Introduction
Henoch-Schonlein purpura (HSP) is common in
children, the main pathological feature is the small blood vessel
inflammation caused by the accumulation of a variety of cytokines,
and it recurs easily, mostly occurring in children aged 3–10 years,
with less incidence among adults (1).
The main clinical features of the disease are
non-thrombocytopenic purpura, arthritis, and internal organs
involved, including gastrointestinal tract and kidney (2). The disease is usually acute and
self-limiting, however, kidney involvement often leads to serious
clinical consequences, and its prognosis depends on the severity of
kidney involvement (3). HSP patients
may occur with secondary renal function impairment, hematuria or
proteinuria, the renal disease at this time is also known as
Henoch-Schonlein purpura nephritis (HSPN) (4). IL-8 is a proinflammatory factor, IL-10
is an anti-inflammatory factor, and both of these are altered with
the change in the degree of inflammation. TNF-α has a very
important regulatory role for the immune function, and the level of
urinary protein is closely related to the degree of renal function
impairment.
In the present study, by detecting the levels of
TNF-α, IL-8 and IL in serum of HSP combined with renal function
impairment patient (HSPN group) and HSP combined without renal
function impairment (NHSPN group) and healthy people, we explored
the relationship between inflammatory factors such as IL-8, IL-10,
TNF-α and HSPN.
Materials and methods
General information
Patients with Henoch-Schonlein purpura (HSP)
admitted into The Affiliated Hospital of Jining Medical University
from June, 2016 to June, 2017 were included, divided into the NHSPN
group (n=58) and HSPN group (n=54) according to whether or not
combined with HSPN. The NHSPN group comprised 31 males and 27
females, aged 7.2±2.5 years, and the HSPN group had 28 males and 26
females, aged 7.1±2.3 years. In addition, 50 healthy individuals
were randomly selected as the control group, including 27 males and
25 females, with an average age of 7.0±2.1 years. There was no
significant difference in sex and age in the three groups
(P>0.05).
This study was approved by the ethics committee of
The Affiliated Hospital of Jining Medical University (Shandong,
China). Signed written informed consents were obtained from all the
participants before the study.
According to the classification of HSPN at the
Zhuhai meeting in 2000, 15 cases of simple proteinuria, 11 cases of
acute nephritis, 13 cases of chronic nephritis, and 15 cases of
nephrotic syndrome were diagnosed in HSPN group. Of these, 15 cases
had light nephritis, and 39 cases had severe nephritis. There was
no significant difference in age, sex and weight between the
subgroups of HSPN group (P>0.05). Specific distribution is
provided in Table I. All the
included cases were consistent with the Henoch-Schonlein purpura
classification criteria of the American Rheumatology Association
(5). Patients were excluded from the
HSPN group if systemic vasculitis, thrombocytopenic purpura, other
systemic immune diseases (such as systemic lupus erythematosus),
dermato-myositis, diabetes was detected, and if renal biopsy
results did not render them eligible for this group.
 | Table I.General information of patients with
HSPN (mean ± SD). |
Table I.
General information of patients with
HSPN (mean ± SD).
| Type of
nephritis | No. of cases | Sex (M/F) | Age (year) | Weight (kg) |
|---|
| Light nephritis |
|
|
|
|
| Simple
proteinuria | 15 | 9/6 | 6.9±3.1 | 23.2±3.5 |
| Severe nephritis |
|
|
|
|
| Acute
nephritis | 11 | 7/4 | 7.0±2.9 | 24.1±3.2 |
| Chronic
nephritis | 13 | 8/5 | 7.1±3.2 | 22.8±3.9 |
| Nephrotic
syndrome | 15 | 8/7 | 7.1±3.1 | 23.5±3.1 |
| t/χ2
test | – | 0.354 | 2.321 | 3.011 |
| P-value | – | P>0.05 | P>0.05 | P>0.05 |
Methods
Venous blood (2 ml) was taken from the subjects in
the morning following fasting, and centrifuged at 2,300 × g for 10
min. The serum at the lower part of the test tube was stored at
−20°C. The collected blood samples were assayed for cytokine level
using the ELISA kit (Sigma, St. Louis, MO, USA). Determination of
the urinary protein level was performed by MA-4210 urine analyzer
(Guilin Huatong Medical Instrument Co., Ltd., Guangxi, China), the
urine reagent strip was immersed in urine for 1 min, and then
removed and placed into the urine analyzer for quantitative
detection.
