Introduction
Renal cell carcinoma (RCC), the most common tumor of
the kidney, accounts for about 3% of adult malignancies (1). Approximately 30% of patients are
diagnosed with metastatic RCC when the disease is discovered and
the 5-year survival rate is estimated to be ~8% (2). Nearly 80% of RCC is clear cell RCC
(ccRCC), which is characterized by frequent genetic mutations of
the von Hippel Lindau (VHL) (3,4).
Patients with metastatic or recurrent RCC are frequently treated by
mTOR inhibitors or receptor tyrosine kinase (RTK), which delays the
progression of cancer rather than curing cancer (5,6).
Surgical therapy is the only definitive treatment when RCC is
resistant to chemotherapy (7),
besides, approximately 20–40% patients still experience recurrence
after the resection (8).
microRNAs (miRNAs, miRs) belong to a group of short,
non-coding RNAs that are 19–22 bases long and mediate translational
repression and/or RNA degradation by binding to the 3′ untranslated
region of the messenger RNA (mRNA) (9). miRNAs are involved in cellular
proliferation, differentiation and apoptosis by regulating 60% of
the protein-coding genes in the human genome (10). miRNAs are aberrantly expressed in
multiple human cancers and stimulate inappropriate cellular
programs such as invasion and metastasis (11–13). As
aberrantly expressed miRNAs are closely related to tumor initiation
and progression (13–15), it is important to elucidate the
function of aberrantly expressed miRNAs.
Previous studies have demonstrated that miR-216a-5p
is dysregulated in a number of malignancies, such as colorectal
cancer, pancreatic cancer, prostate cancer and liver cancer
(16–19). However, the expression of miR-216a-5p
requires quantification and the role of miR-216a-5p in RCC has not
been investigated to date. In the present study, we investigate the
expression of miR-216a-5p in RCC tissues and cells lines, and
explore the effects of miR-216a-5p on cellular proliferation,
viability, motility and apoptosis by wound scratch assay, Transwell
assay, MTT assay, CCK-8 assay and flow cytometry assay.
Materials and methods
Sample collection
A total of 24 RCC and matched normal tissues,
resected at Peking University Shenzhen Hospital (Shenzhen, China)
and reviewed by hematoxylin and eosin staining, were collected for
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). After resection, the specimens were immersed in RNA
later (Qiagen GmbH, Hilden, Germany) for 30 min and stored at −80°C
for further use. Additionally, ethics committees of Peking
University Shenzhen Hospital approved this study and written
informed consent for participation was provided by all patients.
Clinical and pathological characteristics of these twenty-four
patients are listed in Table I.
 | Table I.Clinicopathological features of
patients with renal cell carcinoma. |
Table I.
Clinicopathological features of
patients with renal cell carcinoma.
| Characteristic | Number of
patients |
|---|
| Mean age, range
(years) | 48 (24–63) |
| Males | 8 |
| Females | 16 |
| Histological
type |
|
| Clear
cell | 21 |
|
Papillary | 3 |
| pT-stage |
|
| T1 | 15 |
| T2 | 7 |
| T3 +
T4 | 2 |
| Fuhrmann grade |
|
| I | 5 |
| II | 16 |
|
III | 2 |
| IV | 1 |
| AJCC clinical
stage |
|
| I | 7 |
| II | 15 |
| III +
IV | 2 |
Cell culture
The present study used 293T, ACHN and 786-O RCC cell
lines, cultured in Dulbecco's modified Eagle's medium (DMEM basic;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco), 1%
penicillin streptomycin (pen strep; Gibco) and 1% glutamine (Gibco)
in a humidified incubator containing 5% CO2 at 37°C.
RNA extraction, cDNA synthesis and
RT-qPCR
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
was used to extract total RNA, which was then was purified with the
RNeasy Maxi kit (Qiagen GmbH). NanoDrop 2000/2000c (Thermo Fisher
Scientific, Inc.) was used to determine the concentration of RNA.
To obtain the cDNA templates, 1 µg total RNA of each sample was
used for reverse transcription using miScript Reverse Transcription
kit (Qiagen GmbH), and the temperature for the reverse
transcription reaction was 37°C for 60 min, 95°C for 5 min and
storage at 4°C. To detect the expression level of miR-216a-5p, qPCR
was performed with miScript SYBR®-Green PCR kit (Qiagen
GmbH) on the Roche Lightcycler 480 Real-Time PCR system (Roche
Diagnostics, Basel, Switzerland). The temperature for qPCR was 95°C
for 1 min, 40 cycles of 95°C for 15 sec, 55°C for 30 sec and 72°C
for 30 sec. In addition, the sequences of the primers of
miR-216a-5p and internal control (U6), used in PCR, are indicated
in Table II. The expression of
miR-216-5p was analyzed with the 2−ΔΔCq method (20).
 | Table II.Sequences of primers and
microRNAs. |
Table II.
