Introduction
The occurrence of hepatic ischemia/reperfusion (I/R)
injury is inevitable in cases of trauma, shock, hepatectomy and
hepatic transplantation. These events may lead to the compromise of
liver function and structure, particularly inpatients with hepatic
steatosis or cirrhosis. To date, the exact pathogenesis of I/R
injury remains unclear. I/R injury involves a series of complex
pathophysiological mechanisms, and apoptosis is one of the most
important mechanisms of cell death after hepatic I/R injury
(1). I/R injury induces apoptosis of
hepatic cells and results in the compromise of hepatic function
(1). Therefore, it may be inferred
that inhibition of apoptosis is a promising approach for the
preservation of hepatic function.
The mitochondrial apoptosis pathway is a key step in
the process of apoptosis (2), and
the release of cytochrome c from mitochondria plays a key
role. Under conditions of I/R injury, the fine balance between
mitochondrial fusion and fission within a cell may be disrupted, as
well as mitochondrial homeostasis, predisposing the cell to
apoptosis (3,4). This suggests that protection of
mitochondria may play an important role in the maintenance of
cellular integrity during I/R injury (5). Mitofusin 2 (Mfn2), a protein that is
present in the outer membrane of the mitochondrion, is a
dynamin-like protein that mediates fusion of the mitochondria and
plays an important role in the regulation of mitochondrial
morphology and function (6,7). The fragmentation of mitochondria is
aggravated in apoptotic hepatocytes when the expression of Mfn2 is
downregulated in hepatic I/R injury (8). Therefore, investigating changes in the
expression of Mfn2 may help to determine the role of the
mitochondrial apoptosis pathway in hepatic I/R injury.
Breviscapine, a traditional Chinese medicine, is a
flavonoid derived from the natural plant Erigeron
breviscapus. Scutellarin is the main active ingredient and its
structural formula is 4,5,6-trihydroxyflavone-7-glucuronide
(9). Previous studies have
demonstrated that breviscapine decreases cardiomyocyte apoptosis
and neuroapoptosis during I/R injury (10,11), and
breviscapine is known to possess protective propertiesin myocardial
and cerebral I/R injury. However, studieson its protective effects
against hepatic I/R injury are currently scarce. Our previous study
demonstrated that breviscapine preconditioning attenuates hepatic
I/R injury by inhibiting the development of liver oxidative stress
(12). Therefore, it may be inferred
that the reduction of hepatocellular apoptosis may be involved in
the protective effect of breviscapine against hepatic I/R
injury.
Based on these findings, a hepatic I/R rat model was
established in the present study to investigate the protective
effect of breviscapine against hepatic I/R injury after different
durations of ischemia. The results revealed that breviscapine
reduced cell apoptosis in hepatic I/R injury through upregulation
of the expression of the Mfn2 protein. The aim of the present study
was to provide a theoretical basis for the clinical application of
breviscapine to treat hepatic I/R injury.
Materials and methods
Experimental animals and treatment
design
A total of 40 male Sprague-Dawley rats, weighing
225–250 g, were purchased from Jinan Peng Yue Experimental Animal
Breeding Co., Ltd. (Shandong, China) and maintained in a
temperature-controlled room with alternating 12 h light-dark cycles
under pathogen-free conditions. All animal study protocols used in
the present study were approved by the Laboratory Animal Ethics
Committee of Jinan University and conducted in accordance with
their guidelines. The 40 male Sprague-Dawley rats were randomly
divided into five groups (n=8 per group) as follows: i) Sham group:
The animals underwent midline laparotomy only, without vessel
occlusion. ii) I/R + NS1 group: Equivalent volume of normal saline
was administered via the tail vein 1 h prior to surgery and
immediately postoperatively. Hepatic reperfusion was recovered
after 20 min of ischemia and the abdomen was closed. iii) I/R +
Bre1 group: A breviscapine injection (10 mg/kg; Hunan Hang Seng
Pharmaceutical Co., Ltd., Hong Kong, China) was administered via
the tail vein 1 h prior to surgery and immediately postoperatively.