Statistical analysis
SPSS 11.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Measurement data are
presented as mean ± SD. Comparisons between groups was tested with
ANOVA and SNK test was used as post hoc test. Enumeration data were
analyzed using Chi-square analysis. The correlation between urinary
protein content and serum factor in patients with HSPN combined
with renal impairment was analyzed by Pearson's linear correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
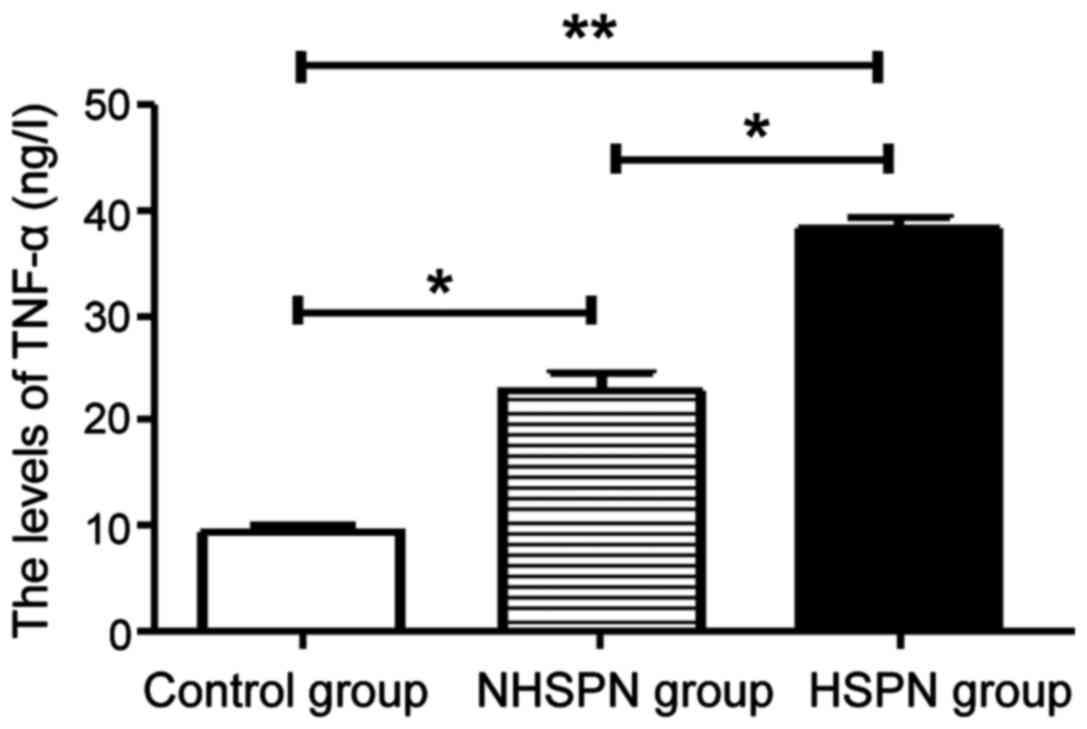
TNF-α levels in the three groups
The levels of TNF-α in the HSPN and NHSPN groups
were significantly higher than those in the control group
(P<0.05). The level of TNF-α in HSPN group was higher than that
in NHSPN group (P<0.05) (Fig.
1).
