Introduction
Diabetes mellitus is a complex metabolic disorder. A
key feature of diabetes mellitus is the reduced function of the β
cells in the islets of Langerhans in the pancreas. These β cells
produce insulin and their reduced function may decrease insulin
secretion and lead to increased blood glucose levels (1). The synthesis of advanced glycosylation
end-products and the induction of oxidative stress may exacerbate
early pathological vascular changes (2). In patients with diabetes, associated
complications may contribute to increased morbidity and premature
mortality (3). Retinopathy,
neuropathy, cardiovascular disease, nephropathy and encephalopathy
are considered to be the primary complications of diabetes
(4) and periodontal disease has been
implicated as the sixth complication of diabetes (5). Periodontitis is a common, chronic,
inflammatory disease that induces the gradual destruction of the
supporting apparatus of the teeth, causing teeth to become mobile,
which may ultimately result in tooth loss (6). It has been reported that the prevalence
of periodontal disease in diabetic patients is >85% (27.3% of
patients had gingivitis and 59.5% had periodontitis) whereas the
prevalence of periodontitis in the general population is 46%
(1,7). Diabetes is a known risk factor of
periodontal disease and patients with diabetes have an increased
prevalence of severe periodontitis compared with healthy adults
(8). Indeed, it has been
demonstrated that the susceptibility to periodontitis increases by
~3-fold in patients with diabetes (6). Among individuals with chronic
periodontitis, patients with diabetes exhibit enhanced
lipopolysaccharide-induced immune responsiveness, implicating an
exacerbated inflammatory response (9).
It has been suggested that diabetes and
periodontitis are biologically linked (10) Hyperglycemia associated with diabetes
has been previously studied due to its association with adverse
periodontal outcomes (3). However,
to the best of our knowledge, the risk factors for periodontitis
specifically in patients with diabetes have not yet been
identified. Therefore, the present study was performed to assess
the risk factors for periodontitis in Korean adults with diabetes
using nationally representative data.
Materials and methods
Survey and subjects
The results of the present study were obtained by
secondary analysis of data obtained during the Korean National
Health and Nutrition Examination Survey (KNHANES) between January
2012 and December 2014. The KNHANES was approved by the
Institutional Review Board of the Korea Center for Disease Control
and all participants provided written informed consent. The
Institutional Review Board at the Catholic University of Korea
approved the present study (KC14EISI0636).
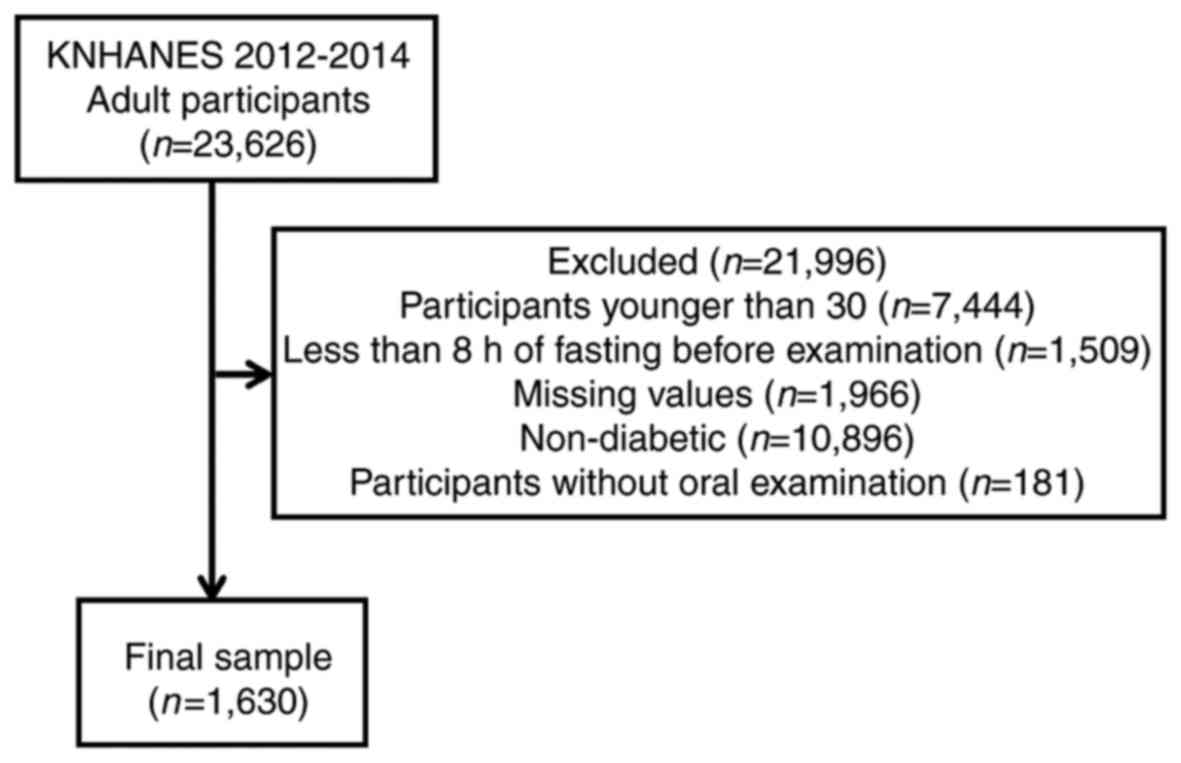
A total of 23,626 individuals participated in the
KNHANES survey. The number of individuals included in the present
study was reduced to 1,630 following the exclusion of participants
<30 years old, individuals that had only undergone <8 h
fasting prior to examination, participants with missing values,
individuals without diabetes mellitus and participants that did not
undergo an oral examination (Fig.
