Introduction
Lung cancer is one of the most common causes of
cancer-associated mortality worldwide (1). Non-small cell lung cancer (NSCLC)
accounts for ~80% of all lung cancer cases (2). In 2014, there were 160,000 mortalities
resulting from lung cancer, accounting for 20% of all
cancer-associated mortalities in the United States (3). Out of all lung cancer-associated
mortalities, ~80% occurred in patients with NSCLC (3–5) and the
main types of NSCLC include adenocarcinoma and squamous cell
carcinoma (6). Recently, the
therapeutic strategies available to treat NSCLC have advanced
greatly; however, patients with advanced NSCLC have a poor
prognosis and the 5-year survival rate of patients with NSCLC is
still only <5% (7,8). By contrast, the 5-year survival rate
for early-stage NSCLC following curative resection is 30–60%
(9). Therefore, it is important to
identify novel biomarkers and determine the molecular mechanisms of
NSCLC to improve the early diagnosis and treatment of patients with
NSCLC, thus improving their prognosis (10).
microRNAs (miRNAs or miRs) are a class of small,
regulatory, non-coding RNAs that are 20–24 nucleotides long. They
are involved in various biological events, including cell growth,
differentiation, apoptosis and migration, as well as pathological
processes, including the development of tumors, and metabolic and
neurodegenerative illnesses (11–14). It
is estimated that one-third of all mammalian genes are directly or
indirectly regulated by miRNAs. These directly bind to the
3′-untranslated region of target mRNA, causing mRNA destabilization
and degradation, and consequently altering the expression of target
proteins (14,15). miRNA deregulation serves an important
role in the pathogenesis of different tumors (16,17).
Furthermore, it has been hypothesized that miRNA levels change
prior to the phenotypic changes that occur during cancer
progression (18). The detection and
quantification of miRNAs is easily performed using standard
diagnostic biological material, including formalin-fixed
paraffin-embedded samples, blood, serum and sputum (19).
It is important to identify novel miRNAs and
determine their roles in tumor development to elucidate the
mechanism of cancer progression and aid in the development of novel
methods to diagnose and treat cancer (20). It has been demonstrated that a
variety of miRNAs are deregulated during NSCLC progression
(21,22). It has been demonstrated that miR-148b
levels are reduced in plasma samples taken from patients with NSCLC
(23). Furthermore, it has been
proposed that miR-148b may be a novel biomarker in NSCLC (9,24).
However, the molecular mechanism underlying the role of miR-148b in
NSCLC remains unclear.
The present study aimed to investigate the effect of
miR-148b on cell proliferation, the epithelial-mesenchymal
transition (EMT) and radiosensitivity in NSCLC cells. It was
revealed that miR-148b expression was reduced in NSCLC tissues and
cell lines, inhibited NSCLC cell proliferation and the EMT and
increased radiosensitivity in NSCLC cells by regulating the
expression Rho-associated protein kinase 1 (ROCK1).
Materials and methods
Clinical specimens
A total of 16 cases (mean age, 53; 9 males and 7
females) of NSCLC were retrieved from the Departments of Oncology 2
Division and Respiratory Medicine, Foshan Nanhai District People's
Hospital (Foshan, China) between January and March 2016. Patients
did not receive any chemo- or radiotherapy prior to surgery to
resect clinical specimens. Tumor tissues and adjacent non-tumor
tissues were resected from patients. The present study was approved
by the Ethical Review Committee of People's Hospital of Nanhai
District Guangdong Province (Foshan, China) and complied with the
Declaration of Helsinki. Informed consent was obtained from all
patients.
Cell culture
The human bronchial cell line (HBE1) and NSCLC cell
lines, including H1299, H1650, H460 and A549, were obtained from
the American Type Culture Collection (Manassas, VA, USA). Cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml
streptomycin and 100 U/ml penicillin in an incubator with 5%
CO2 at 37°C. The culture medium was replenished every
2–3 days and the cells were passaged at 1:6 every 4 days following
trypsinization with 0.05% trypsin-EDTA. Cells were cultured with
plasmids for 3 days.
Cell transfection
The miR-148b mimic (5′-UACUAGACAUCGCAUACACUA-3′;
5′-GCAUAUACUAUGUCAUGACUU-3′), NC-mimic
(5′-UUCUCCGAACGUGUCACGUTT-3′; 5′-ACGUGACACGUUCGGAGAATT-3′);
miR-148b inhibitor (5′-ACAAAGUUCUGUGAUGCACUGA-3′) and anti-NC
(5′-CAGUACUUUUGUGUAGUACAA-3′) were synthesized commercially using
pCMV-miR by Guangzhou RiboBio Co., Ltd., Guangzhou, China). The
ROCK1 sequence was cloned into the pCMV vector. The expression
vector pCMV (Invitrogen; Thermo Fisher Scientific, Inc.) was used
to construct the ROCK1 expression vector. The genomic sequence of
ROCK1 (NC_000018.10) was cloned from 293 cell cDNA and the PCR
products were digested by EcoRI and BamHI. PCR
amplication was performed using a High Yield PCR EcoDry™ Premix
(Takara Biotechnology Co., Ltd., Dalian, China). Thermocycling
conditions were as follows: Initial denaturation at 95°C for 10 min
followed by 40 cycles at 95°C for 1 min, annealing at 53°C for 1
min, extension at 72°C for 1 min and final extension at 72°C for 5
min. The primer sequences for ROCK1 were as follows: Forward
5′-TGGATCCATGATGGCTCTGGGCGCAGCGGGAG-3′ and reverse,
5′-CGAATTCTTAGTGTCTCTGACAAGTGTGAAGCCTAGAAG-3′. The amplified
product was then subcloned into the pCMV vector. A549 cells were
transfected with plasmids. Transient transfection of 100 nM
miR-148b mimic, 100 nM NC-mimic, 100 nM miR-148b inhibitor, 100 nM
anti-NC, and 100 nM pCMV-ROCK1 was performed using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
following the manufacturer's protocols. A total of 6 h following
transfection, the cell growth medium was removed and cells were
incubated in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) containing 5% FBS for another 24–72 h. A total of 48 h
following transfection, RT-qPCR was performed to measure the level
of miR-148b, 24–72 h following transfection, cell proliferation was
determined and 72 h following transfection, the expression of EMT
markers, apoptosis and radiosensitivity were evaluated.