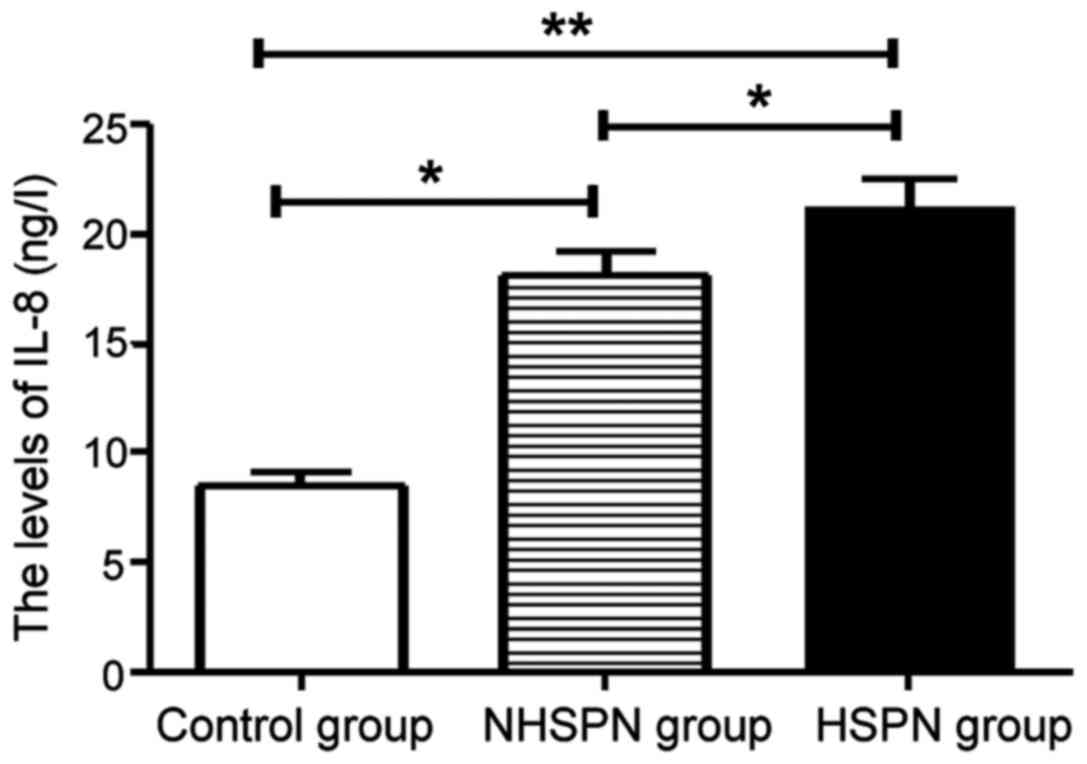
IL-8 levels in three groups
The levels of IL-8 in the HSPN and NHSPN groups were
significantly higher than those in the control group, and the
difference was statistically significant (P<0.05). There was no
significant difference in IL-8 level between HSPN group and NHSPN
group (P>0.05) (Fig. 2).
IL-10 level in the three groups
The levels of IL-10 in the HSPN and NHSPN groups
were significantly higher than those in the control group, and the
difference was statistically significant (P<0.05). There was no
significant difference in IL-10 level between the HSPN and NHSPN
groups (P>0.05) (Fig. 3).
Levels of TNF-α, IL-8 and IL-10 in
HSPN group
The levels of TNF-α, IL-8 and IL-10 in the acute
nephritis, chronic nephritis and nephrotic syndrome groups were
higher than those in the simple proteinuria group (P<0.05,
P<0.01). Levels of TNF-α, IL-8 and IL-10 in the acute nephritis
group were significantly higher than those in the chronic nephritis
and nephrotic syndrome groups (P<0.05). There was no significant
difference between the chronic nephritis and nephrotic syndrome
groups (P>0.05) (Fig. 4).