1).
Demographic variables
The sociodemographic and lifestyle variables of the
participants were evaluated by a self-administered questionnaire
that assessed education level, household income, smoking, alcohol
intake and physical exercise. Regarding smoking status, patients
were classified as non-smokers, ex-smokers or current smokers.
Non-smokers were those who had never smoked or had smoked <100
cigarettes during their lifetime, whereas ex-smokers were those who
had smoked >100 cigarettes in their entire life but were not
smoking at the time of the study. Regarding alcohol consumption,
patients were categorized as non-drinkers, mild to moderate
drinkers (1–30 g/day) or heavy drinkers (>30 g/day). Physical
activity was measured using the international physical activity
questionnaire as part of the KNHANES. Subjects who exercised for ≥5
occasions per week for 30 min per session, or those who
participated in strenuous physical activity for ≥3 occasions per
week for 20 min per session, were defined as regular exercisers.
Education level was categorized as either high school education
(≥10 years schooling) or middle school graduate or lower (<10
years schooling). The number of household members was evaluated.
Household income was divided by the number of included family
members and categorized into quartiles. The lowest quartile of
household income was <USD 1,092.40 per month and patients were
categorized as being in the lowest quartile or not. The
participants self-reported health status was categorized as good or
not good (which consisted of patients reporting their health as
average or bad).
Anthropometric measurements
Anthropometric measurements of individuals that
participated in the present study were taken by the trained staff
members. Participants wore light, indoor clothing without shoes for
the measurement of body weight and height, as previously described
(11). The narrowest point between
the iliac crest and the lower border of the rib cage was used to
determine waist circumference. The following formula of
weight/height2 (kg/m2) was used to calculate
body mass index. A standard mercury sphygmomanometer (Baumanometer;
W.A. Baum Co., Inc., Copiague, NY, USA) was used to measure
systolic and diastolic blood pressure in the right arm. Blood
pressure measurements were performed twice with a 5 min interval
and the average of the two measurements was used for analysis.
Blood samples were collected from the antecubital vein of each
participant to measure the white blood cell count. The white blood
cell count was measured using an automated hematology analyzer
(XE-2100D; Sysmex Corporation, Kobe, Japan).
Definition of diabetes mellitus and
hypertension
A high waist circumference was categorized as ≥90 cm
in men and ≥80 cm in women. Hypertension was diagnosed if an
individual had a systolic blood pressure of >160 mm Hg or a
diastolic blood pressure of >90 mm Hg, or if the individual was
using systemic antihypertensive drugs. A fasting blood cholesterol
level >240 mg/ml or the use of medication for the condition was
considered to indicate the presence of hypercholesterolemia. The
level of kidney function was determined by estimating the
glomerular filtration rate (eGFR) using the following equations:
eGFR (ml/min/1.73 m2)=186.3× (serum
creatinine−1.154) × (age−0.203) for males and
186.3× (serum creatinine−1.154) × (age−0.203)
×0.742 for females. Serum creatinine was measured by the
colorimetric method using a Hitachi 7600 modular chemistry analyzer
(Hitachi, Ltd., Tokyo, Japan). The eGFR was categorized as either
≥60 or not as previously described (12). Cardiovascular disease was considered
present if the individual had experienced a stroke or had a
congenital heart defect (13).
Diabetes was defined as a fasting blood sugar
>126 mg/dl or the individual was currently using anti-diabetic
medication (14). The recognition,
treatment and control of diabetes was self-evaluated, as well as
insulin injection. Glucose and glycated hemoglobin levels were
categorized into five levels. Patients were classified into the
following 5 groups based on their glycated hemoglobin levels:
<6, 6≤x<6.5, 6.5≤x<7, 7≤x<7.9 and ≥8 and into the
following 5 groups based on their glucose levels: <100,
100≤x<120, 120≤x<140, 140≤x<160 and >160.