Cell proliferation
Cell proliferation was determined using the Cell
Counting Kit-8 assay kit (Beyotime Institute of Biotechnology,
Haimen, China) following the manufacturer's protocols. A total of
4×104 cells were seeded in the plates and transfected
with miR-148b mimic, NC-mimic, miR-148b inhibitor, anti-NC, with or
without pCMV-ROCK1 for 24–72 h. Absorbance at 450 nm was measured
using a microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells of all
transfection groups using the PARIS™ kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA templates were synthesized by
MultiScribe Reverse Transcriptase (42°C for 15 min, 75°C for 3 min;
Applied Biosystems; Thermo Fisher Scientific, Inc.) and qPCR was
conducted using the Maxima SYBR Green/ROX qPCR Master Mix Assays
(Fermentas; Thermo Fisher Scientific, Inc.) in an Applied
Biosystems 7500 detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). qPCR was performed as follows: Initial
denaturation at 95°C for 10 min followed by 40 cycles at 95°C for 1
min, annealing at 53°C for 1 min, extension at 72°C for 1 min and
final extension at 72°C for 5 min. U6 and β-actin were used as
loading controls. Relative expression levels were normalized to the
expression of β-actin mRNA using the 2−ΔΔCq method
(25). Primer sequences used in the
current study were as follows: E-cadherin, forward,
5′-CTGCTGCAGGTCTCCTCTTG-3′ and reverse, 5′-TGTCGACCGGTGCAATCTTC-3′;
Vimentin, forward, 5′-AAGGCGAGGAGAGCAGGATT-3′ and reverse
5′-GGTCATCGTGATGCTGAGAAG-3′; N-cadherin, forward,
5′-ACAGTGGCCACCTACAAAGG-3′ and reverse, 5′-TGATCCCTCAGGAACTGTCC-3′;
ROCK1, forward, 5′-ATGAGTTTATTCCTACACTCTACCACTTTC-3′ and reverse,
5′-TAACATGGCATCTTCGACACTCTAG-3′; β-actin, forward,
5′-CCTGGGCATGGAGTCCTGTG-3′ and reverse, 5′-TCTTCATTGTGCTGGGTGCC-3′;
miR-148b, forward, 5′-TCAGTGCATCACAGAACTTTGTAA-3′ and reverse,
5′-GCTGTCAACGATACGCTACGT-3′; and U6, forward,
5′-CGCTTCGGCAGCACATATAC-3′ and reverse, 5′-TTCACGAATTTGCGTGTCAT-3′.
Individual experiments were performed in triplicate and results
were presented as a proportion of the control.
Western blot analysis
Cells were lysed using radioimmunoprecipitation
assay lysis buffer (Thermo Fisher Scientific, Inc.) supplemented
with protease inhibitor cocktails (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Following protein extraction, protein
concentration was determined using a bicinchoninic acid assay
(Thermo Fisher Scientific, Inc.). Total protein samples (2 µg/lane)
were separated by 10% SDS-PAGE and transferred onto PVDF membranes
(EMD Millipore, Billerica, MA, USA). Membranes were blocked with 8%
skimmed milk in Tris-buffered saline Tween-20 (TBST) buffer at 37°C
for 1 h and subsequently incubated overnight at 4°C with primary
antibodies (β-actin; cat no. 4970, 1:1,000; ROCK1, cat no. 4035,
1:1,000; both Cell Signaling Technology, Inc. Danvers, MA, USA).
Following four washes (10 min/wash) in TBST, membranes were
incubated with a horseradish peroxidase-conjugated secondary
antibody (1:1,000; cat no. 31460; Thermo Fisher Scientific, Inc.)
at 37°C for 30 min. Bands were visualized with an enhanced
chemiluminescence kit (cat no. 32106; Thermo Fisher Scientific,
Inc.) and images were captured using ChemiDoc™ (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and analysed using the
Quantity One 4.6 (Bio-Rad Laboratories, Inc.).
Radiosensitivity
Cells were exposed to 0, 2, 4, 6, 8 and 10 Gy
irradiation using the Primus K linear accelerator (Siemens AG,
Munich, Germany) and a clonogenic assay was then conducted.