Hepatic reperfusion was recovered after 20 min of ischemia and the
abdomen was closed. iv) I/R + NS2 group: All surgical procedures
were performed as in the I/R + NS1 group, but the time of ischemia
was increased to 60 min. v) I/R + Bre2 group (n=8): All surgical
procedures were performed as in the I/R + NS2 group, but
breviscapine (10 mg/kg) instead of normal saline was injected via
the tail vein. All the rats were fasted but allowed free access to
water for 12 h prior to surgery and were anesthetized with 10%
chloralhydrate (4 ml/kg, i.p.). Surgery was performed as previously
described (13). Briefly, laparotomy
was performed through a small midline incision and the hilum of the
liver was exposed. The hepatic artery, portal vein and bile duct to
the left anterior and median hepatic lobes were clamped using a
non-invasive vascular clamp, leading to ischemia of almost 70% of
the liver. The clamps were removed after the aforementioned times.
After 6 h of reperfusion, the animals were sacrificed, and blood
and liver tissue samples were collected and immediately stored at
−80°C.
Serum aspartate and alanine
aminotransferase (AST and ALT) levels
In order to determine the degree of hepatic injury,
blood was collected in sterile syringes without anticoagulant and
then centrifuged to separate the serum. The serum AST and ALT
levels were determined using a Selectra-E auto analyzer.
Histological assessment
The liver tissue wasfixed in 10% neutral buffered
formalin and the specimens were embedded in paraffin. The tissue
were sliced into 4-µm sections and stained with hematoxylin and
eosin (H&E) for histopathological examination. The histological
severity of the hepatic injury was evaluated under a light
microscope by a pathologist who was blinded to the research.
Histological examination was based on the standard procedures
reported previously (14).
Tissue apoptosis assayed by TUNEL
Hepatocellular apoptosis was assessed using the
terminal deoxynucleotidyl transferase deoxyuridine triphosphate
(dUTP) nick end labeling (TUNEL). TUNEL staining was performed
according to the manufacturer's instructions using an in
situ apoptosis detection kit (Bio-Techne China Co., Ltd.,
Shanghai, China). Apoptotic cells exhibiteda brown-stained nucleus
and were identified among viablecells. The results were expressed
as the proportion of TUNEL-positive cells amongthe total number of
hepatocytes in 5 non-overlapping serial scopes taken from each
slide, with a random start, at ×200 magnification.
Western blotting
The levels of cleaved caspase-3, cytoplasm
cytochrome c and Mfn2 were determined using western blot analysis.
The levels of cytochrome c were determined in cytoplasmic extracts
as previously described (15).
Briefly, liver tissues were ground and lysed in lysis buffer
(Promega Corporation, Madison, WI, USA). The lysates were
centrifuged at 850 × g for 10 min at 4°C to remove the nuclei and
cell debris, and the supernatants were further centrifuged at
10,000 × g for 10 min at 4°C. Subsequently, the supernatants were
collected for cytoplasmic cytochrome c analysis. Protein
concentration was determined by the BCA protein assay (Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). An equal amount
of protein from each sample was separated by homogeneous 10%
SDS-polyacrylamide gels and then transferred onto PVDF membranes.
The membranes were blocked with 5% fat-free milk blocking buffer at
room temperature and then incubated with primary antibodies
overnight at 4°C. After being washed with TBST buffer, the
corresponding secondary antibodies were used to identify primary
antibody binding. After washing, the bands were visualized using
enhanced chemiluminescence reagents (ECL; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and the FluorChem 5500 imaging system
(Alpha Innotech Corp., San Leandro, CA, USA). The band intensities
were evaluated by densitometric analysis using Image J 1.50
software (National Institutes of Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from hepatic tissues using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocols. The RNA purity and
concentration were determined spectrophotometrically using the
NanoDrop ND-1000 (NanoDrop Technologies; Thermo Fisher Scientific,
Inc., Wilmington, DE, USA). RNA (100 ng) was reverse-transcribed
into complementary DNA (cDNA) with PrimeScript RT Master Mix
(Takara Biotechnology Co., Ltd., Dalian, China) in a 20 ml final
reaction volume, according to the manufacturer's protocol. The
primer sequences (5′-3′) were as follows: β-actin (forward)
TGCTATGTTGCCCTAGACTTCG, (reverse) GTTGGCATAGAGGTCTTTACGG; Mfn2
(forward) GATGACAGAGGAAGTGGAAAGGC, (reverse)
ACAGACACAGGAAGAAGGGGCT. Relative expression was measured by qPCR
using SYBR Green PCR Master Mix SYBR Premix Ex Taq™ II (Takara
Biotechnology Co., Ltd.,) on a Mastercycler ep realplex4
(Eppendorf, Hamburg, Germany). Mfn2 gene expression profiles were
normalized to β-actin and calculated using real-time quantitative
PCR and the 2−ΔΔCq method.