Periodontitis and oral health
behaviors
The oral health status of the participants was
evaluated using the World Health Organization Community Periodontal
Index (CPI) (15,16). The index teeth according to the
Federation Dentaire Internationale system were 11, 16, 17, 26, 27,
31, 36, 37, 46 and 47 (17).
Periodontitis was defined as CPIs of 3 and 4. The CPI score was 3
in cases of a shallow pocket with a depth of 3.5–5.5 mm and 4 in
cases of a deep pocket with a depth ≥5.5 mm (18). Moderate periodontitis was defined as
a CPI of 3 and severe periodontitis was defined as a CPI of 4.
The frequency of daily tooth brushing and the use of
secondary oral products were used to evaluate oral health behavior.
Dental floss, interdental brushes, electric toothbrushes,
irrigation devices, end-tufted brushes, tongue cleaners, mouthwash
and special devices for dentures were considered to be secondary
oral products. The survey also recorded the participants
self-reported oral status, the presence of any toothache, chewing
discomfort, speech discomfort and whether patients had undergone a
dental checkup within that year.
Statistical analysis
The results are presented as the mean ± standard
error of the mean for continuous variables and as proportions
(standard errors) for categorical variables. To assess differences
in characteristics as categorized by body mass index, a
χ2 test was performed to assess categorical variables
and an independent t test was used to assess continuous variables.
The risk factors for periodontitis in diabetes were evaluated
following adjustments for age, sex, smoking, drinking, number of
household members, income, self-reported health, medication,
self-reported oral status, tooth pain, frequency of tooth brushing
per day and the use of secondary oral products. Multiple logistic
regression analyses were used to assess the associations between
periodontitis and age, sex, diabetic control and duration of
diabetes, following adjustment for confounding factors. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using SAS software version
9.2 for Windows (SAS Institute, Inc., Cary, NC, USA).
Results
Baseline characteristics
Table I details the
baseline characteristics of the participants included in the study
according to the presence of periodontitis. The odds ratio (OR) of
periodontitis was higher in individuals aged ≥65 years old compared
with individuals aged 30–65 years old (OR 1.152). The OR of
periodontitis was significantly higher in males compared with
females, with a 95% confidence interval of 1.387–2.269 (P<0.05).
Being male, smoking, drinking, have a higher number of household
members, being in the lowest income quartile, have an average or
bad health status, having problematic self-reported oral status,
experiencing tooth pain, experiencing chewing or speech discomfort,
having a lower frequency of tooth brushing and not using secondary
oral products were significantly associated with the incidence of
periodontitis in the diabetic population.
 | Table I.Baseline characteristics of study
participants with moderate and severe periodontitis. |
Table I.
Baseline characteristics of study
participants with moderate and severe periodontitis.
| Characteristic | No periodontitis,
n=838, % (SEM) | Periodontitis, n=792,
% (SEM) | P-value | Odds ratio (95%
CI) |
|---|
| Age (years) |
|
| 0.4059 |
|
|
30–64 | 67.4 (1.8) | 65.3 (2.1) |
| 1 (reference) |
|
≥65 | 32.6 (1.8) | 34.7 (2.1) |
| 1.152 (0.916,
1.449) |
| Sex |
|
|
<0.0001a |
|
|
Male | 49.3 (2.1) | 60.9 (2) |
| 1.774 (1.387,
2.269) |
|
Female | 50.7 (2.1) | 39.1 (2) |
| 1 (reference) |
| Smoking |
|
|
<0.0001a |
|
| No | 60.4 (2.2) | 43.5 (2) |
