potential miR-let-7e binding site,
suggesting that SOCS4 may be a direct target of miR-let-7e in AR
(Fig. 4A). Subsequently, a
dual-luciferase reporter assay was conducted to further validate
whether miR-let-7e is able to bind to the 3′-UTR of SOCS4 in NECs.
The results demonstrated that overexpression of miR-let-7e by
transfection with miR-let-7e mimic resulted in significant
suppression of the SOCS4-3′-UTR reporter luciferase activity
(P<0.05), while the mutant SOCS4-3′-UTR abrogated the
suppressive effect of the miR-let-7e mimic (Fig. 4B). In addition to the luciferase
activity analysis, the mRNA and protein levels of SOCS4 in NECs
were detected by RT-qPCR and western blot analysis, respectively.
It was identified that miR-let-7e overexpression significantly
inhibited the endogenous mRNA and protein expression levels of
SOCS4 (P<0.05; Fig. 4C and D),
indicating that miR-let-7e may negatively regulate SOCS4 expression
in NECs.
Effects of SOCS4 overexpression on
inflammatory factor expression
The study then investigated the effect of SOCS4
overexpression on the expression levels of various inflammatory
factors by transfection of NECs with pcDNA3.1-SOCS4. As shown in
Fig. 5A and B, SOCS4 was
significantly overexpressed following the transfection (P<0.01).
The expression levels of histamine, IgE and TNF-α subsequent to
SOCS4 overexpression were detected using ELISA. As presented in
Fig. 5C, the results revealed that
the inflammatory factors were significantly increased by IL-13
compared with the control, but this effect was significantly
reversed by simultaneous overexpression of miR-let-7e and IL-17.
The results demonstrated that overexpressed SOCS4 abrogated the
effect of miR-let-7e overexpression on the inflammatory factors
induced by IL-13 (P<0.05). This suggested that miR-let-7e may
inhibit inflammation by regulating the expression of SOCS4.
Effect of miR-let-7e overexpression on
JAK1/STAT signaling pathway
To further examine the underlying mechanisms of
miR-let-7e regulating SOCS4, the effect of miR-let-7e on JAK1/STAT3
pathway was determined. It was observed that miR-let-7e
overexpression significantly increased the mRNA and protein
expression levels of p-JAK1 and p-STAT3, as well as decreased the
SOCS4 expression (P<0.01; Fig.
6). These results suggested that miR-let-7e may regulate the
SOCS4 expression via activating the JAK1/STAT3 pathway.
Discussion
The present study demonstrated that miR-let-7e was
downregulated in patients and mice with AR when compared with the
controls. miR-let-7e overexpression inhibited the expression levels
of various inflammatory factors in AR mice and IL-13-stimulated
NECs. Further experiments identified that SOCS4 was a potential
target gene of miR-let-7e and was negatively regulated by
miR-let-7e. In particular, overexpressed SOCS4 was able to abrogate
the anti-inflammatory activity of miR-let-7e overexpression.
Furthermore, it was observed that miR-let-7e overexpression
activated the JAK1/STAT3 signaling pathway.
Previous studies have suggested the role of miRNAs
in controlling allergic airway inflammation (10,11). The
miR-let-7 family of miRNAs is highly conserved across animal
species, serving important roles in the regulation of cell
proliferation and differentiation (18). Notably, altered expression of
miR-let-7 has also been reported in inflammation. For instance,
Iliopoulos et al (19) have
demonstrated that miR-let-7 is repressed in inflammation, resulting
in increased expression of pro-inflammatory cytokines and enhanced
inflammatory responses. The results of the present study revealed
the lower expression of miR-let-7 in patients with AR and a mouse
model of AR, which was consistent with a previous study (1). The results also indicated that
overexpression of miR-let-7 may exert an anti-inflammatory effect
on the development of AR. Kumar et al (20) administrated miR-let-7 mimics to mice
and observed that overexpression of miR-let-7 repressed IL-13
production and reduced the inflammation in the allergic airway
inflammation. IL-13 is a cytokine produced by T helper type 2 cells
and it has been confirmed that IL-13 is required for
allergen-induced airway inflammation and the production of mucus
(21). In accordance with the
aforementioned studies, the results of the current study also
observed that administration of IL-13 significantly increased
inflammatory factors, whereas miR-let-7e overexpression inhibited
the expression levels of the inflammatory factors histamine, IgE
and TNF-α, suggesting the anti-inflammatory activity of
miR-let-7e.
SOCS4 belongs to the SOCS family that is composed of
eight members, including cytokine-inducible Src-homology 2 protein
(CIS), as well as SOCS1 to 7 (22,23).
These proteins are known to be cytokine-inducible negative
regulators of cytokine signaling, as well as key regulators of
innate and adaptive immunity (24).