Statistical analysis
Data analysis was performed using the SPSS
statistical package, version 13 (SPSS, Inc., Chicago, IL, USA). The
results are expressed as the mean ± standard deviation and one-way
analysis of variance was used to compare groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Breviscapine alleviates I/R-induced
deterioration of hepatic function
The serum AST and ALT levels in each group are shown
in Fig. 1. The levels of serum ALT
and AST in the sham group were 81.63±14.49 and 409.8±23.06 U/l,
respectively. After 20 and 60 min of ischemia and 6 h of
reperfusion, the levels of serum ALT and AST were significantly
increased in the two groups compared with the sham group
(P<0.01). The serum levels of ALT and AST were significantly
decreased in the I/R + Bre groups compared with those in the I/R +
NS groups (P<0.05), particularly in the I/R + Bre2 group
(P<0.01). These results indicated that breviscapine can reduce
the serum level of ALT and AST compared with normal saline. These
data were associated with histological tissue alterations and
H&E staining revealed that the structure of hepatic tissue in
the sham group appeared normal (Fig.
2A). Compared with the sham group, the livers of rats in the
normal saline control groups exhibited extensive hepatocyte
swelling with significantly more prominent vacuolar degeneration,
unclear boundaries, narrower hepatic sinusoids and hepatocellular
necrosis, accompanied by a large number of infiltrating
neutrophils, particularly in the I/R + NS2 group. Tissues from the
I/R + Bre groups exhibited less prominent neutrophil infiltration,
necrosis and hepatocyte swelling, particularly in the I/R + Bre2
group. As shown in Fig. 2B, tissues
from the normal saline control groups exhibited a higher
pathological score compared with the sham group. The level of
hepatic function injury improved in the I/R + Bre groups,
particularly in the I/R + Bre2 group (P<0.01) when compared with
the normal saline control groups. These results demonstrated that
breviscapine improved the compromised hepatic function induced by
I/R, and this protective effect became more pronounced with
prolongation of the time of ischemia.
Breviscapine reduces the apoptotic
index (AI) of hepatocytes following I/R injury
A TUNEL assay was used to assess the level of
apoptosis and AI was used to assess the protective effect of
breviscapine. As shown in Fig. 3,
the AI observably increased in the normal saline groups compared