| 1 (reference) |
| Ex | 21 (1.8) | 24.9 (1.8) |
| 1.514 (0.993,
2.31) |
|
Current | 18.6 (1.6) | 31.6 (2.1) |
| 2.503 (1.67,
3.752) |
| Drinking |
|
| 0.0362a |
|
|
Non-drinker | 37.3 (1.9) | 30.8 (2) |
| 1 (reference) |
| Mild to
moderate drinker | 54 (2.1) | 57.1 (2.1) |
| 1.176 (0.9,
1.535) |
| Heavy
drinker | 8.7 (1.3) | 12.1 (1.4) |
| 1.388 (0.855,
2.255) |
| Exercise |
|
| 0.3669 |
|
| No | 83.9 (1.8) | 86.1 (1.8) |
| 1 (reference) |
|
Yes | 16.1 (1.8) | 13.9 (1.8) |
| 0.832 (0.559,
1.237) |
| Number of household
members |
|
| 0.0079a |
|
| 1 | 10.5 (1.2) | 13.6 (1.5) |
| 1 (reference) |
| 2 | 30.9 (1.8) | 36.7 (2.2) |
| 0.793 (0.544,
1.156) |
| ≥3 | 58.6 (2) | 49.7 (2.5) |
| 0.626 (0.414,
0.949) |
| Education |
|
| 0.2500 |
|
|
Middle-school graduate or
lower | 51.2 (2.2) | 54.8 (2.2) |
| 1 (reference) |
|
High-school education or
higher | 48.8 (2.2) | 45.2 (2.2) |
| 0.924 (0.689,
1.24) |
| Income (the lowest
quartile) |
|
| 0.0157a |
|
| No | 76.1 (1.7) | 69.8 (2.2) |
| 1 (reference) |
|
Yes | 23.9 (1.7) | 30.2 (2.2) |
| 1.312 (0.979,
1.757) |
| Self reported
health status |
|
| 0.0122a |
|
|
Good | 17.4 (1.6) | 12.3 (1.3) |
| 1 (reference) |
| Average
or bad | 82.6 (1.6) | 87.7 (1.3) |
| 1.67 (1.19,
2.343) |
| Body mass index
(kg/m2) |
|
| 0.2601 |
|
|
<25 | 47.8 (2.1) | 51.2 (2.1) |
| 1 (reference) |
|
≥25 | 52.2 (2.1) | 48.8 (2.1) |
| 0.939 (0.735,
1.199) |
| High waist
circumference, ≥90 cm in men and ≥80 cm in women |
|
| 0.0793a |
|
| No | 44 (2.1) | 49 (2.1) |
| 1.091 (0.852,
1.396) |
|
Yes | 56 (2.1) | 51 (2.1) |
| 1 (reference) |
| Hypertension |
|
| 0.1316 |
|
| No | 47 (2.1) | 42.4 (2.2) |
| 1 (reference) |
|
Yes | 53 (2.1) | 57.6 (2.2) |
| 1.052 (0.809,
1.367) |
|
Hypercholesterolemia |
|
| 0.8652 |
|
| No | 69.2 (1.9) | 69.7 (1.9) |
| 1 (reference) |
|
Yes | 30.8 (1.9) | 30.3 (1.9) |
| 1.04 (0.79,
1.37) |
| Estimated
glomerular filtration rate (ml/min/1.73 m2) |
|
| 0.7636 |
|
|
≥60 | 91.7 (1.1) | 92.1 (1) |
| 1.197 (0.796,
1.801) |
|
<60 | 8.3 (1.1) | 7.9 (1) |
| 1 (reference) |
| Cardiovascular
disease |
|
| 0.9456 |
|
| No | 90.9 (1.1) | 90.8 (1.1) |
| 1 (reference) |
|
Yes | 9.1 (1.1) | 9.2 (1.1) |
| 0.912 (0.615,
1.352) |
| Diabetes
(recognition) |
|
| 0.3699 |
|
| No | 37.6 (2.2) | 40.2 (2.2) |
| 1 (reference) |
|
Yes | 62.4 (2.2) | 59.8 (2.2) |
| 0.785 (0.611,
1.008) |
| Diabetes
(treated) |
|
| 0.1644 |
|
| No | 43.4 (2.2) | 47.7 (2.2) |
| 1 (reference) |
|
Yes | 56.6 (2.2) | 52.3 (2.2) |
| 0.732 (0.567,
0.944) |
| Diabetes
(controlled) |
|
| 0.9387 |
|
| No | 78.2 (1.9) | 78 (1.9) |
| 1 (reference) |
|
Yes | 21.8 (1.9) | 22 (1.9) |
| 0.993 (0.728,
1.353) |
| Insulin
injection |
|
| 0.4330 |
|
| No | 94.8 (0.8) | 93.8 (1) |
| 1 (reference) |
|
Yes | 5.2 (0.8) | 6.2 (1) |
| 1.150 (0.663,
1.994) |
| Diabetic
medication |
|
| 0.234 |
|
| No | 45 (2.2) | 48.6 (2.2) |
| 1 (reference) |
|
Yes | 55 (2.2) | 51.4 (2.2) |
| 0.758 (0.59,
0.973) |
| Glucose level |
|
| 0.9500 |
|
|
<100 | 9.8 (1.1) | 9.4 (1.2) |
| 1 (reference) |
|
100≤x<120 | 22.5 (1.7) | 22.2 (1.9) |
| 1.095 (0.725,
1.655) |
|
120≤x<140 | 33.9 (2.1) | 32.6 (2.1) |
| 1.079 (0.721,
1.615) |
|
140≤x<160 | 15.6 (1.6) | 15.9 (1.4) |
| 1.146 (0.724,
1.812) |
|
≥160 | 18.2 (1.7) | 19.9 (1.7) |
| 1.326 (0.852,
2.065) |
| Glycated
hemoglobin |
|
| 0.4800 |
|
|
<6 | 5.8 (1.1) | 7.8 (1.1) |
| 1 (reference) |
|
6≤x<6.5 | 16 (1.6) | 14.2 (1.6) |
| 0.701 (0.4,
1.231) |
|
6.5≤x<7 | 27.6 (1.9) | 28.6 (2.1) |
| 0.805 (0.468,
1.386) |
|
7≤x<7.9 | 31.1 (1.9) | 28.1 (1.9) |
| 0.699 (0.418,
1.168) |
| ≥8 | 19.5 (1.7) | 21.3 (1.7) |
| 0.91 (0.526,
1.577) |
| Dental checkup
within a year |
|
| 0.2490 |
|
| No | 76.7 (1.7) | 73.8 (1.9) |