Galic et al (25) also
reported that the SOCS family proteins serve an critical role in
mediating inflammatory responses in immune cells and metabolic
organs. In the present study, SOCS4 was a potential target gene of
miR-let-7e, and was negatively regulated by this miRNA. SOCS4
overexpression was able to abrogate the anti-inflammatory activity
of miR-let-7e, which suggested that the anti-inflammatory function
of miR-let-7e may be achieved by regulating SOCS4. The results
demonstrated that SOCS4 overexpression may serve as an important
pro-inflammatory factor in the development of AR.
The JAK1/STAT3 pathway is a conserved signaling
pathway employed by diverse cytokines, growth factors, interferons
and associated molecules (26). This
pathway transmits information from extracellular chemical signals
to the nucleus, which leads to the expression of genes involved in
immunity, proliferation, apoptosis and differentiation. Disrupted
JAK1/STAT3 functionality results in immune deficiency syndromes
(27). It has been reported that
cytokine receptors are constitutively associated with members of
the JAK family of protein tyrosine kinases. In addition, the STAT
family of transcription factors serves a critical role in the
regulation of physiological responses to cytokine stimulation
(28). JAK1/STAT3 signaling has been
reported to be closely involved in inflammation (29,30).
Once activated, JAK1/STAT3 signaling can lead to the expression of
inflammation-associated genes (31).
Notably, Alexander (32) has
identified that the SOCS proteins are key negative regulators of
the JAK1/STAT3 pathway. In the present study, miR-let-7e
overexpression activated the JAK1/STAT3 pathway and decreased the
SOCS4 expression, which suggested that miR-let-7e may negatively
regulate SOCS4 in order to activate the JAK1/STAT3 signaling
pathway.
In conclusion, the present study observed that
miR-let-7e may serve an important role in the progression and
development of AR. Overexpression of miR-let-7e exerted an
anti-inflammatory effect by targeting SOCS4 in the progression and
development of AR, which may be achieved by activating the
JAK1/STAT3 signaling pathway. Therefore, miR-let-7e and SOCS4 may
be used as biomarkers in the diagnosis and treatment of AR.
Acknowledgements
This study was supported by a grant from the Jiangxi
Provincial Natural Science Foundation (no. 20151BAB205028).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Suojalehto H, Toskala E, Kilpeläinen M,
Majuri ML, Mitts C, Lindström I, Puustinen A, Plosila T, Sipilä J,
Wolff H and Alenius H: MicroRNA profiles in nasal mucosa of
patients with allergic and nonallergic rhinitis and asthma. Int
Forum Allergy Rhinol. 3:612–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luo Y, Deng Y, Tao Z, Chen S, Xiao B, Ren
J, Chen Z, Han J, Kong Y, Xu Y and Deng M: Regulatory effect of
microRNA-135a on the Th1/Th2 imbalance in a murine model of
allergic rhinitis. Exp Ther Med. 8:1105–1110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walker S, Khan-Wasti S, Fletcher M,
Cullinan P, Harris J and Sheikh A: Seasonal allergic rhinitis is
associated with a detrimental effect on examination performance in
United Kingdom teenagers: Case-control study. J Allergy Clin
Immunol. 120:381–387. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nihlén U, Greiff L, Montnémery P, Löfdahl
CG, Johannisson A, Persson C and Andersson M: Incidence and
remission of self-reported allergic rhinitis symptoms in adults.
Allergy. 61:1299–1304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miadonna A, Milazzo N, Gibelli S, Salmaso
C, Lorini M and Tedeschi A: Nasal response to a single antigen
challenge in patients with allergic rhinitis-inflammatory cell
recruitment persists up to 48 h. Clin Exp Allergy. 29:941–949.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ding H, Wu YL, Wang YX and Zhu FF:
Characterization of the microRNA expression profile of cervical
squamous cell carcinoma metastases. Asian Pac J Cancer Prev.
15:1675–1679. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen RF, Huang HC, Ou CY, Hsu TY, Chuang
H, Chang JC, Wang L, Kuo HC and Yang KD: MicroRNA-21 expression in
neonatal blood associated with antenatal immunoglobulin E
production and development of allergic rhinitis. Clin Exp Allergy.
40:1482–1490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang X: The emerging role of microRNAs in
asthma. Mol Cell Biochem. 353:35–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shaoqing Y, Ruxin Z, Guojun L, Zhiqiang Y,
Hua H, Shudong Y and Jie Z: Microarray analysis of differentially
expressed microRNAs in allergic rhinitis. Am J Rhinol Allergy.