with the sham group (P<0.001). Furthermore, the AI in the I/R +
Bre1 and I/R + Bre2 groups was markedly reduced compared with the
normal saline groups (P<0.01). These results suggest that
breviscapine protected the hepatocytes against I/R-induced
apoptosis.
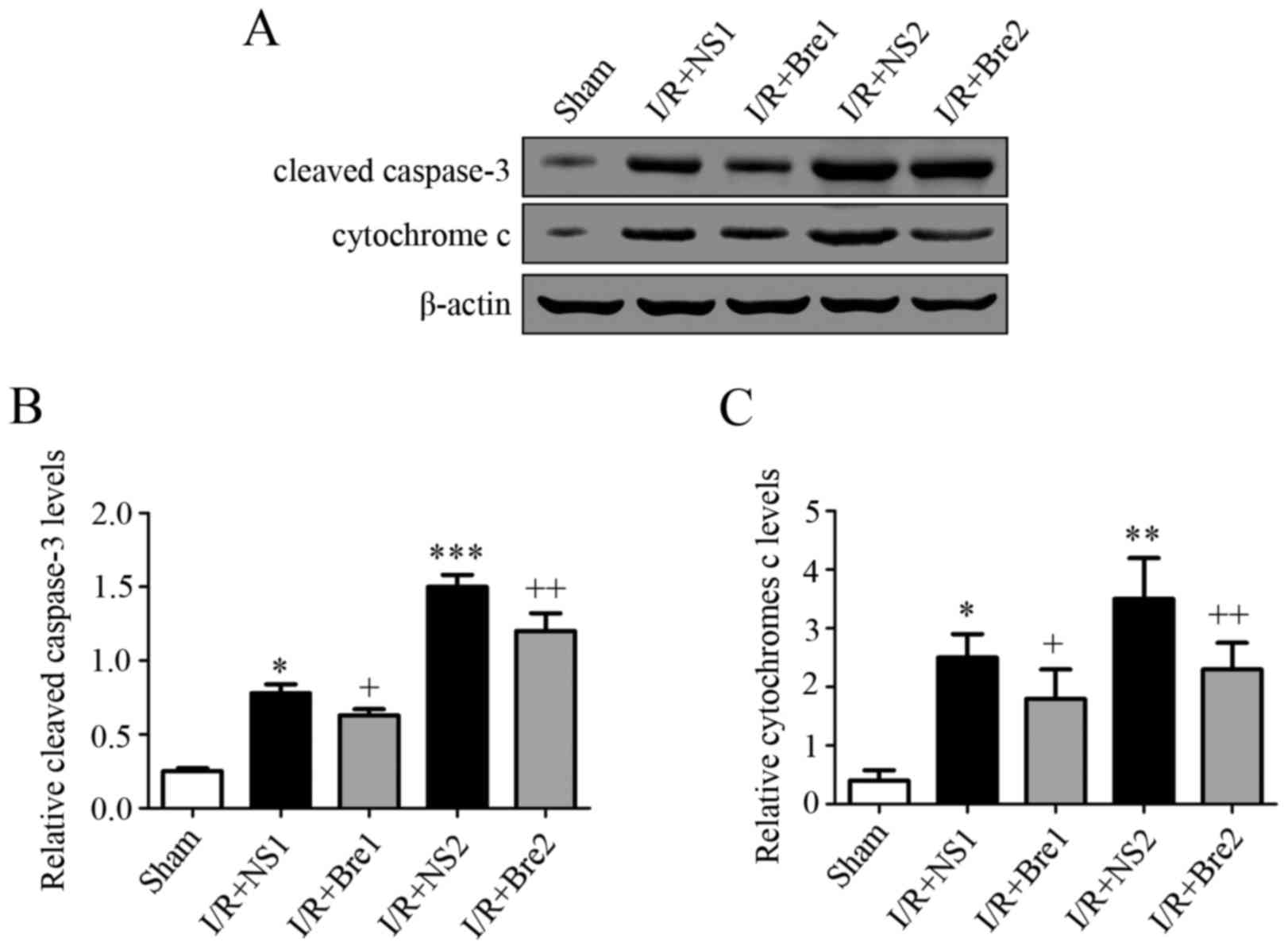
Breviscapine restores elevated cleaved
caspase-3 expression in the hepatic and cytosolic translocation of
cytochrome c
To further explore the possible mechanism by which
breviscapine reduces hepatocyte apoptosis, the expression level of
caspase-3 and cytochrome c was detected by western blotting. As
shown in Fig. 4, the normal saline
groups exhibited a higher expression of cytochrome c and cleaved
caspase-3 compared with the sham group (P<0.05), and this
discrepancy became more evident with prolongation of the time of
ischemia, particularly in the I/R + NS2 group (P<0.01). These
results demonstrated that breviscapine reduced the release of
mitochondrial cytochrome c and the expression of cleaved caspase-3
compared with the normal saline groups (P<0.05). It is well
established that the release of cytochrome c is associated with
caspase family activation. Our findings suggest that breviscapine
plays a role in preventing mitochondrial-related hepatocyte
apoptosis by suppressing cytochrome c release and caspase
activation during I/R injury.
Breviscapine downregulates the
expression of Mfn2 in hepatocytes during I/R injury
Mfn2 is a mediator during mitochondrial fusion, an
evolutionarily conserved process responsible for the surveillance
of mitochondrial homeostasis. To further explore the effect of
breviscapine on mitochondrial function, changes in the expression
of Mfn2 were detected by western blotting. The results demonstrated
that the expression of Mfn2 in the I/R + NS groups was
significantly lower compared with the sham group (P<0.05). The
expression of Mfn2 in the I/R + Bre groups was significantly higher
compared with the I/R + NS groups (P<0.05; Fig. 5). Finally, RT-qPCR was performed to
further confirm the hypothesis that the mRNA concentration of Mfn2
in the normal saline groups wassignificantly decreased compared
with that in the sham group (P<0.05). However, the mRNA
concentrations of Mfn2 in the I/R + Bre groups were increased
compared with those in the normal saline groups (Fig. 5). Our findings indicate that
breviscapine protects hepatocytes against I/R injury by
upregulating the expression of Mfn2.