| 1 (reference) |
|
Yes | 23.3 (1.7) | 26.2 (1.9) |
| 1.184 (0.887,
1.579) |
| Self-reported oral
status |
|
|
<0.0001a |
|
|
Favorable | 14.9 (1.6) | 9.9 (1.2) |
| 1 (reference) |
|
Average | 36.6 (2.1) | 26.5 (1.7) |
| 1.179 (0.772,
1.801) |
|
Problematic | 48.6 (2.2) | 63.6 (1.9) |
| 2.019 (1.346,
3.027) |
| Tooth pain |
|
|
<0.0001a |
|
| No | 65.4 (2) | 47.6 (2.1) |
| 1 (reference) |
|
Yes | 34.6 (2) | 52.4 (2.1) |
| 2.086 (1.653,
2.632) |
| Chewing
discomfort |
|
|
<0.0001a |
|
| No | 69.7 (2) | 56.9 (2.1) |
| 1 (reference) |
|
Yes | 30.3 (2) | 43.1 (2.1) |
| 1.642 (1.271,
2.12) |
| Speech
discomfort |
|
| 0.0339a |
|
| No | 85.5 (1.4) | 81.1 (1.6) |
| 1 (reference) |
|
Yes | 14.5 (1.4) | 18.9 (1.6) |
| 1.277 (0.946,
1.724) |
| Frequency of tooth
brushing per day |
|
| 0.0019a |
|
| ≤1 | 12.5 (1.4) | 18.5 (1.7) |
| 1 (reference) |
| 2 | 42.6 (2.2) | 46.1 (2) |
| 0.773 (0.534,
1.119) |
| ≥3 | 44.9 (2.2) | 35.4 (2) |
| 0.583 (0.393,
0.865) |
| Use of secondary
oral products |
|
| 0.0020a |
|
| No | 54.1 (2.2) | 63.6 (2.1) |
| 1 (reference) |
|
Yes | 45.9 (2.2) | 36.4 (2.1) |
| 0.757 (0.583,
0.981) |
The odds ratio of periodontitis
following adjustments for confounding factors
ORs and their 95% confidence intervals for moderate
and severe periodontitis categorized by various variables following
adjustments for age, sex, smoking, drinking, number of household
members, income, self-reported health, medication, self-reported
oral status, tooth pain, frequency of tooth brushing per day and
use of secondary oral products are presented in Fig. 2. The ORs and 95% confidence intervals
of periodontitis were 1.60 (1.05, 2.46) and 2.28 (1.47, 3.52) for
ex-smokers and current smokers, respectively when non-smokers were
considered as a reference. The ORs and 95% confidence intervals of
periodontitis for the individuals were 1.51 (1.01, 2.26) and 1.20
(0.91, 1.58) for the individuals with a toothbrushing frequency of
≤1 and 2, respectively when participants with toothbrushing
frequency of 3 was considered as a reference. These results
indicate that individuals with current smoking had higher ORs of
periodontitis. Similarly, participants with a toothbrushing
frequency of ≤1 had higher ORs of periodontitis.
The percentage of moderate and severe
periodontitis categorized by different variables
Fig. 3 presents the
percentage of moderate and severe periodontitis categorized by age,
sex, duration of diabetes and glycated hemoglobin. The percentages
of moderate periodontitis in individuals aged 30–39 and ≥70 were
24.1 and 36.8%, respectively. The percentages of moderate
periodontitis in male and female were 35.5 and 30.2%, respectively.
The percentage of patients with periodontitis differed
significantly between different age groups (P=0.0074) and
significant differences were also noted between males and females
(P<0.0001). There were no significant differences between
patients with moderate and severe periodontitis.
Discussion
The present study used nationally representative
data to determine that age, sex and oral health behavior were risk
factors for periodontitis in Korean adults with diabetes. The
effect of age on the prevalence of periodontitis may partially be
explained by the onset of chronic inflammation, which is a common
feature of aging and age-associated diseases, including
periodontitis (19). It has been
previously revealed that the prevalence and severity of periodontal
disease increased as age increased (1). Similarly, periodontitis occurred at a
higher rate in diabetic participants compared with non-diabetic
individuals and this phenomenon was positively correlated with age
in individuals with diabetes (19).
