Introduction
With the aging of the population, there is an
ongoing increase in the number of patients with osteoporosis
(1). Reportedly, >50% of women in
their 80s are osteoporotic (2),
while 40% have vertebral compression fractures (3). Vertebral fractures commonly occur at
the thoracolumbar junction. These fractures can be treated with
mild residual deformity in many cases, but are reportedly
associated with increased risk of secondary vertebral fractures
(4–6). In patients with osteoporosis-associated
vertebral fractures, the risk of secondary vertebral fractures
appears to increase with each additional fracture (2). Compared with patients without vertebral
fractures, patients with one vertebral fracture and those with
multiple vertebral fractures are reported to have a 3.2- and
6.7-fold higher risk of experiencing secondary vertebral fractures,
respectively (7).
Various therapies and clinical/experimental studies
have been reported for vertebral compression fractures (8–12). For
the prevention of osteoporotic vertebral fractures, it is reported
that bisphosphonates, including etidronate, alendronate, minodronic
acid, risedronate, ibandronate suppress the onset of vertebral
fractures by 36–62% (13–17), teriparatide suppresses onset of
vertebral fracture by 65–80% (18–19) and
denosumab suppressed onset of vertebral fracture by 68% (20). Regarding the treatment of
osteoporotic vertebral fractures, alignment can be corrected by
performing vertebroplasty or spinal shortening osteotomy, but in
the case of conservative treatment, there is a possibility that
kyphotic deformity will remain (21,22).
Regarding alignment abnormalities following osteoporotic vertebral
fractures, it is possible to evaluate the mechanism of secondary
vertebral fractures by creating a fracture model using the finite
element method and performing mechanical analysis, however to the
best of our knowledge, this is the first study to create a
three-dimensional whole spine model directly from a medical image
and perform a mechanical analysis. The authors propose that it is
important to extract a model from each patient's medical images and
to perform individualized analysis in future clinical practice. The
aim of the present study was to construct a normal whole spine
model and compression fracture models with vertebral deformities at
T11, T12 and L1 vertebrae, which are commonly affected by
compression fractures, from the medical images of a patient using
FEM. Furthermore, the validity of the models and the mechanism of
development of secondary compression fractures were determined.
Materials and methods
Patient images
Computed tomography (CT) images (0.67-mm slice
thickness) of the whole spine, ranging from the cervical spine to
the pelvis, of an adult male (Japanese, aged 32 years) were