Discussion
With the growing number of patients undergoing liver
transplantation or hepatectomy, hepatic I/R injury is inevitable;
this type of injury may also occur in other clinical settings,
including trauma and hemorrhagic shock. Hepatic I/R injury not only
results in liver dysfunction, but also increases mortality rate and
the period of hospitalization. Therefore, alleviating the effects
of hepatic I/R injury is urgent in clinical practice.
Breviscapine is a widely used traditional Chinese
medicine that is derived from the natural plant Erigeron
breviscapus. It is extensively used in clinical settings for
treating cerebral infarction, cardiovascular disease and stroke in
China. It was reported that breviscapine decreased cardiomyocyte
apoptosis and neuroapoptosis during I/R injury (10,11).
However, whether breviscapine is able to suppress hepatic I/R
injury remains obscure. In the present study, breviscapine was
found to decrease ALT and AST levels, ameliorate histological
findings and inhibit subsequent hepatocyte apoptosis following
hepatic I/R injury in rats. Therefore, it was hypothesized that
breviscapine protects hepatocytes from I/R-induced injury and the
mitochondrial-dependent apoptotic pathway may be involved in its
protective effect.
The serum levels of ALT and AST reflect the degree
of liver function damage. Any injury to the cellular membrane of
hepatocytes, such as I/R-induced hepatic injury, may result in a
rapid increase of the serum ALT level (16). If the hepatic injury persists, AST
expression in the mitochondria and circulation would be increased
(17). The present study
demonstrated that liver tissues from the breviscapine-treated
groups exhibited a smaller increase in ALT and AST levels compared
with the normal saline-treated groups. The protective effect of
breviscapine was also supported by the fact that the
pathomorphological changes of the liver tissue in the I/R + Bre
groups were milder compared with those in the normal saline groups.
These results suggest that breviscapine exerted a protective effect
on hepatocytes and reduced I/R injury.
Apoptosis is the major type of cell death and it
plays a crucial role in hepatic I/R injury (1). Mitochondria play a key role in the
regulation of cell apoptosis. It was previously demonstrated that
I/R injury leads to the opening of a non-specific pore in the inner
mitochondrial membrane, referred to as the mitochondrial
permeability transition pore (mPTP) (18). The opening of the mPTP results in
dissipation of the mitochondrial membrane potential (ΔΨm), followed
by progressive mitochondrial swelling and the loss of soluble
components of the respiratory chain, which eventually leads to
rupture of the outer mitochondrial membrane and leakage of the
pro-apoptotic protein cytochrome c from the mitochondria to
the cytosol (19). I/R-induced mPTP
opening leads to ΔΨm collapse and cytochrome c release from
mitochondria to the cytosol, where it interacts with the apoptosis
protease-activating factor-1 (APAF-1) that causes activation of
caspase-9, which in turn activates caspase-3 (20,21).
Caspase-3 is a major factor in the process of cell apoptosis and
its activated form, cleaved caspase-3, marks the irreversible phase
of cell apoptosis.
We herein hypothesized that breviscapine may be able
to reduce apoptosis in hepatic I/R injury via reducing the activity
of mitochondria-related apoptotic pathways. In the present study,
it was observed that breviscapine effectively downregulated the
expression of cleaved caspase-3 in hepatocytes and reduced the
translocation of cytochrome c from mitochondria to the
cytosol. The results suggested that breviscapine may exert
anti-apoptotic effects against hepatic I/R injury.
Mitochondria are dynamic organelles and their
morphological transitions are mainly effected by undergoing fission
and fusion, processes essential to mitochondrial homeostasis
(21,22). The fine balance between mitochondrial
fusion and fission within a cell may be affected by I/R injury
(3), which promotes mPTP opening
(23) and predisposes the cell to
apoptosis (4). Mfn2, a mitochondrial
outer membrane protein, is essential for mitochondrial fusion and
it regulates mitochondrial metabolism and cell death (6). The fragmentation of mitochondria is
aggravated when the expression of Mfn2 is suppressed, and it
observably increases the sensitivity of the cell to apoptotic
signals (24). Mfn2 is vital for
maintaining the mitochondrial structure. It has been demonstrated
that Mfn2 not only preserves mitochondrial morphology in
hepatocytes, but also is associated with the opening of mPTP in the
liver under conditions of I/R injury (25). Ong et al (22) also reported that the overexpression
of Mfn2 prevented mPTP opening induced by I/R injury in the HL-1
cardiac cell line. As mentioned above, mPTP opening leads to
cytochrome c release from the mitochondria to the cytosol.