Following 12 days incubation, the colonies formed were fixed with
100% methanol at room temperature for 5 min and stained with 1%
crystal violet at room temperature for 2 h. Colonies of >50
cells were scored as survivors. The cells were then harvested and
assessed using a multifunctional microplate reader at 546 nm. The
clonogenic fractions of irradiated cells were normalized to the
plating efficiencies of the non-irradiated controls.
Apoptosis
Following treatment, cells were fixed with 10%
paraformaldehyde for 10 min at room temperature and apoptosis was
measured using a terminal deoxynucleotidyl transferase-mediated
dUTP nick-end labeling assay kit (Roche Diagnostics, Basel,
Switzerland) following the manufacturer's protocol. Detection and
analysis of apoptosis were performed using a FACScalibur Flow
Cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Antifade
Mounting Medium (Beyotime Institute of Biotechnology) was used and
six random fields of view was observed. Triplicate individual
experiments were performed and results were provided as a
proportion of the control.
Statistical analysis
All data are presented as the mean ± standard error
of the mean and data analysis was performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). The significance among ≥2
groups was analyzed using one-way analysis of variance followed by
a Tukey test. Differences between two groups were analyzed using
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-148b levels are decreased in NSCLC
tissues and cell lines
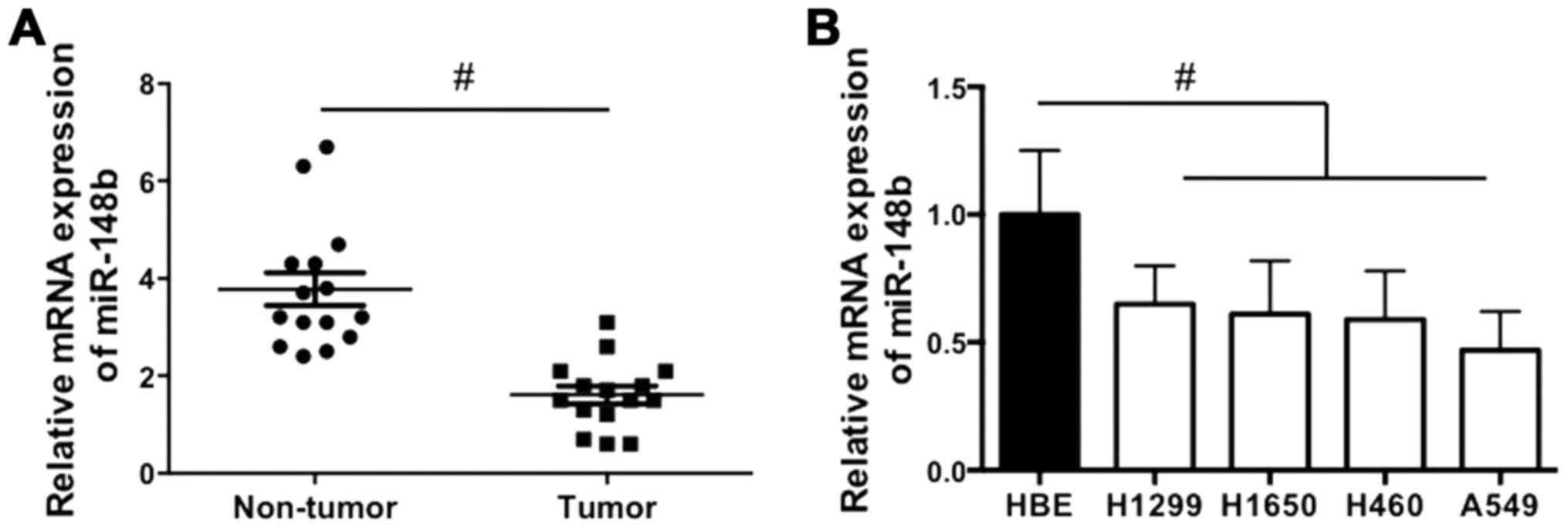
The expression of miR-148b in NSCLC tissues and cell
lines was examined. It was demonstrated that miR-148b expression
was significantly decreased in NSCLC tissues compared with
corresponding adjacent normal lung tissues (P<0.05; Fig. 1A). In addition, miR-148b expression
was significantly decreased in all NSCLC cell lines compared with
the HBE cell line (all P<0.05; Fig.
1B). The results indicate that miR-148b expression is increased
in NSCLC tissue.