obtained with the Brilliance 64 CT scanner (Philips Healthcare,
Amsterdam, The Netherlands). The use of these CT images was
approved by the ethics committee at the Center for Clinical
Research, Yamaguchi University Hospital (Ube, Japan; approval no.
H29-052).
Model construction
Model construction was performed with FEM analysis
software, Simpleware ScanIP version M-2017.06 (Synopsys, Inc.
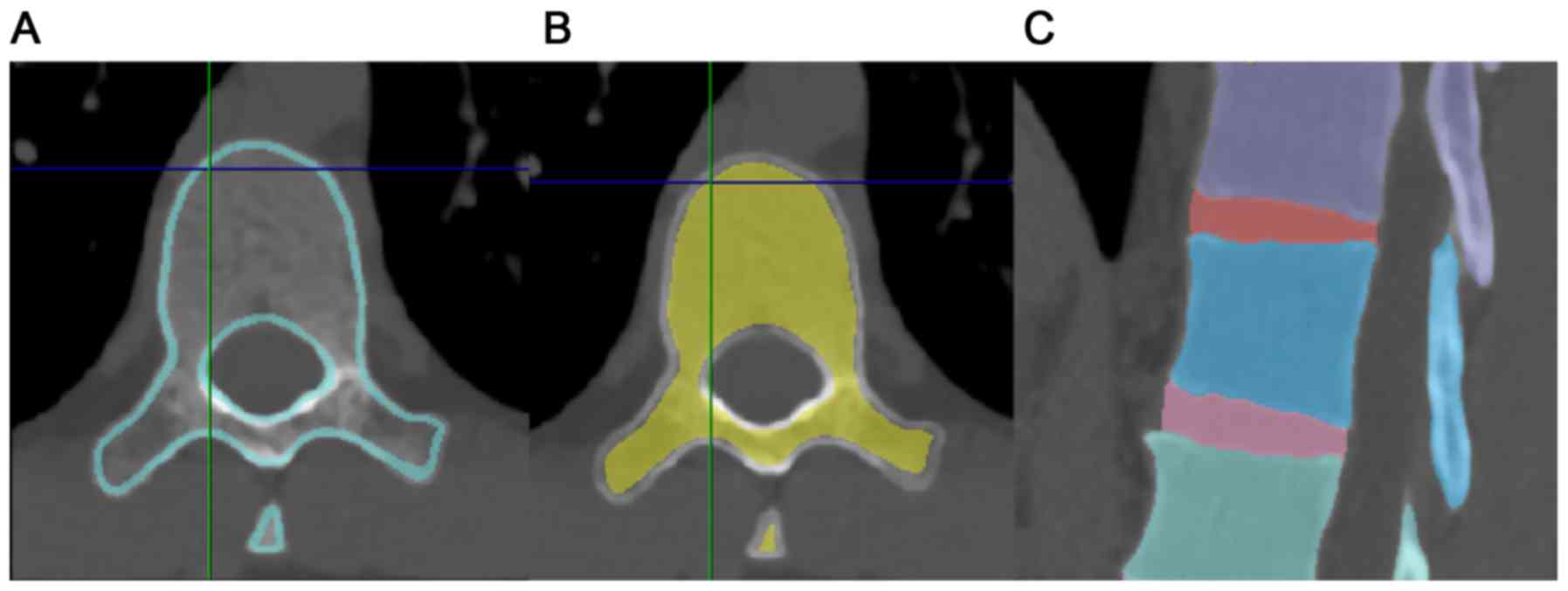
Mountain View, CA, USA. After the spine was extracted, vertebrae
were mapped into cancellous and cortical bones and the sizes of
intervertebral discs (IVDs) were adjusted to match the sizes of end
plates of each vertebra (Fig. 1). A
3D whole spine model was constructed by individually mapping all
vertebrae and IVDs from the cervical to sacral regions. The gap
between each vertebra and IVD was regarded as completely restricted
in movement. Facet joint spaces were created at all levels so that
each vertebra could move independently. The model without
compression fracture was defined as the normal spine model. Whole
spine models with compression fractures were created by trimming
the cranial and caudal surfaces of the T11, T12 or L1 vertebrae by
5° and 10° to make the angle formed by the cranial and caudal
surfaces of each vertebra 10° and 20°, respectively, and also by
rotating IVDs on the cranial and caudal sides of each vertebra
(Fig. 2). These were defined as T11
10°, T11 20°, T12 10°, T12 20°, L1 10° and L1 20° compression
fracture models. Considering that in normal sagittal alignment, a
perpendicular line from the center of the C7 vertebra passes
through the center of the upper surface of the sacral vertebrae
(23), the standing position was
reproduced by rotating the sacral vertebrae to compensate for
kyphosis.
In the normal model, the total number of elements
and nodes was 405,335 and 1,875,549, respectively. In this
analysis, all elements were considered to be linear elastic
materials. Young's modulus was set as cortical bone: 12,000 MPa,
cancellous bone: 1,500 MPa, IVD: 10 MPa. Poisson's ratio was set as
cortical bone: 0.3, cancellous bone: 0.3, IVD: 0.4, according to a
previously published paper (24).
Dynamic analysis was performed assuming that the volunteer fell on
his/her buttocks and load was applied to the spine.
Load application
Assuming that the pelvis was in a consistent
position during the fall and the sacroiliac joint was fixed, a
1,200-N load, corresponding to two-thirds of the body weight (60
kg) excluding the feet, was applied in a vertical direction,
distributed according to the number of nodes of the whole spine.
The load rise time was set at 0.002 sec. Analysis was performed
using Jvision version 3.3.0 (JSOL Corporation, Tokyo, Japan) and
LS-DYNA version R9.1.0 (JSOL Corporation) software.