It has been suggested that patients with diabetes aged ≥35 years
old experience more rapid destruction of the periodontium (20). Furthermore, it has been demonstrated
that age is significantly associated with the increased prevalence
and greater severity of destructive periodontal disease in patients
with diabetes (21). The results of
present study confirmed the importance of age in determining the
prevalence of periodontitis in patients with diabetes.
Differences between the two sexes have also been
noted in previous studies. A previous report revealed that diabetic
males had worse periodontal conditions compared with diabetic
females (22). A previous study also
revealed statistically significant associations between males and
severe periodontitis (23). These
results therefore suggest that there is a greater need for the
regular periodontal evaluation and effective oral hygiene care
among males with diabetes than in females with diabetes to decrease
the risk of developing periodontitis and progression of
periodontitis into a more severe form (24). The present study revealed that the
percentage of patients with moderate and severe periodontitis did
not significantly differ between different duration of diabetes or
diabetic control. In a previous study, diabetic status was
associated with an increased severity and prevalence of destructive
periodontal disease in the diabetic population (21). Furthermore, glycemic status
influences the prevalence and severity of periodontal disease in
diabetic participants (1).
Individuals with poorly controlled diabetes and periodontitis are
more likely to suffer from gingival bleeding than those with good
or moderate control (25).
Conversely, no significant correlation was observed between
periodontitis and glycated hemoglobin levels in non-diabetic
subjects (26). Similarly, glycemic
control, defined by fasting plasma glucose (140 mg/dl) and
glycohemoglobin value (6.5%), was not significantly associated with
periodontal status (27). Diabetic
control did not reveal any differences in the periodontal condition
(20). A previous report
demonstrated that the glycated hemoglobin levels were not
significantly correlated with periodontal values in diabetic
participants (28), which was
similar to trends observed in the present study. The association
between the duration of diabetes and periodontitis was evaluated in
the present study; however no significant association was observed.
The association between the duration of diabetes and periodontitis
is controversial as some reports have demonstrated that the two
factors were associated, while others have reported no association
(19,20,25,27,28). A
previous study reported that the number of years since the
diagnosis of diabetes was more significant than age for predicting
the severity of periodontal disease in diabetic patients (29). A previous study observed that the
duration of diabetes mellitus had an association with the
prevalence and severity of periodontal disease (1). It should be noted that the number of
participants in these previous reports were relatively small, being
composed of only 100 patients (46 males and 54 females) (29) and 1,500 patients (751 males and 749
females), respectively (1). However,
no correlation was observed between periodontitis in individuals
with diabetes and the length of time from the diagnosis of diabetes
(19). Several previous studies have
reported that the duration of diabetes was not associated with the
periodontal condition (20,27,28). A
previous report revealed that no significant correlation was
observed between diabetic duration and gingival bleeding (25). An advantage of the present study is
that it was based on a nationally representative sample of Korean
patients with diabetes, which allows for an effective investigation
into whether age and sex are associated with periodontitis. The
sampling units were based on the population and housing census
conducted by the National Census Registry in Korea and survey
sample weights were adjusted for participation and response rates
(30). To the best of our knowledge,
the present study is the first to demonstrate the importance of age
and sex as risk indicators for the prevalence of periodontitis in
individuals with diabetes (31). The
present study assessed the effects of various risk indicators by
subgroup analysis, including age, sex, control of diabetes and
duration of diabetes. However, the present study did have certain
limitations. Firstly, as it was a cross-sectional study it was not
possible to identify a cause-and-effect association. Secondly, the
present study used partial-mouth recording protocols of CPI, which
may underestimate the prevalence of periodontal disease. To
overcome these limitations a prospective study using full-mouth
recording may be designed, however this type of study requires far
greater resources to complete.
A bidirectional association has been reported
between diabetes and periodontitis (3). Patients with diabetes have a higher
predisposition to periodontitis (9)
and periodontitis is a risk factor in a number of systemic
diseases, including diabetes and cardiovascular pathologies
(2). In diabetic patients, the
elimination of periodontal infections and a reduction in
periodontal inflammation produces a noticeable short-term reduction
in glycated hemoglobin (32).
Individuals with periodontitis had an increased rate of
microvascular complications ketoacidosis and of hospitalizations
associated with hyperglycemia with odds ratio and 95% confidence
intervals of [2.43 (1.74–3.40)], [2.72 (1.53–4.80)] and [2.76
(1.72–4.42)], respectively (33).