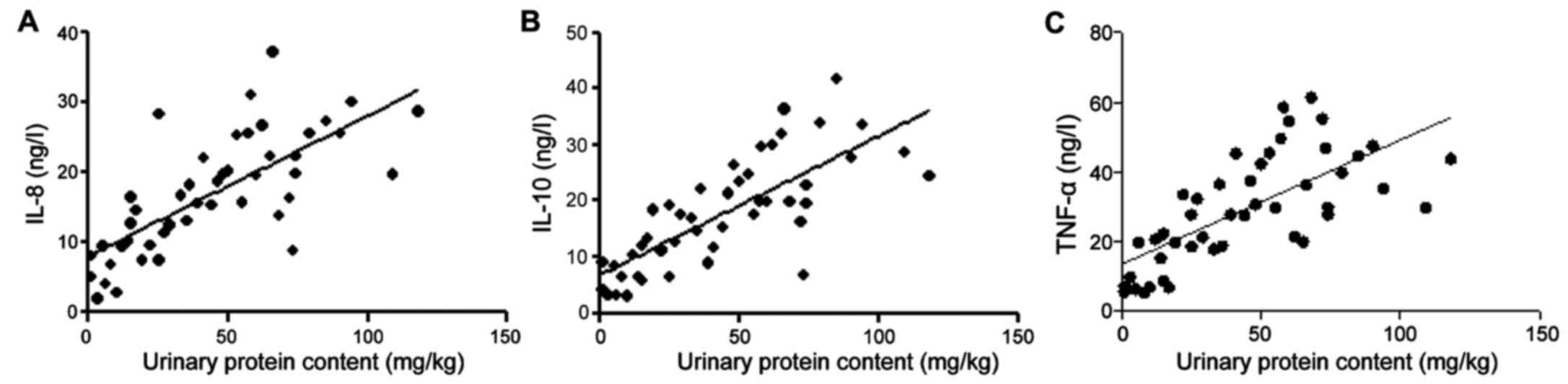
Correlation between urinary protein
content and serum TNF-α, IL-8 and IL-10 in HSPN patients
The r values of IL-8, IL-10, TNF-α and urinary
protein were 0.61, 0.413 and 0.428, respectively, and the test
standard was α=0.05 (Table II and
Fig. 5).
 | Table II.Pearson's linear correlation analysis
of HSPN and serum TNF-α, IL-8 and IL-10. |
Table II.
Pearson's linear correlation analysis
of HSPN and serum TNF-α, IL-8 and IL-10.
| Group | r | Pearson | Sig |
|---|
| IL-8 | 0.61 | 0.017 | 0.001 |
| IL-10 | 0.413 | 0.045 | 0.012 |
| TNF-α | 0.428 | 0.520 | 0.038 |
Discussion
Henoch-Schonlein purpura (HSP) is the most common
small vasculitis in childhood, which mainly causes skin, bowel and
kidney changes (6,7). Although the pathogenesis is not clear
currently, in various related research on the pathogenesis,
cytokines have proven to have a certain role on the development of
the disease. Clinical experiments have confirmed that cytokines
such as TNF-α, IL-8 and IL-10 are involved in the pathogenesis of
HSP (8,9). They are likely to be released by
vascular endothelial cells, which trigger and promote the
inflammatory response. These proinflammatory factors are stimulated
and release chemokines, adsorbing inflammatory cells, inducing the
expression of endothelial cell adhesion molecules, promoting the
adhesion of endothelial cells to vascular walls (10,11).
IL-8 is a cytokine that appears in the acute phase
of HSP, mainly infiltrated by peripheral leukocytes and
polymorphonuclear cells, released to activate endothelial cells
(12), which is also a protein
peptide. Elevated levels of IL-8 in serum cause neutrophil
chemotaxis and release associated proteases, causing systemic small
vessel damage (13). The results of
the present study show that serum IL-8 levels in patients with HSPN
are significantly higher than those of NHSPN and normal healthy
individuals, and IL-8 is correlated with HSPN urine protein
content. Since the content of urine protein and the degree of renal
function impairment were positively correlated, IL-8 is closely
associated with the pathogenesis of HSP with nephritis. IL-10, as
an immune regulator secreted by TH2 cells, mainly plays
anti-inflammatory and immunosuppressive roles (14). Kimura et al have confirmed
that serum levels of IL-10 in patients with HSP mainly exert an
inhibitory effect on the antigen-presenting function of
macrophages, and can effectively inhibit the production of other
proinflammatory factors (15).