Results
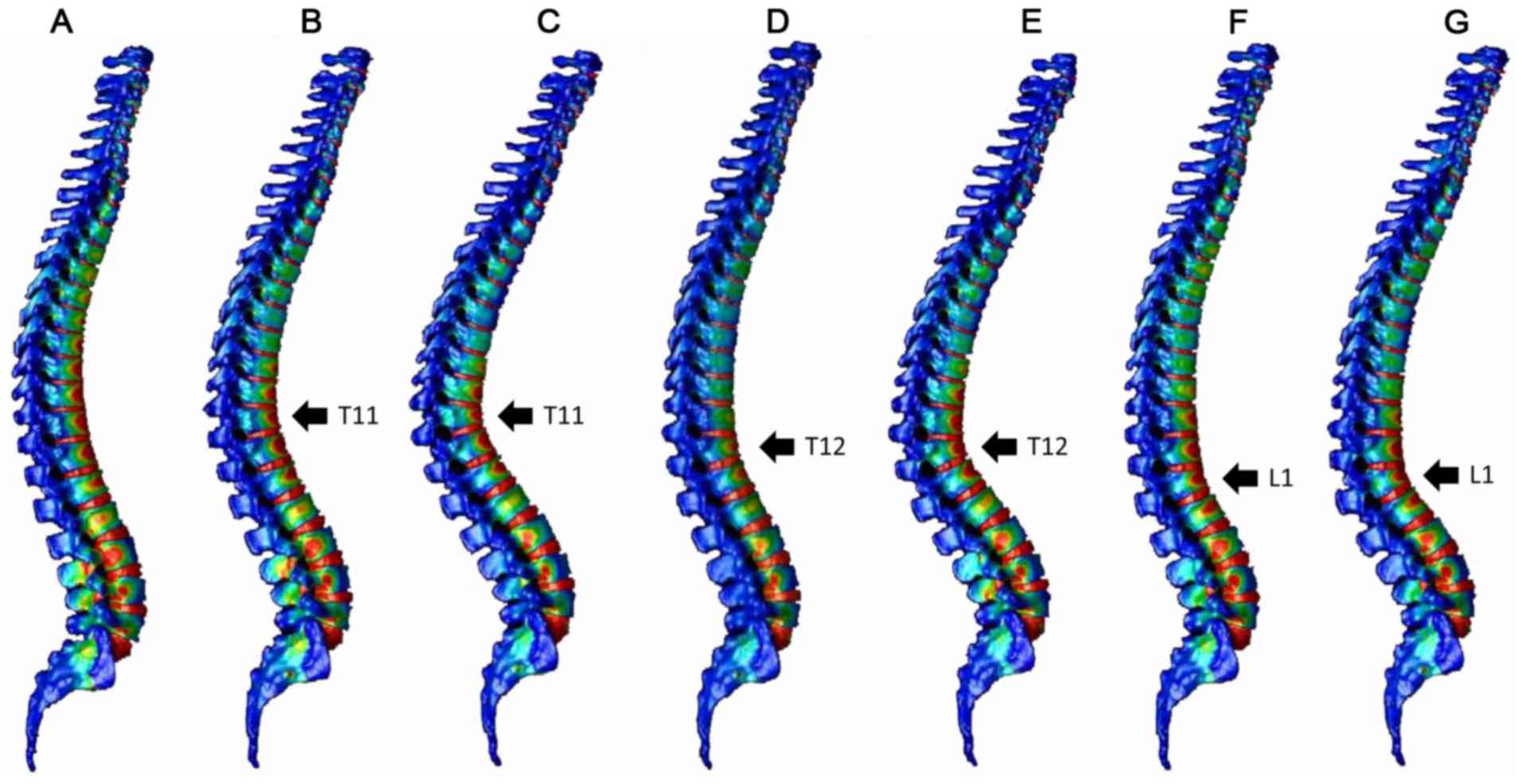
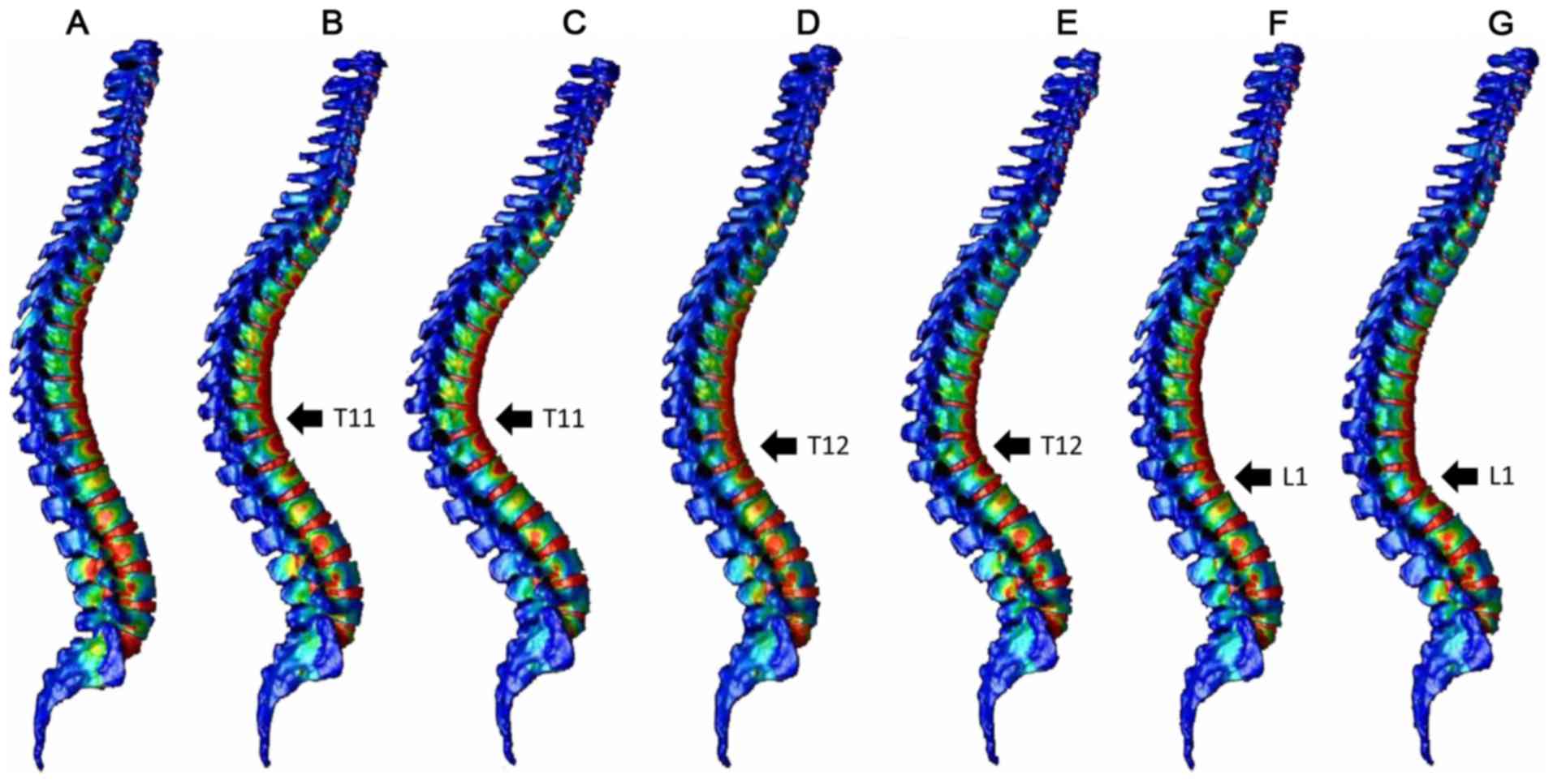
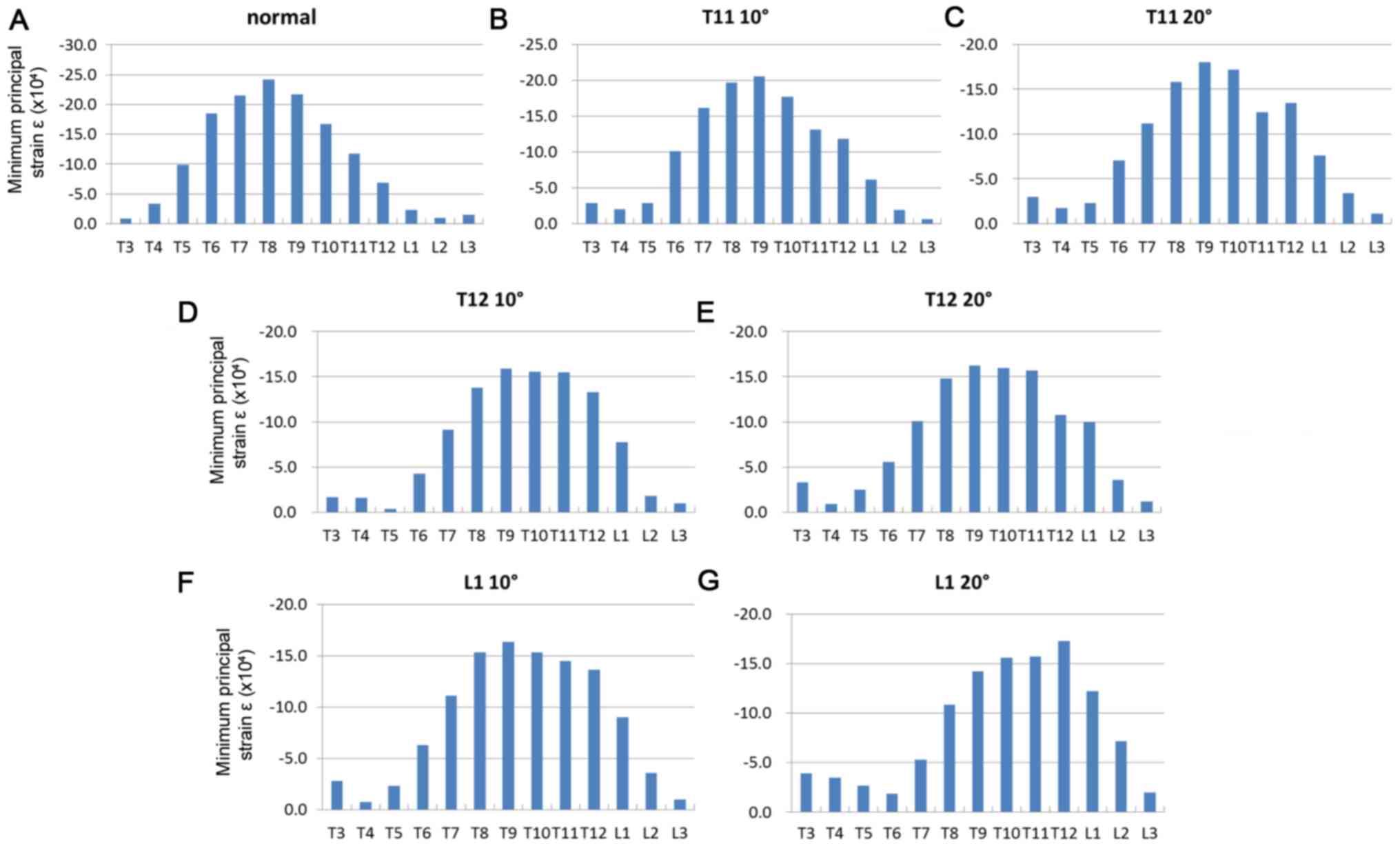
Fig. 3 indicates the
distribution of the minimum principal strain of the spinal model
after 0.004 sec and Fig. 4 indicates
the distribution of the minimum principal strain of the spinal
model after 0.01 sec. Fig. 5
presents graphs of the minimum principal strain after 0.004 sec and
Fig. 6 presents graphs of the
minimum principal strain after 0.01 sec.
At 0.004 sec after load application, peak strain was
observed not only at the thoracolumbar junction, but also in the
middle thoracic spine in the normal spine and compression fracture
models (T11 10°, T11 20°, T12 10°, T12 20° and L1 10°; Figs. 3 and 5)
At 0.01 sec after load application, peak strain
shifted to the thoracic spine in the normal spine model. In the
compression fracture models, peak strain moved to the thoracic
vertebrae, but the larger the fracture angle, the less the movement
to the thoracic vertebrae. (T 11 20°, T 12 20° L 1 20°>T 11 10°,
T 12 10°, L 1 10°>normal; Figs. 4
and 6).
Discussion
Spinal compression fractures commonly occur at the
thoracolumbar junction (T11 to L2 level), which is biomechanically
vulnerable to stress and can lead to kyphotic deformity of the
spine at the fracture site (25).
Lumbar kyphosis is compensated for by reduced thoracic kyphosis to
maintain the sagittal balance, while thoracic kyphosis is
compensated for by increased lordosis of the lumbar spine (26). This results in altered alignment of
the whole spine and altered load application to each vertebra,
which is a suggested cause of secondary compression fracture.