miR-148b inhibits the proliferation of
A549 cells
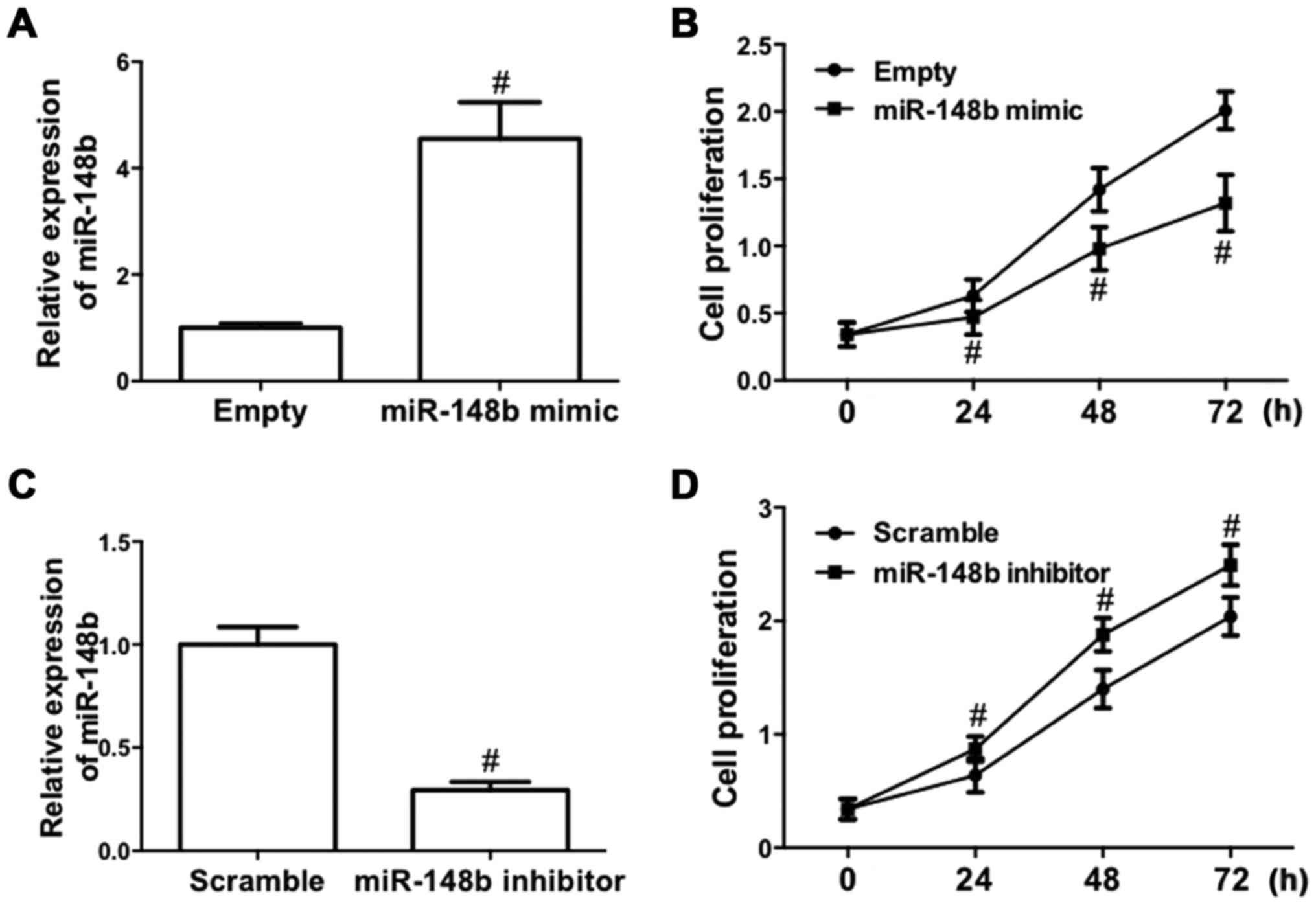
To determine the role of miR-148b in NSCLC, the
effect of deregulated expression of miR-148b on cell proliferation
in A549 cells was evaluated, as A549 cells are one of the most
common cell models used for the study of tumor biology in NSCLC
(26). A549 cells were transfected
with a miR-148b mimic or inhibitor. The results demonstrated that
transfection of the miR-148b mimic significantly increased miR-148b
expression (P<0.05; Fig. 2A).
Furthermore, following transfection with miR-148b mimic, the
proliferation of A549 cells was significantly inhibited (P<0.05;
Fig. 2B). By contrast, transfection
of miR-148b inhibitor significantly decreased miR-148b expression
(P<0.05; Fig. 2C) and
significantly increased the proliferation of A549 cells (P<0.05;
Fig. 2D). These results demonstrate
that miR-148b increases the proliferation of NSCLC cells.