This study confirmed that serum IL-10 levels in HSPN
patients were significantly higher than those in NHSPN and healthy
individuals, and its content was positively correlated with the
level of urine protein, which indicates the degree of renal
function impairment, suggesting that IL-10 is involved in the
pathogenesis of HSP, especially playing a greater role in the
combination of renal dysfunction. TNF-α is an effective
proinflammatory cytokine produced by a variety of cell types,
including monocytes/macrophages, mesangial cells and renal
epithelial cells. TNF-α can also cause changes in renal endothelial
and mesangial cells (16). Previous
findings have found that serum TNF-α levels are elevated in HSP
patients, suggesting that cytokine TNF-α may be involved in
vascular injury (17). Yang et
al have shown that the activation of inflammatory cells in
glomerular and renal interstitial cells can lead to local TNF-α
production, leading to glomerular barrier damage and increased
permeability (18).
The present findings have shown that serum TNF-α
levels in patients with HSPN were significantly higher than the
NHSPN and control group. TNF-α levels in the HSPN group serum was
significantly higher than that in the NHSPN group. Urinary protein
content also increased correspondingly, indicating that TNF-α and
HSP renal function impairment is closely related (19,20).
Urine protein is an indicator of renal dysfunction, and with the
increase in urinary protein level, the degree of renal function
damage also increases. Thus, the above three inflammatory immune
factors are involved in kidney function impairment. Our results
showed that the levels of TNF-α, IL-8 and IL-10 in the severe
nephritis group (acute nephritis, chronic nephritis and nephrotic
syndrome groups) were higher than those in the light nephritis
group (i.e., simple proteinuria group) (P<0.05, P<0.01). The
levels of TNF-α, IL-8 and IL-10 in the acute nephritis group were
higher than those in the chronic nephritis and nephrotic syndrome
groups.
In summary, the pathogenesis of HSP is complex,
serum TNF-α, IL-8, and IL-10 in acute phase HSP patients was
significantly higher than that in the healthy group. The TNF-α
level in the HSPN group was significantly higher than that in the
NHSPN group, indicating that TNF-α may cause a series of functions
and morphological changes in glomerular cells. Therefore, serum
TNF-α concentration can be used as an indicator of disease
activity.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LY and QW analyzed and interpreted the patients
data. SZ wrote the manuscript. LZ revised the manuscript for
important intellectual content. SZ and LZ contributed to the
conception and design of the study. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Affiliated Hospital of Jining Medical University (Shandong,
China). Signed written informed consents were obtained from all the
participants before the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saulsbury FT: Henoch-Schönlein purpura.
Curr Opin Rheumatol. 13:35–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang YH, Hung CF, Hsu CR, Wang LC, Chuang
YH, Lin YT and Chiang BL: A nationwide survey on epidemiological
characteristics of childhood Henoch-Schönlein purpura in Taiwan.
Rheumatology (Oxford). 44:618–622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ballinger S: Henoch-Schonlein purpura.
Curr Opin Rheumatol. 15:591–594. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sano H, Izumida M, Shimizu H and Ogawa Y:
Risk factors of renal involvement and significant proteinuria in
Henoch-Schönlein purpura. Eur J Pediatr. 161:196–201. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mills JA, Michel BA, Bloch DA, Calabrese
LH, Hunder GG, Arend WP, Edworthy SM, Fauci AS, Leavitt RY, Lie JT,
et al: The American College of Rheumatology 1990 criteria for the
classification of Henoch-Schönlein purpura. Arthritis Rheum.