Treatment of periodontitis reduces the risk of cardiovascular
disease in diabetic patients (34).
Therefore, periodontitis may stimulate inflammatory changes in
adipose tissue and this may create a self-generating cycle of
morbidity that links diabetes and periodontal disease (35).
Dental management has a beneficial effect on the
health of patients with diabetes (34). Maintaining oral health helps prevent
oral chronic diseases and ameliorate the consequences of chronic
inflammatory processes (36).
Patients with diabetes are likely to visit a physician more
frequently than they visit a dentist and it is the physician's
responsibility to educate and motivate the patient to seek dental
treatment (37).
In conclusion, the results of the present study
indicate that age, sex and oral health behavior are risk indicators
of periodontitis in Korean adults with diabetes. The present study
suggests that that an increased age, being male and engaging in
poor oral health behavior increases the risk of periodontitis in
participants with diabetes. Further prospective studies involving a
larger sample size of diabetic subjects over a longer period of
time are required to evaluate the cause-and-effect association. The
present study suggests that individuals of a certain age with
diabetes should be recommended for regular periodontal evaluation,
particularly males.
Acknowledgements
The authors thank the Korea Centers for Disease
Control and Prevention for providing the data.
Funding
The present study was supported by Research Fund of
Seoul St. Mary's Hospital, The Catholic University of Korea (2017).
This work was partly supported by Basic Science Research Program
through the National Research Foundation of Korea funded by the
Ministry of Science, ICT & Future Planning (grant no.
NRF-2017R1A1A1A05001307; Daejeon, Republic of Korea).
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
KH and JP designed the research, analyzed the data
and wrote the manuscript. Both authors reviewed the final
manuscript.
Ethics approval and consent to
participate
The KNHANES was approved by the Institutional Review
Board of the Korea Center for Disease Control and all participants
provided written informed consent. The Institutional Review Board
at the Catholic University of Korea approved the present study
(KC14EISI0636).
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Rajhans NS, Kohad RM, Chaudhari VG and
Mhaske NH: A clinical study of the relationship between diabetes
mellitus and periodontal disease. J Indian Soc Periodontol.
15:388–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Napora M, Grabowska E and Górska R:
Prospective analysis of the relationship between the state of
periodontal tissues and changes in selected cardiovascular
parameters in patients with type 2 diabetes. Adv Clin Exp Med.
25:879–886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chapple IL and Genco R: working group 2 of
the joint EFP/AAP workshop: Diabetes and periodontal diseases:
Consensus report of the joint EFP/AAP workshop on periodontitis and
systemic diseases. J Periodontol. 84 4 Suppl:S106–S112. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khanuja PK, Narula SC, Rajput R, Sharma RK
and Tewari S: Association of periodontal disease with glycemic
control in patients with type 2 diabetes in Indian population.
Front Med. 11:110–119. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saini R, Saini S and Sugandha R:
Periodontal disease: The sixth complication of diabetes. J Family
Community Med. 18:312011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Preshaw PM and Bissett SM: Periodontitis:
Oral complication of diabetes. Endocrinol Metab Clin North Am.
42:849–867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eke PI, Dye BA, Wei L, Slade GD,
Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD and
Genco RJ: Update on prevalence of periodontitis in adults in the
United States: NHANES 2009 to 2012. J Periodontol. 86:611–622.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lakschevitz F, Aboodi G, Tenenbaum H and
Glogauer M: Diabetes and periodontal diseases: Interplay and links.
Curr Diabetes Rev. 7:433–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mesia R, Gholami F, Huang H, Clare-Salzler
M, Aukhil I, Wallet SM and Shaddox LM: Systemic inflammatory
responses in patients with type 2 diabetes with chronic
periodontitis. BMJ Open Diabetes Res Care. 4:e0002602016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shetty A, Bhandary R, Thomas B and Ramesh
A: A comparative evaluation of serum magnesium in diabetes mellitus
type 2 patients with and without periodontitis-a
clinico-biochemical study. J Clin Diagn Res. 10:ZC59–ZC61.
2016.PubMed/NCBI
|
|
11
|
Nam GE, Kim DH, Cho KH, Park YG, Han KD,
Choi YS, Kim SM, Ko BJ, Kim YH and Lee KS: Estimate of a predictive
cut-off value for serum 25-hydroxyvitamin D reflecting abdominal
obesity in Korean adolescents. Nutr Res. 32:395–402. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chapter 1: Definition and classification
of CKD. Kidney Int Suppl (2011). 3:19–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang MS, Kim CH, Jeong SJ and Park TS:
Dietary sodium intake in people with diabetes in Korea: The Korean
national health and nutrition examination survey for 2008 to 2010.