25:e242–e246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Teng Y, Zhang R, Liu C, Zhou L, Wang H,
Zhuang W, Huang Y and Hong Z: miR-143 inhibits
interleukin-13-induced inflammatory cytokine and mucus production
in nasal epithelial cells from allergic rhinitis patients by
targeting IL13Rα1. Biochem Biophys Res Commun. 457:58–64. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bousquet J, Lund VJ, van Cauwenberge P,
Bremard-Oury C, Mounedji N, Stevens MT and El-Akkad T:
Implementation of guidelines for seasonal allergic rhinitis: A
randomized controlled trial. Allergy. 58:733–741. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi J, Luo Q, Chen F, Chen D, Xu G and Li
H: Induction of IL-6 and IL-8 by house dust mite allergen Der p1 in
cultured human nasal epithelial cells is associated with
PAR/PI3K/NFkappaB signaling. ORL J Otorhinolaryngol Relat Spec.
72:256–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peterson SM, Thompson JA, Ufkin ML,
Sathyanarayana P, Liaw L and Congdon CB: Common features of
microRNA target prediction tools. Front Genet. 5:232014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi H, Chen J, Li Y, Li G, Zhong R, Du D,
Meng R, Kong W and Lu M: Identification of a six microRNA signature
as a novel potential prognostic biomarker in patients with head and
neck squamous cell carcinoma. Oncotarget. 7:21579–21590.
2016.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pasquinelli AE, Reinhart BJ, Slack F,
Martindale MQ, Kuroda MI, Maller B, Hayward DC, Ball EE, Degnan B,
Müller P, et al: Conservation of the sequence and temporal
expression of let-7 heterochronic regulatory RNA. Nature.
408:86–89. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iliopoulos D, Hirsch HA and Struhl K: An
epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and
IL6 links inflammation to cell Transformation. Cell. 139:693–706.
2011. View Article : Google Scholar
|
|
20
|
Kumar M, Ahmad T, Sharma A, Mabalirajan U,
Kulshreshtha A, Agrawal A and Ghosh B: Let-7 microRNA-mediated
regulation of IL-13 and allergic airway inflammation. J Allergy
Clin Immunol. 128:1077–1085.e1-10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wills-Karp M, Luyimbazi J, Xu X, Schofield
B, Neben TY, Karp CL and Donaldson DD: Interleukin-13: Central
mediator of allergic asthma. Science. 282:2258–2261. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hilton DJ, Richardson RT, Alexander WS,
Viney EM, Willson TA, Sprigg NS, Starr R, Nicholson SE, Metcalf D
and Nicola NA: Twenty proteins containing a C-terminal SOCS box
form five structural classes. Proc Natl Acad Sci USA. 95:pp.
114–119. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jin HJ, Shao JZ, Xiang LX, Wang H and Sun
LL: Global identification and comparative analysis of SOCS genes in
fish: Insights into the molecular evolution of SOCS family. Mol
Immunol. 45:1258–1268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kedzierski L, Linossi EM, Kolesnik TB, Day
EB, Bird NL, Kile BT, Belz GT, Metcalf D, Nicola NA, Kedzierska K
and Nicholson SE: Suppressor of cytokine signaling 4 (SOCS4)
protects against severe cytokine storm and enhances viral clearance
during influenza infection. PLoS Pathog. 10:e10041342014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Galic S, Sachithanandan N, Kay TW and
Steinberg GR: Suppressor of cytokine signalling (SOCS) proteins as
guardians of inflammatory responses critical for regulating insulin
sensitivity. Biochem J. 461:177–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
O'Shea JJ, Schwartz DM, Villarino AV,
Gadina M, Mcinnes IB and Laurence A: The JAK-STAT pathway: Impact
on human disease and therapeutic intervention. Ann Rev Med.
66:311–328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aaronson DS and Horvath CM: A road map for
those who don't know JAK-STAT. Science. 296:1653–1655. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Krebs DL and Hilton DJ: SOCS:
Physiological suppressors of cytokine signaling. J Cell Sci.
113:2813–2819. 2000.PubMed/NCBI
|
|
29
|
Darnell JE Jr: STATs and gene regulation.
Science. 277:1630–1635. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kessler DS, Veals SA, Fu XY and Levy DE:
Interferon-alpha regulates nuclear translocation and DNA-binding
affinity of ISGF3, a multimeric transcriptional activator. Genes
Dev. 4:1753–1765. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim OS, Park EJ, Joe EH and Jou I:
JAK-STAT signaling mediates gangliosides-induced inflammatory
responses in brain microglial cells. J Biol Chem. 277:40594–40601.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alexander WS: Suppressors of cytokine
signalling (SOCS) in the immune system. Nat Rev Immunol. 2:410–416.
2002. View
Article : Google Scholar : PubMed/NCBI
|