Regarding reduced quality of life (QOL) due to
compression fracture, Glassman et al (27,28) have
reported that increased anterior inclination of the body trunk is
associated with greater impairment of QOL. Miyakoshi et al
(29) have reported reduced QOL due
to poor sagittal balance, where kyphosis of the thoracic or lumbar
spine is associated with marked reduction in QOL. Takahashi et
al (30) reported that worsening
of gait disturbance due to spinal kyphosis is associated with
reduced frequency of going outdoors, leading to reduced
satisfaction with daily life. These reports suggest the importance
of preventing secondary compression fractures. For the prevention
of secondary compression fractures, the importance of osteoporosis
treatment has been emphasized and various surgical therapies,
including balloon kyphoplasty, have been proposed (13–17,31).
While the usefulness of FEM analysis of the spine
has been demonstrated in many studies (8–12), only
a few studies have analyzed spinal compression fracture by FEM.
Imai et al (32) compared the
results of FEM analysis and an actual compression experiment using
samples of the thoracolumbar junction collected from fresh cadavers
and demonstrated that bone strength and fracture sites can be
predicted by FEM analysis. Tawara et al (33–35)
reported the usefulness of FEM analysis for examining osteoporotic
spines using CT images of bisphosphonate-treated patients, although
they only analyzed limited intervertebral spaces and did not take
into account the anatomical variability across the whole spine.
Although used in a limited number of reports as
described above, FEM analysis has been demonstrated to be effective
for the clinical analysis of compression fractures and other
conditions. Based on the proposal that FEM analysis of compression
fractures using whole spine models extracted from medical images of
patients could be effective for the prevention of secondary
compression fractures, models were created in the current study. It
was investigated whether the obtained findings were consistent with
those reported in clinical and experimental studies.
Osteoporotic spinal compression fractures have been
reported to occur most commonly at the thoracolumbar junction
(4–6). The current results also demonstrated
focused strain at the thoracolumbar junction in the normal spine
model, which was consistent with previous findings. Secondary
compression fractures have been reported to occur most commonly in
adjacent vertebrae, followed by the middle thoracic spine (36). The compression fracture models of the
present study revealed focused strain in the fractured vertebra and
adjacent vertebrae within a short time, more prominently as
compared with the normal spine model, and spread of strain up to
the middle thoracic spine over time, which was consistent with
previous clinical findings.
The present study had certain limitations. First,
the present models did not consider ligaments, particularly the
supraspinatus and interspinatus ligaments, joint capsule, muscles
and ribs. Second, anterior inclination associated with spinal
compression fracture was compensated for only by rotation of the
pelvis in our models; however, in actual patients, it is
compensated for by inclination, in addition to rotation, of the
pelvis and the IVD-lower limb alignment (26,37).
Other limitations include the assumption that the material
constants of vertebrae and IVDs and bone mineral density were
fixed, and that consistent load was applied to the spine when the
patient fell on his/her buttocks. Finally, the analysis did not
consider the patient's posture, the hardness of the ground or the
time elapsed during the fall.
Nevertheless, the findings obtained from the whole
spine model and compression fracture models created from medical
images in the present study support previous findings. The
development of improved models that overcome the aforementioned
limitations may contribute to the prevention of damage to adjacent
IVDs and progression to burst fractures, and also to the
development of rehabilitation programs to compensate for stress
applied to the fractured vertebrae.
In conclusion, FEM models were created of the whole
spine from medical images and strain analysis was performed using
compression fracture models. The normal spine model exhibited a
shift of high strain region from the thoracolumbar junction to the
middle thoracic spine, while the compression fracture models
exhibited focused strain at the fracture site and adjacent
vertebrae. These results supported the previous findings, and
suggested that whole spine models created from medical images could
be used for various types of analysis.
Acknowledgements
The authors would like to thank the members of the
Medical and Mechanical Engineering Laboratory of Yamaguchi
University, and graduate students from this laboratory, for their
assistance with this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoshimura N, Kinoshita H, Oka H, Muraki S,
Mabuchi A, Kawaguchi H and Nakamura K: Cumulative incidence and
changes in the prevalence of vertebral fractures in a rural
Japanese community: A 10-year follow-up of the Miyama cohort. Arch
Osteoporos. 1:43–49. 2006. View Article : Google Scholar
|
|
2
|
Yoshimura N, Muraki S, Oka H, Mabuchi A,
En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, et
al: Prevalence of knee osteoarthritis, lumbar spondylosis, and
osteoporosis in Japanese men and women: The research on
osteoarthritis/osteoporosis against disability study. J Bone Miner
Metab. 27:620–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitazawa A, Kushida K, Yamazaki K and
Inoue T: Prevalence of vertebral fractures in a population-based
sample in Japan. J Bone Miner Metab. 19:115–118. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wasnich RD: Vertebral fracture
epidemiology. Bone. 18 3 Suppl:179S–183S. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ismail AA, Cooper C, Felsenberg D, Varlow
J, Kanis JA, Silman AJ and O'Neill TW: Number and type of vertebral
deformities: Epidemiological characteristics and relation to back
pain and height loss. European vertebral osteoporosis study group.