Diabetes Metab J. 40:290–296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH,
Kim CS, Song KH, Won JC, Lim S, Choi SH, et al: Prevalence of
diabetes and prediabetes according to fasting plasma glucose and
HbA1c. Diabetes Metab J. 37:349–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kingman A, Susin C and Albandar JM: Effect
of partial recording protocols on severity estimates of periodontal
disease. J Clin Periodontol. 35:659–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park JB, Han K, Park YG and Ko Y:
Association between alcohol consumption and periodontal disease:
The 2008 to 2010 Korea national health and nutrition examination
survey. J Periodontol. 85:1521–1528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blinkhorn AS, Choi CL and Paget HE: An
investigation into the use of the FDI tooth notation system by
dental schools in the UK. Eur J Dent Educ. 2:39–41. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Islam SA, Seo M, Lee YS and Moon SS:
Association of periodontitis with insulin resistance, β-cell
function, and impaired fasting glucose before onset of diabetes.
Endocr J. 62:981–989. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Albrecht M, Bánóczy J and Tamás G Jr:
Dental and oral symptoms of diabetes mellitus. Community Dent Oral
Epidemiol. 16:378–380. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bacić M, Plancak D and Granić M: CPITN
assessment of periodontal disease in diabetic patients. J
Periodontol. 59:816–822. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Emrich LJ, Shlossman M and Genco RJ:
Periodontal disease in non-insulin-dependent diabetes mellitus. J
Periodontol. 62:123–131. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schulze A and Busse M: Gender differences
in periodontal status and oral hygiene of non-diabetic and type 2
diabetic patients. Open Dent J. 10:287–297. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kongstad J, Enevold C, Christensen LB,
Fiehn NE and Holmstrup P: Impact of periodontitis case criteria: A
cross-sectional study of lifestyle. J Periodontol. 88:602–609.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strauss SM and Stefanou LB: Interdental
cleaning among persons with diabetes: Relationships with individual
characteristics. Int J Dent Hyg. 12:127–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ervasti T, Knuuttila M, Pohjamo L and
Haukipuro K: Relation between control of diabetes and gingival
bleeding. J Periodontol. 56:154–157. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wahi S, Tripathi A, Wahi S, Mishra VD,
Singh AP and Sinha N: Assessment of levels of glycosylated
hemoglobin in patients with periodontal pathologies: A comparative
study. J Contemp Dent Pract. 18:506–509. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bridges RB, Anderson JW, Saxe SR, Gregory
K and Bridges SR: Periodontal status of diabetic and non-diabetic
men: Effects of smoking, glycemic control, and socioeconomic
factors. J Periodontol. 67:1185–1192. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rylander H, Ramberg P, Blohme G and Lindhe
J: Prevalence of periodontal disease in young diabetics. J Clin
Periodontol. 14:38–43. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cerda J, Vázquez de la Torre C, Malacara
JM and Nava LE: Periodontal disease in non-insulin dependent
diabetes mellitus (NIDDM). The effect of age and time since
diagnosis. J Periodontol. 65:991–995. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han K and Park JB: Association between
oral health behavior and periodontal disease among Korean adults:
The Korea national health and nutrition examination survey.
Medicine (Baltimore). 96:e61762017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song IS, Han K, Park YM, Ji S, Jun SH, Ryu
JJ and Park JB: Severe periodontitis is associated with insulin
resistance in non-abdominal obese adults. J Clin Endocrinol Metab.
101:4251–4259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grossi SG, Skrepcinski FB, DeCaro T,
Zambon JJ, Cummins D and Genco RJ: Response to periodontal therapy
in diabetics and smokers. J Periodontol. 67 10 Suppl:S1094–S1102.
1996. View Article : Google Scholar
|
|
33
|
Oliveira LS, Lira-Junior R, Figueredo CM,
Gomes MB and Fischer RG: Self-reported periodontitis and
complications in type 1 diabetes patients: A Brazilian nationwide
survey. Braz Dent J. 27:599–603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng CH, Yang YS, Chan KC, Kornelius E,
Chiou JY and Huang CN: Periodontal treatment and the risks of
cardiovascular disease in patients with type 2 diabetes: A
retrospective cohort study. Intern Med. 56:1015–1021. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Levine RS: Obesity, diabetes and
periodontitis-a triangular relationship? Br Dent J. 215:35–39.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lifshitz F, Casavalle PL, Bordoni N,
Rodriguez PN and Friedman SM: Oral health in children with obesity
or diabetes mellitus. Pediatr Endocrinol Rev. 14:159–167.
2016.PubMed/NCBI
|
|
37
|
Ummadisetty T, Chava VK and Bhumanapalli
VR: Diabetes and periodontitis: How well are the patients aware
about an established relation? J Indian Soc Periodontol.
20:472–475. 2016. View Article : Google Scholar : PubMed/NCBI
|