miR-148b inhibits the EMT in A549
cells
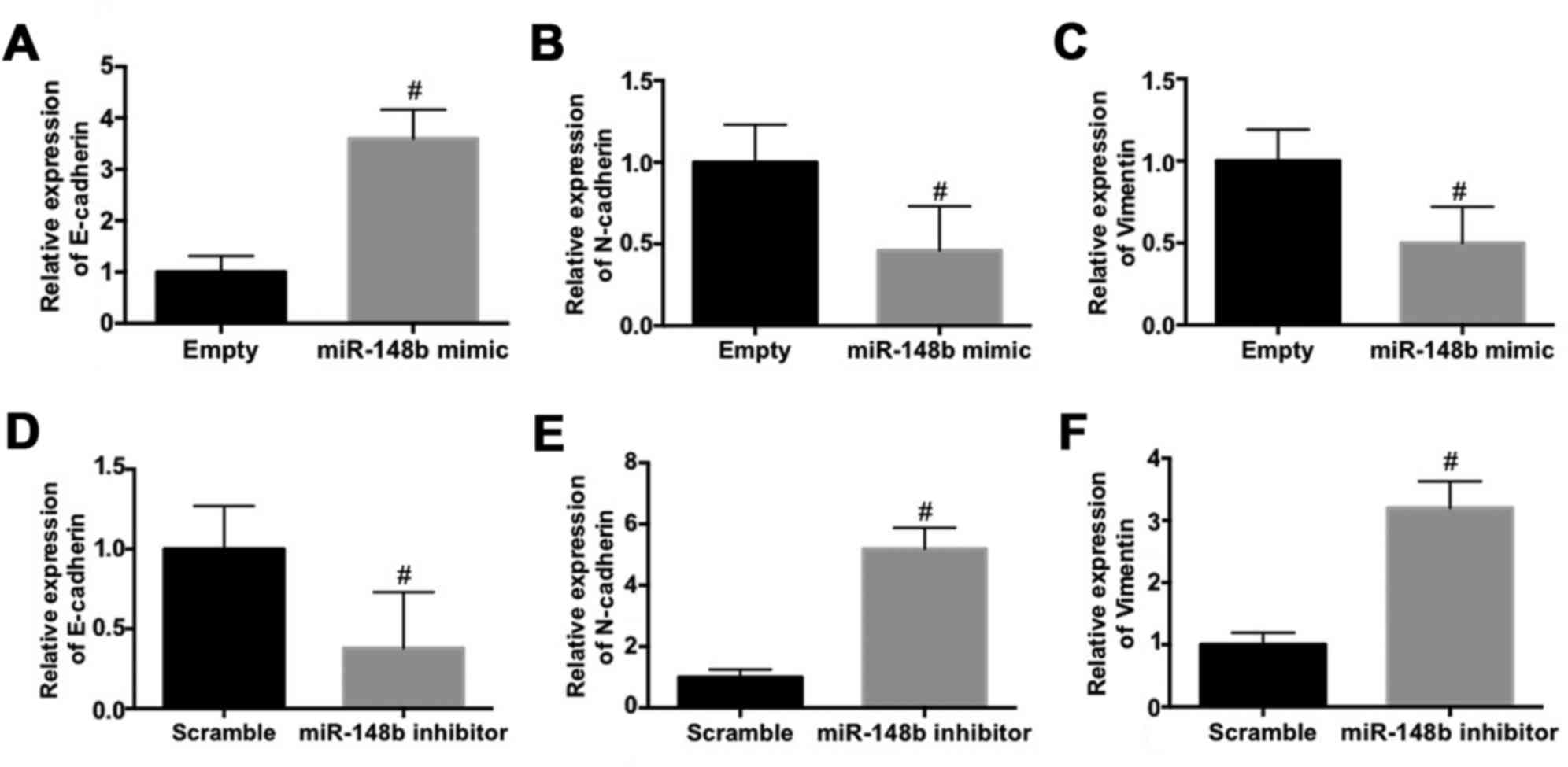
The effect of miR-148b on the expression EMT
biomarkers in A549 cells was evaluated. The mRNA expression of
E-cadherin was significantly increased (P<0.05; Fig. 3A) whereas the mRNA expression of
N-cadherin (Fig. 3B) and vimentin
(Fig. 3C) were significantly
decreased (P<0.05) following transfection with miR-148b mimic.
By contrast, transfection with the miR-148b inhibitor significantly
decreased the mRNA expression of E-cadherin (P<0.05; Fig. 3D) and significantly increased the
mRNA expression of N-cadherin (P<0.05; Fig. 3E) and vimentin (P<0.05; Fig. 3F). These results demonstrate that
miR-148b inhibits the EMT and the invasiveness of A549 cells.
miR-148b enhances radiosensitivity and
promotes apoptosis in A549 cells
The effect of the deregulated expression of miR-148b
on irradiation-induced cell death was determined. Transfection with
the miR-148b mimic significantly promoted irradiation-induced cell
death (P<0.05; Fig. 4A). By
contrast, irradiation-induced cell death was significantly
inhibited following transfection with the miR-148b inhibitor
(P<0.05; Fig. 4B). Furthermore,
irradiation of A549 cells transfected with the miR-148b mimic
significantly increased the percentage of apoptotic cells, compared
with negative controls (Fig. 4C).
The miR-148b mimic promoted irradiation-induced apoptosis,
indicating that miR-148b expression enhances radiosensitivity.