Osteoporos Int. 9:206–213. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panjabi MM: Physical properties and
functional biomechanics of the spine = Clinical Biomechanics of the
Spine. Panjabi MM and White AA: LWW Corp.; Philadelphia, PA: pp.
1–76. 1990
|
|
7
|
Lindsay R, Silverman SL, Cooper C, Hanley
DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K,
et al: Risk of new vertebral fracture in the year following a
fracture. JAMA. 285:320–323. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu CC, Chao CK, Wang JL, Hou SM, Tsai YT
and Lin J: Increase of pullout strength of spinal pedicle screws
with conical core: Biomechanical tests and finite element analyses.
J Orthop Res. 23:788–794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kilinçer C, Inceoglu S, Sohn MJ, Ferrara
LA and Benzel EC: Effects of angle and laminectomy on triangulated
pedicle screws. J Clin Neurosci. 14:1186–1191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashemi A, Bednar D and Ziada S: Pullout
strength of pedicle screws augmented with particulate calcium
phosphate: An experimental study. Spine J. 9:404–410. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sairyo K, Goel VK, Masuda A, Vishnubhotla
S, Faizan A, Biyani A, Ebraheim N, Yonekura D, Murakami R and Terai
T: Three-dimensional finite element analysis of the pediatric
lumbar spine. Part I: pathomechanism of apophyseal bony ring
fracture. Eur Spine J. 15:923–929. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sairyo K, Goel VK, Masuda A, Vishnubhotla
S, Faizan A, Biyani A, Ebraheim N, Yonekura D, Murakami R and Terai
T: Three dimensional finite element analysis of the pediatric
lumbar spine. Part II: Biomechanical change as the initiating
factor for pediatric isthmic spondylolisthesis at the growth plate.
Eur Spine J. 15:930–935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wells GA, Cranney A, Peterson J, Boucher
M, Shea B, Robinson V, Coyle D and Tugwell P: Etidronate for the
primary and secondary prevention of osteoporotic fractures in
postmenopausal women. Cochrane Database Syst Rev.
23:CD0033762008.
|
|
14
|
Wells GA, Cranney A, Peterson J, Boucher
M, Shea B, Robinson V, Coyle D and Tugwell P: Alendronate for the
primary and secondary prevention of osteoporotic fractures in
postmenopausal women. Cochrane Database Syst Rev.
23:CD0011552008.
|
|
15
|
Matsumoto T, Hagino H, Shiraki M, Fukunaga
M, Nakano T, Takaoka K, Morii H, Ohashi Y and Nakamura T: Effect of
daily oral minodronate on vertebral fractures in Japanese
postmenopausal women with established osteoporosis: A randomized
placebo-controlled double-blind study. Osteoporos Int.
20:1429–1437. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wells G, Cranney A, Peterson J, Boucher M,
Shea B, Robinson V, Coyle D and Tugwell P: Risedronate for the
primary and secondary prevention of osteoporotic fractures in
postmenopausal women. Cochrane Database Syst Rev.
23:CD0045232008.
|
|
17
|
Chesnut CH III, Skag A, Christiansen C,
Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride
J, Schimmer RC, et al: Effects of oral ibandronate administered
daily or intermittently on fracture risk in postmenopausal
osteoporosis. J Bone Miner Res. 19:1241–1249. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neer RM, Arnaud CD, Zanchetta JR, Prince
R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S,
Genant HK, et al: Effect of parathyroid hormone (1–34) on fractures
and bone mineral density in postmenopausal women with osteoporosis.