33:1114–1121. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu JJ, Zhu YT and Hu YM: Mechanism of
feedback regulation of neutrophil inflammation in Henoch-Schönlein
purpura. Eur Rev Med Pharmacol Sci. 20:4277–4285. 2016.PubMed/NCBI
|
|
7
|
Knight JF: The rheumatic poison: A survey
of some published investigations of the immunopathogenesis of
Henoch-Schönlein purpura. Pediatr Nephrol. 4:533–541. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng WJ, Chen MG, Chen XY, Yang Q and Lin
RX: Renal expression of macrophage migration inhibitory factor in
children with Henoch-Sch-nlein purpura nephritis. Zhongguo Dang Dai
Er Ke Za Zhi. 12:120–122. 2010.(In Chinese). PubMed/NCBI
|
|
9
|
Wu TH, Wu SC, Huang TP, Yu CL and Tsai CY:
Increased excretion of tumor necrosis factor alpha and interleukin
1 beta in urine from patients with IgA nephropathy and
Schönlein-Henoch purpura. Nephron. 74:79–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gattorno M, Vignola S, Barbano G, Sormani
MP, Sabatini F, Buoncompagni A, Picco P and Pistoia V: Tumor
necrosis factor induced adhesion molecule serum concentrations in
Henoch-Schönlein purpura and pediatric systemic lupus
erythematosus. J Rheumatol. 27:2251–2255. 2000.PubMed/NCBI
|
|
11
|
Bradley JR, Lockwood CM and Thiru S:
Endothelial cell activation in patients with systemic vasculitis.
QJM. 87:741–745. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montefort S, Holgate ST and Howarth PH:
Leucocyte-endothelial adhesion molecules and their role in
bronchial asthma and allergic rhinitis. Eur Respir J. 6:1044–1054.
1993.PubMed/NCBI
|
|
13
|
Rostoker G, Rymer JC, Bagnard G,
Petit-Phar M, Griuncelli M and Pilatte Y: Imbalances in serum
proinflammatory cytokines and their soluble receptors: A putative
role in the progression of idiopathic IgA nephropathy (IgAN) and
Henoch-Schönlein purpura nephritis, and a potential target of
immunoglobulin therapy? Clin Exp Immunol 114: 468–476, 1998? Clin
Exp Immunol 114: 468–476, 1998. 114: 468–476, 1998:468-476,
1998–476, 1998. 1998.
|
|
14
|
Gardner-Medwin JM, Dolezalova P, Cummins C
and Southwood TR: Incidence of Henoch-Schönlein purpura, Kawasaki
disease, and rare vasculitides in children of different ethnic
origins. Lancet. 360:1197–1202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimura S, Takeuchi S, Soma Y and Kawakami
T: Raised serum levels of interleukins 6 and 8 and antiphospholipid
antibodies in an adult patient with Henoch-Schönlein purpura. Clin
Exp Dermatol. 38:730–736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tong YF, Liu Y, Hu ZX, Li ZC and Agula A:
Protocatechuic aldehyde inhibits TNF-α-induced fibronectin
expression in human umbilical vein endothelial cells via a c-Jun
N-terminal kinase dependent pathway. Exp Ther Med. 11:277–282.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
López-Mejías R, Genre F, Pérez BS,
Castañeda S, Ortego-Centeno N, Llorca J, Ubilla B, Remuzgo-Martínez
S, Mijares V, Pina T, et al: HLA-DRB1 association with
Henoch-Schonlein purpura. Arthritis Rheumatol. 67:823–827. 2014.
View Article : Google Scholar
|
|
18
|
Yang YH, Huang MT, Lin SC, Lin YT, Tsai MJ
and Chiang BL: Increased transforming growth factor-beta
(TGF-beta)-secreting T cells and IgA anti-cardiolipin antibody
levels during acute stage of childhood Henoch-Schönlein purpura.
Clin Exp Immunol. 122:285–290. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Furukawa S, Matsubara T, Yone K, Hirano Y,
Okumura K and Yabuta K: Kawasaki disease differs from anaphylactoid
purpura and measles with regard to tumour necrosis factor-alpha and
interleukin 6 in serum. Eur J Pediatr. 151:44–47. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Royall JA, Berkow RL, Beckman JS,
Cunningham MK, Matalon S and Freeman BA: Tumor necrosis factor and
interleukin 1 alpha increase vascular endothelial permeability. Am
J Physiol. 257:L399–L410. 1989.PubMed/NCBI
|