Downregulation of ROCK1 is involved in
the miR-148b-induced inhibition of cell proliferation and EMT, and
the increase in radiosensitivity in A549 cells
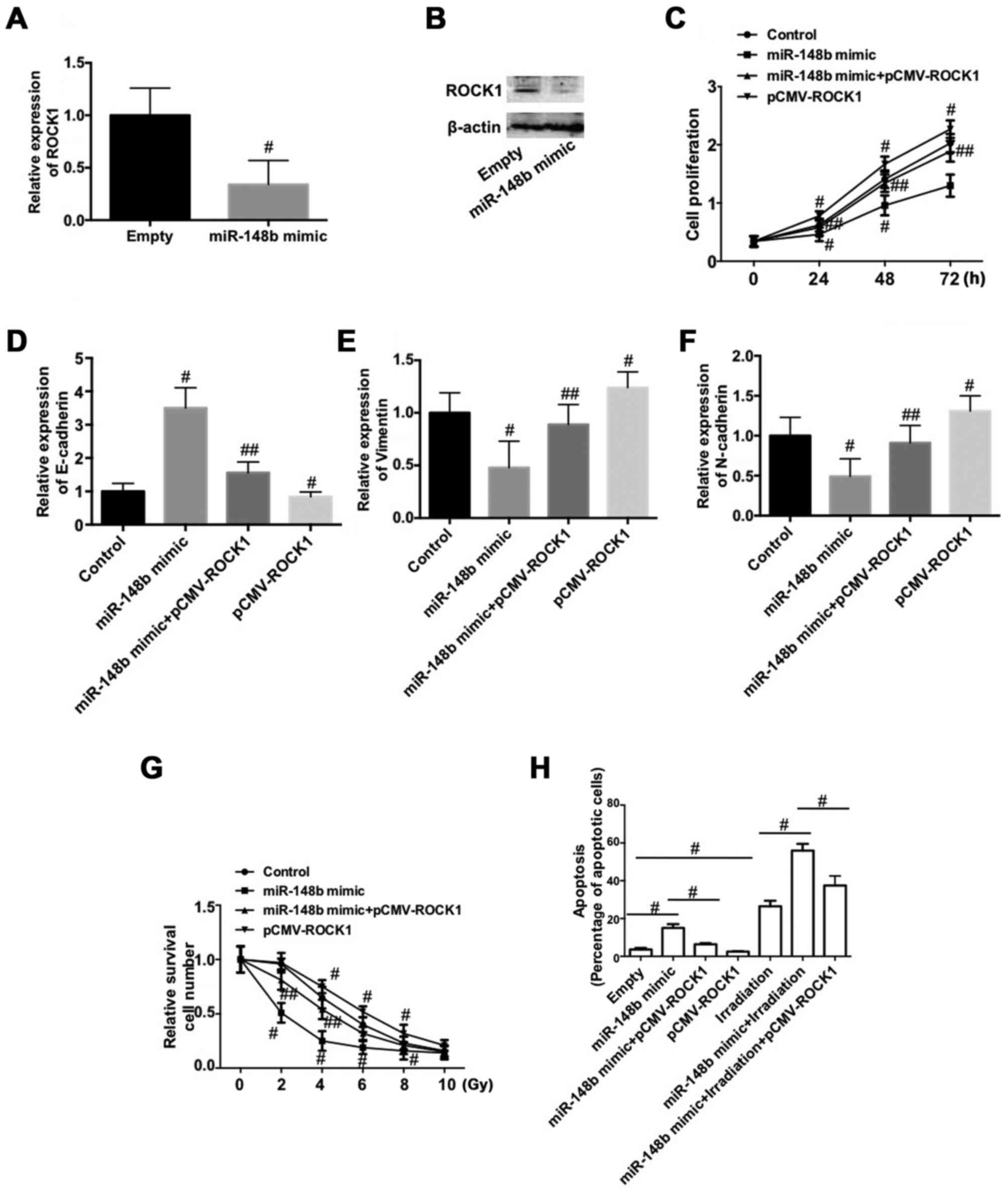
To determine the mechanism by which miR-148b
decreases the proliferation of NSCLC cells, inhibits the EMT and
increases radiosensitivity, the expression of ROCK1 was determined
following transfection with miR-148b mimic. The expression of ROCK1
mRNA and protein was significantly decreased following transfection
with miR-148b mimic (P<0.05; Fig. 5A
and B). To test whether the downregulation of ROCK1 is involved
in the negative role of miR-148b in NSCLC, A549 cells were
co-transfected with miR-148b and pCMV-ROCK1. The results
demonstrate that overexpression of ROCK1 significantly reversed the
miR-148b-induced decrease in cell proliferation (P<0.05;
Fig. 5C). Furthermore,
overexpression of ROCK1 per se significantly reduced cell
proliferation compared with the control (P<0.05; Fig. 5C). The miR-148b-induced increase in
E-cadherin expression (Fig. 5D) and
decreases in vimentin (Fig. 5E), and
N-cadherin (Fig. 5F) expression were
significantly inhibited following overexpression of ROCK1 (all
P<0.05). Additionally, overexpression of ROCK1 significantly
decreased the expression of E-cadherin and increased the expression
of vimentin and N-cadherin, compared with the control (P<0.05).
Overexpression of ROCK1 inhibited the irradiation-induced decrease
in A549 cell survival (P<0.05) and significantly reversed the
miR-148b-induced decrease in cell survival following irradiation
(P<0.05; Fig. 5G). ROCK1
overexpression significantly reversed the increase in apoptosis
that occurred following transfection with miR-148b mimic
(P<0.05; Fig. 5H) and
significantly reversed the miR-148b-induced increase in apoptosis
that occurred following irradiation (P<0.05; Fig. 5H). These data demonstrate that the
downregulation of ROCK1 is involved in the miR-148b-induced
inhibition of cell proliferation and the EMT, and increases the
radiosensitivity of A549 cells.
Discussion
The present study investigated the role of miR-148b
in the proliferation of NSCLC cells, the EMT and radiosensitivity
in order to elucidate its potential molecular mechanisms of action.
It has been previously reported that miR-148b serves a role in
regulating several types of tumors (27–29).