N Engl J Med. 344:1434–1441. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura T, Sugimoto T, Nakano T,
Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H,
Nishizawa Y, et al: Randomized Teriparatide [human parathyroid
hormone (PTH) 1–34] once-weekly efficacy research (TOWER) trial for
examining the reduction in new vertebral fractures in subjects with
primary osteoporosis and high fracture risk. J Clin Endocrinol
Metab. 97:3097–3106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cummings SR, San Martin J, McClung MR,
Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A,
et al: Denosumab for prevention of fractures in postmenopausal
women with osteoporosis. N Engl J Med. 361:756–765. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Andrei D, Popa I, Brad S, Iancu A, Oprea
M, Vasilian C and Poenaru DV: The variability of vertebral body
volume and pain associated with osteoporotic vertebral fractures:
Conservative treatment versus percutaneous transpedicular
vertebroplasty. Int Orthop. 41:963–968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanchiku T, Imajo Y, Suzuki H, Yoshida Y,
Nishida N, Funaba M and Taguchi T: Operative methods for delayed
paralysis after osteoporotic vertebral fracture. J Orthop Surg
(Hong Kong). 25:1–7. 2017. View Article : Google Scholar
|
|
23
|
Gelb DE, Lenke LG, Bridwell KH, Blanke K
and McEnery KW: An analysis of sagittal spinal alignment in 100
asymptomatic middle and older aged volunteers. Spine (Phila Pa
1976). 20:1351–1358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie F, Zhou H, Zhao W and Huang L: A
comparative study on the mechanical behavior of intervertebral disc
using hyperelastic finite element model. Technol Health Care.
25:177–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gertzbein SD: Scoliosis research society.
Multicenter spine fracture study. Spine (Phila Pa 1976).
17:528–540. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jackson RP and Hales C: Congruent
spinopelvic alignment on standing lateral radiographs of adult
volunteers. Spine (Phila Pa 1976). 25:2808–2815. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Glassman SD, Berven S, Bridwell K, Horton
W and Dimar JR: Correlation of radiographic parameters and clinical
symptoms in adult scoliosis. Spine (Phila Pa 1976). 30:682–688.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Glassman SD, Bridwell K, Dimar JR, Horton
W, Berven S and Schwab F: The impact of positive sagittal balance
in adult spinal deformity. Spine (Phila Pa 1976). 30:2024–2029.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyakoshi N, Kasukawa Y, Sasaki H, Kamo K
and Shimada Y: Impact of spinal kyphosis on gastroesophageal reflux
disease symptoms in patients with osteoporosis. Osteoporos Int.
20:1193–1198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi T, Ishida K, Hirose D, Nagano Y,
Okumiya K, Nishinaga M, Matsubayashi K, Doi Y, Tani T and Yamamoto
H: Trunk deformity is associated with a reduction in outdoor
activities of daily living and life satisfaction in
community-dwelling older people. Osteoporos Int. 16:273–279. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wardlaw D, Van Meirhaeghe J, Ranstam J,
Bastian L and Boonen S: Balloon kyphoplasty in patients with
osteoporotic vertebral compression fractures. Expert Rev Med
Devices. 9:423–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Imai K, Ohnishi I, Bessho M and Nakamura
K: Nonlinear finite element model predicts vertebral bone strength
and fracture site. Spine (Phila Pa 1976). 31:1789–1794. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tawara D, Sakamoto J, Murakami H, Kawahara
N, Oda J and Tomita K: Mechanical evaluation by patient-specific
finite element analyses demonstrates therapeutic effects for
osteoporotic vertebrae. J Mech Behav Biomed Mater. 3:31–40. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tawara D, Sakamoto J, Murakami H, Kawahara
N, Oda J and Tomita K: Mechanical therapeutic effects in
osteoporotic L1-vertebrae evaluated by nonlinear patient-specific
finite element analysis. JBSE. 5:499–514. 2010. View Article : Google Scholar
|
|
35
|
Tawara D, Sakamoto J, Murakami H, Kawahara
N and Tomita K: Patient-specific finite element analyses detect
significant mechanical therapeutic effects on osteoporotic
vertebrae during a three-year treatment. JBSE. 6:248–261. 2011.
View Article : Google Scholar
|
|
36
|
Itoi E, Sakurai M, Mizunashi K, Sato K and
Kasama F: Long-term observations of vertebral fractures in spinal
osteoporotics. Calcif Tissue Int. 47:202–208. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iyer S, Lenke LG, Nemani VM, Albert TJ,
Sides BA, Metz LN, Cunningham ME and Kim HJ: Variations in sagittal
alignment parameters based on age: A prospective study of
asymptomatic volunteers using full-body radiographs. Spine (Phila
Pa 1976). 41:1826–1836. 2016. View Article : Google Scholar : PubMed/NCBI
|