It has been reported that upregulating the expression of Mfn2 may
reduce the release of cytochrome c (26), while downregulating the expression of
Mfn2 exacerbates ceramide-induced mitochondrial dysfunction and
release of cytochrome c (27). These findings indicate that Mfn2
reduces apoptosis through inhibiting the mitochondrial apoptosis
pathway. In the present study, the expression of Mfn2 was found to
be significantly decreased in the normal saline groups compared
with that in the sham group, while the level of Mfn2 in the I/R +
Bre groups increased compared with that in the normal saline
groups. These results suggest that the protective effect of
breviscapine against hepatic I/R maybe effected via upregulating
the expression of Mfn2.
In summary, our results demonstrated the protective
effect of breviscapine against hepatic I/R injury in an animal
model. The mechanism underlying this protective effect is likely
through inhibiting the mitochondrial apoptosis pathway and
upregulating Mfn2 expression, which reduces hepatocyte apoptosis.
Therefore, breviscapine may have potential as a novel therapeutic
agent for the treatment of hepatic I/R injury and may be of value
in a clinical setting. However, further studies are required to
fully elucidate the mechanisms underlying its effects.
Acknowledgements
The present study was supported by the Science and
Technology Program of Guangzhou, China (grant no.
2011j4100078).
References
|
1
|
Kong R, Gao Y, Sun B, Chen H, Wang G, Wang
X, Zhu H, Pan S, Xue D and Jiang H: The strategy of combined
ischemia preconditioning and salvianolic acid-B pretreatment to
prevent hepatic ischemia-reperfusion injury in rats. Dig Dis Sci.
54:2568–2576. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brady NR, Hamacher-Brady A and Gottlieb
RA: Proapoptotic BCL-2 family members and mitochondrial dysfunction
during ischemia/reperfusion injury, a study employing cardiac HL-1
cells and GFP biosensors. Biochim Biophys Acta. 1757:667–678. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frank S, Gaume B, Bergmann-Leitner ES,
Leitner WW, Robert EG, Catez F, Smith CL and Youle RJ: The role of
dynamin-related protein 1, a mediator of mitochondrial fission, in
apoptosis. Dev Cell. 1:515–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Novgorodov SA and Gudz TI: Ceramide and
mitochondria in ischemia/reperfusion. J Cardiovasc Pharmacol.
53:198–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zorzano A, Liesa M, Sebastián D, Segalés J
and Palacín M: Mitochondrial fusion proteins: Dual regulators of
morphology and metabolism. Semin Cell Dev Biol. 21:566–574. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang P, Galloway C and Yoon Y: Control of
mitochondrial morphology through differential interactions of
mitochondrial fusion and fission proteins. PLoS One. 6:e206552011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Ke W, Zhou Q, Wu Y, Luo H, Zhou H,
Yang B, Guo Y, Zheng Q and Zhang Y: Tumour necrosis factor-α
promotes liver ischaemia-reperfusion injury through the PGC-1α/Mfn2
pathway. J Cell Mol Med. 18:1863–1873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lou XY, Cheng JL and Zhang B: Therapeutic
effect and mechanism of breviscapine on cisplatin-induced
nephrotoxicity in mice. Asian Pac J Trop Med. 8:873–877. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Ji SY, Liu SZ, Jing R and Lou WJ:
Cardioprotective effect of breviscapine: Inhibition of apoptosis in
H9c2 cardiomyocytes via the PI3K/Akt/eNOS pathway following
simulated ischemia/reperfusion injury. Pharmazie. 70:593–597.
2015.PubMed/NCBI
|
|
11
|
Yiming L, Wei H, Aihua L and Fandian Z:
Neuroprotective effects of breviscapine against apoptosis induced
by transient focal cerebral ischaemia in rats. J Pharm Pharmacol.
60:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin YZ, Lu ZY, Liang XH, Li K, Peng B and
Gong J: Effect of breviscapine against hepatic ischemia reperfusion
injury. J Surg Res. 203:268–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nauta RJ, Tsimoyiannis E, Uribe M, Walsh
DB, Miller D and Butterfield A: Oxygen-derived free radicals in
hepatic ischemia and reperfusion injury in the rat. Surg Gynecol
Obstet. 171:120–125. 1990.PubMed/NCBI
|
|
14
|
Wei Y, Chen P, de Bruyn M, Zhang W, Bremer
E and Helfrich W: Carbon monoxide-releasing molecule-2 (CORM-2)
attenuates acute hepatic ischemia reperfusion injury in rats. BMC
Gastroenterol. 10:422010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gomez L, Paillard M, Thibault H, Derumeaux
G and Ovize M: Inhibition of GSK3beta by postconditioning is
required to prevent opening of the mitochondrial permeability
transition pore during reperfusion. Circulation. 117:2761–2768.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Sun JJ, Chen GY, Wang WW, Xie ZT,
Tang GF and Wei SD: Carnosic acid nanoparticles suppress liver
ischemia/reperfusion injury by inhibition of ROS, Caspases and
NF-κB signaling pathway in mice. Biomed Pharmacother. 82:237–246.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kamiike W, Fujikawa M, Koseki M, Sumimura
J, Miyata M, Kawashima Y, Wada H and Tagawa K: Different patterns
of leakage of cytosolic and mitochondrial enzymes. Clin Chim Acta.
185:265–270. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Halestrap AP, Clarke SJ and Javadov SA:
Mitochondrial permeability transition pore opening during
myocardial reperfusion-a target for cardioprotection. Cardiovasc
Res. 61:372–385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baines CP, Kaiser RA, Purcell NH, Blair
NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA,
Dorn GW, et al: Loss of cyclophilin D reveals a critical role for
mitochondrial permeability transition in cell death. Nature.
434:658–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hüttemann M, Helling S, Sanderson TH,
Sinkler C, Samavati L, Mahapatra G, Varughese A, Lu G, Liu J,
Ramzan R, et al: Regulation of mitochondrial respiration and
apoptosis through cell signaling: Cytochrome c oxidase and
cytochrome c in ischemia/reperfusion injury and inflammation.
Biochim Biophys Acta. 1817:598–609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakagawa T, Shimizu S, Watanabe T,
Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T and Tsujimoto Y:
Cyclophilin D-dependent mitochondrial permeability transition
regulates some necrotic but not apoptotic cell death. Nature.
434:652–658. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ong SB, Subrayan S, Lim SY, Yellon DM,
Davidson SM and Hausenloy DJ: Inhibiting mitochondrial fission
protects the heart against ischemia/reperfusion injury.
Circulation. 121:2012–2022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong D, Xu L, Yu Y, Zhu W, Andrews DW,
Yoon Y and Kuo TH: Regulation of Ca2+-induced permeability
transition by Bcl-2 is antagonized by Drpl and hFis1. Mol Cell
Biochem. 272:187–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Feng L, Yan M, Xu K, Yu Y and Zheng
X: Changes in mitochondrial dynamics during amyloid β-induced PC12
cell apoptosis. Mol Cell Biochem. 344:277–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Biel TG, Lee S, Flores-Toro JA, Dean JW,
Go KL, Lee MH, Law BK, Law ME, Dunn WA Jr, Zendejas I, et al:
Sirtuin 1 suppresses mitochondrial dysfunction of ischemic mouse
livers in a mitofusin 2-dependent manner. Cell Death Differ.
23:279–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Neuspiel M, Zunino R, Gangaraju S,
Rippstein P and McBride H: Activated mitofusin 2 signals
mitochondrial fusion, interferes with Bax activation, and reduces
susceptibility to radical induced depolarization. J Biol Chem.
280:25060–25070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Papanicolaou KN, Khairallah RJ, Ngoh GA,
Chikando A, Luptak I, O'Shea KM, Riley DD, Lugus JJ, Colucci WS,
Lederer WJ, et al: Mitofusin-2 maintains mitochondrial structure
and contributes to stress-induced permeability transition in
cardiac myocytes. Mol Cell Biol. 31:1309–1328. 2011. View Article : Google Scholar : PubMed/NCBI
|