Furthermore, it has been demonstrated that miR-148b expression is
reduced in the plasma samples of patients with NSCLC (23). In addition, miR-148b is a potential
prognostic biomarker and predictor of the response to radiotherapy
in patients with NSCLC (9,24,29).
However, the mechanism by which miR-148b regulates the
proliferation and radiosensitivity of NSCLC cells remains
unclear.
The present study demonstrated that miR-148b
expression is reduced in NSCLC tumor tissue and cell lines.
Furthermore, the overexpression of miR-148b decreased the
proliferation of A549 cells, whereas downregulation of miR-148b
increased cell proliferation, confirming that miR-148b decreases
the proliferation of NSCLC cells. Subsequently, the effect of
miR-148b expression on the EMT in NSCLC cells was determined. The
EMT is a process that is critical for inducing the metastatic
progression of cancer by regulating the migration and invasion of
cells (30,31). During the EMT, epithelial cells lose
their characteristics and switch to a mesenchymal phenotype,
consequently exhibiting increased migratory and invasive
capabilities and promoting tumor progression (32,33). The
molecular mechanism of the EMT involves the alteration of various
crucial regulators, including the downregulation of E-cadherin and
upregulation of the mesenchymal markers N-cadherin and vimentin
(34). The present study
demonstrated that miR-148b overexpression inhibited the EMT, as
indicated by the upregulation of E-cadherin, and downregulation of
N-cadherin and vimentin expression that occurred. Furthermore,
downregulation of miR-148b expression decreased E-cadherin
expression but increased N-cadherin and vimentin expression. The
results indicate that miR-148b inhibits the EMT in NSCLC cells.
The development of radioresistance in NSCLC cells
limits the effectiveness of radiotherapy to treat patients with
NSCLC (35). Therefore, the present
study examined the effect of miR-148b expression on
irradiation-induced cell death. It was confirmed that miR-148b
overexpression increased the sensitivity of NSCLC cells to
irradiation. Furthermore, miR-148b overexpression significantly
increased irradiation-induced apoptosis. Overall, the results
indicated that miR-148b induces radiosensitivity in NSCLC
cells.
The present study aimed to identify the protein that
mediated the effect of miR-148b on NSCLC cells. The results
demonstrated that miR-148b overexpression markedly decreased the
expression of ROCK1. Furthermore, overexpression of ROCK1
significantly inhibited the effects of miR-148b on A549 cell
proliferation, EMT and irradiation-induced cell apoptosis. ROCK1 is
a Rho-GTPase effector that regulates key aspects of the actin
cytoskeleton and is essential for cell cycle progression,
senescence and tumorigenesis (36).
ROCK1 is required for NSCLC anchorage-independent growth and
invasion (37). A previous study has
revealed that miR-148a suppresses the EMT by targeting ROCK1 in
NSCLC cells (38). Additionally, it
has been demonstrated that miR-148b targets ROCK1 expression in
breast cancer and hepatocellular carcinoma cells (39). Based on these results and the results
of the present study, it was indicated that the downregulation of
ROCK1 mediates the inhibitory effect of miR-148b on NSCLC cells.
Therefore, ROCK1 may be a target of miR-148b in different types of
cancer.
In conclusion, the results of the present study
determined that miR-148b inhibits the proliferation of NSCLC cells
and the EMT, and increases radiosensitivity by inhibiting ROCK1.
Furthermore, miR-148b/ROCK1 signaling was identified as a novel
therapeutic target for the inhibition of NSCLC and the enhancement
of radiotherapy to treat patients with NSCLC.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murray N: Reality check for pemetrexed and
maintenance therapy in advanced non-small-cell lung cancer. J Clin
Oncol. 32:482–483. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst RS, Heymach JV and Lippma SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Claassens L, van Meerbeeck J, Coens C,
Quinten C, Ghislain I, Sloan EK, Wang XS, Velikova G and Bottomley
A: Health-related quality of life in non-small-cell lung cancer: An
update of a systematic review on methodologic issues in randomized
controlled trials. J Clin Oncol. 29:2104–2120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Z, Chen X, Zhao Y, Tian T, Jin G, Shu
Y, Chen Y, Xu L, Zen K, Zhang C and Shen H: Serum microRNA
signatures identified in a genome-wide serum microRNA expression
profiling predict survival of non-small-cell lung cancer. J Clin
Oncol. 28:1721–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shtivelman E, Hensing T, Simon GR, Dennis
PA, Otterson GA, Bueno R and Salgia R: Molecular pathways and
therapeutic targets in lung cancer. Oncotarget. 5:1392–1433. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scagliotti GV and Novello S: Adjuvant
therapy in completely resected non-small-cell lung cancer. Curr
Oncol Rep. 5:318–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Chen YY, Li SQ, Huang C and Qin YZ:
Expression of miR-148/152 family as potential biomarkers in
non-small-cell lung cancer. Med Sci Monit. 21:1155–1161. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Socinski MA: Seeking new options for the
treatment of small-cell lung cancer. Lung cance. 50 Suppl
1:S25–S26. 2005. View Article : Google Scholar
|
|
11
|
Ranganathan K and Sivasankar V:
MicroRNAs-Biology and clinical applications. J Oral Maxillofac
Pathol. 18:229–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li MH, Fu SB and Xiao HS: Genome-wide
analysis of microRNA and mRNA expression signatures in cancer. Acta
Pharmacol Sin. 36:1200–1211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rutnam ZJ and Yang BB: The involvement of
microRNAs in malignant transformation. Histol Histopathol.
27:1263–1270. 2012.PubMed/NCBI
|
|
14
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zimmerman AL and Wu S: MicroRNAs, cancer
and cancer stem cells. Cancer Lett. 300:10–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kishikawa T, Otsuka M, Ohno M, Yoshikawa
T, Takata A and Koike K: Circulating RNAs as new biomarkers for
detecting pancreatic cancer. World J Gastroenterol. 21:8527–8540.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Skrzypski M, Dziadziuszko R and Jassem J:
MicroRNA in lung cancer diagnostics and treatment. Mutat Res.
717:25–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang P, Zhuang L, Zhang J, Fan J, Luo J,
Chen H, Wang K, Liu L, Chen Z and Meng Z: The serum miR-21 level
serves as a predictor for the chemosensitivity of advanced
pancreatic cancer and miR-21 expression confers chemoresistance by
targeting FasL. Mol Oncol. 7:334–345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X and Chen Z: MicroRNA-19a functions
as an oncogenic microRNA in non-small cell lung cancer by targeting
the suppressor of cytokine signaling 1 and mediating STAT3
activation. Int J Mol Med. 35:839–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun W, Ma Y, Chen P and Wang D:
MicroRNA-10a silencing reverses cisplatin resistance in the
A549/cisplatin human lung cancer cell line via the transforming
growth factor-beta/Smad2/STAT3/STAT5 pathway. Mol Med Rep.
11:3854–3859. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang MX: Down-expression of circulating
micro ribonucleic acid (miRNA)-148/152 family in plasma samples of
non-small cell lung cancer patients. J Cancer Res Ther. 12:671–675.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang JS, Li BJ, Lu HW, Chen Y, Lu C, Zhu
RX, Liu SH, Yi QT, Li J and Song CH: Serum miR-152, miR-148a,
miR-148b and miR-21 as novel biomarkers in non-small cell lung
cancer screening. Tumour Biol. 36:3035–3042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie WY, Zhou XD, Yang J, Chen LX and Ran
DH: Inhibition of autophagy enhances heat-induced apoptosis in
human non-small cell lung cancer cells through ER stress pathways.
Arch Biochem Biophys. 607:55–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Orso F, Quirico L, Virga F, Penna E,
Dettori D, Cimino D, Coppo R, Grassi E, Elia AR, Brusa D, et al:
miR-214 and miR-148b Targeting Inhibits dissemination of melanoma
and breast cancer. Cancer Res. 76:5151–5162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song Y, Sun J, Xu Y, Liu J, Gao P, Chen X,
Zhao J and Wang Z: Microarray analysis of long non-coding RNAs
related to microRNA-148b in gastric cancer. Neoplasma. 64:199–208.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang R, Ye F, Zhen Q, Song T, Tan G, Chu
W, Zhang Y, Lv B, Zhao X and Liu J: MicroRNA-148b is a potential
prognostic biomarker and predictor of response to radiotherapy in
non-small-cell lung cancer. J Physiol Biochem. 72:337–343. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kharbanda A, Rajabi H, Jin C, Alam M, Wong
KK and Kufe D: MUC1-C confers EMT and KRAS independence in mutant
KRAS lung cancer cells. Oncotarget. 5:8893–8905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karamitopoulou E, Zlobec I, Panayiotides
I, Patsouris ES, Peros G, Rallis G, Lapas C, Karakitsos P,
Terracciano LM and Lugli A: Systematic analysis of proteins from
different signaling pathways in the tumor center and the invasive
front of colorectal cancer. Human Pathol. 42:1888–1896. 2011.
View Article : Google Scholar
|
|
34
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin, and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
You S, Li R, Park D, Xie M, Sica GL, Cao
Y, Xiao ZQ and Deng X: Disruption of STAT3 by niclosamide reverses
radioresistance of human lung cancer. Mol Cancer Ther. 13:606–616.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kumper S, Mardakheh FK, McCarthy A, Yeo M,
Stamp GW, Paul A, Worboys J, Sadok A, Jørgensen C, Guichard S and
Marshall CJ: Rho-associated kinase (ROCK) function is essential for
cell cycle progression, senescence and tumorigenesis. ELife.
5:e129942016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vigil D, Kim TY, Plachco A, Garton AJ,
Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL and
Der CJ: ROCK1 and ROCK2 are required for non-small cell lung cancer
anchorage-independent growth and invasion. Cancer Res.
72:5338–5347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Song Y, Wang Y, Luo J and Yu W:
MicroRNA-148a suppresses epithelial-to-mesenchymal transition by
targeting ROCK1 in non-small cell lung cancer cells. Mol Cell
Biochem. 380:277–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cimino D, De Pittà C, Orso F, Zampini M,
Casara S, Penna E, Quaglino E, Forni M, Damasco C, Pinatel E, et
al: miR148b is a major coordinator of breast cancer progression in
a relapse-associated microRNA signature by targeting ITGA5, ROCK1,
PIK3CA, NRAS and CSF1. FASEB J. 27:1223–1235. 2013. View Article : Google Scholar : PubMed/NCBI
|