Sequences of primers and
microRNAs.
|
Primer/microRNA | Sequence
(5′-3′) |
|---|
| miR-216a-5p |
|
| F |
TAATCTCAGCTGGCAACTGTGA |
| R | Provided by the
miScript SYBR®-Green |
| U6 |
|
| F |
CTCGCTTCGGCAGCACA |
| R |
ACGCTTCACGAATTTGCGT |
| miR-216a-5p
mimics |
|
| F |
UAAUCUCAGCUGGCAACUGUGA |
| R |
ACAGUUGCCAGCUGAGAUUAUU |
| NC |
|
| F |
UUCUCCGAACGUGUCACGUTT |
| R |
ACGUGACACGUUCGGAGAATT |
| miR-216a-5p
inhibitor |
UCACAGUUGCCAGCUGAGAUUA |
| NC inhibitor |
CAGUACUUUUGUGUAGUACAA |
Cell transfection
The synthesized miR-216a-5p mimics (GenePharma,
Shanghai, China) were transfected into the 786-O and ACHN cells to
upregulate the expression levels of miR-216a-5p. Similarly,
synthesized miR-216a-5p inhibitor (GenePharma) was transfected into
the 786-O and ACHN cells to decrease the cellular levels of active
miR-216a-5p. Lipofectamine 2000 (Invitrogen), mixed in the
Opti-MEM® I Reduced Serum Medium (Gibco), was used for
transfection. RT-qPCR was performed to analyze the changes of
miR-216a-5p expression after transfection.
Wound scratch assay
The cell migration of 786-O and ACHN cells was
assessed by a wound scratch assay. Each well of the 12-well plate
was seeded with ~300,000 cells and the cells were incubated for 24
h. Then Lipofectamine® 2000 was used for transfection of
cells with 40 pmol of miR-216a-5p mimics, negative control (NC),
inhibitors or inhibitor NC. A scratch was introduced in the cell
monolayer with a 200 µl pipette tip 6 h after transfection. The
initial images of the scratch at 0 h and the residual length of the
scratch at 12 h were captured by a digital camera system. At least
three pictures were taken for each experiment. Each experiment was
performed in triplicates and repeated at least three times.
Transwell assay
The migratory and invasive ability of 786-O and ACHN
cells were assessed by Transwell assay. Transwell chamber inserts
(BD Biosciences, Franklin Lakes, NJ, USA) with or without Matrigel
(for invasion) were used to analyze cell migration and invasive
ability respectively. In total, ~1×104 transfected cells
were seeded in the upper compartment of the chamber with 200 µl
serum-free medium, while medium with 10% FBS was added to the lower
chamber. Following migration for 36 h, enough cells migrated to the
bottom of chamber, and the invasion time was 48 h. A microscope was
used for counting the crystal violet stained migrated cells.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
(MTT assay)
The viability of 786-O and ACHN cells were assessed
by MTT assay. Each well of the 96-well plate was seeded with ~5,000
cells. The cells were transfected with 5 pmol miR-216a-5p mimics,
NC, inhibitors and inhibitor NC. A total of 4 days after
transfection, 20 µl MTT (5 mg/ml; Sigma-Aldrich, St. Louis, MO,
USA) was added to each well and incubated for 4 h. The medium with
MTT was replaced by 100 µl dimethyl sulfoxide (Sigma-Aldrich; Merck
Millipore). Subsequently, the 96-well plate was shaken using a by
reciprocating decolorization shaking table (TSB-108; Qilinbeier,
Jiangsu, China) for 10 min in a dark room and the optical density
(OD) was determined by an ELISA microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at 595 nm. MTT assay was
performed in triplicates and repeated at least three times.
Cell counting kit-8 assay (CCK-8
assay)
The proliferative ability of 786-O and ACHN cells
were assessed by CCK-8 (Beyotime Institute of Biotechnology,
Haimen, China). The 96-well plate with ~5,000 cells/well was
transfected with 5 pmol miR-216a-5p mimics, NC, inhibitors and
inhibitor NC after 24 h. At t 0, 24, 48 and 72 h after
transfection, 10 µl of CCK-8 was added to each well for 30 min and
the OD at a wavelength of 490 nm was recorded using an ELISA
microplate reader. CCK-8 assay was performed in triplicates and
repeated at least three times.
Flow cytometry assay
The apoptotic rates of 786-O and ACHN cells were
assessed by flow cytometry assay. Approximately 3×105
cells transfected with 200 pmol miR-216a-5p mimics, NC, inhibitors
or inhibitor NC were harvested from a 6-well plate and the rates of
apoptosis were detected by Annexin V-FITC apoptosis detection kit
(Invitrogen). The cells were mixed with 100 µl 1X binding buffer
and stained with 5 µl Annexin V-fluorescein isothiocyanate and 5 µl
propidium iodide in a dark at room temperature. After 15 min, 400
µl 1X binding buffer was added to each cell suspension. The
apoptotic rate of each cell suspension was analyzed by flow
cytometry (EPICS, Xl-4; Beckman Coulter, Inc., Brea, CA, USA). Flow
cytometry assay was performed in triplicates and repeated at least
three times.
Statistical analysis
All data for expression levels of miR-216a-5p in
matched tumor/normal tissues and different cells were analyzed by
paired t-tests. However, relative expression of miR-216a-5p in
cells was analyzed by one-way analysis of variance followed by
Dunnett's test. All data characterizing the phenotypes of cells
were analyzed by Student's t-test using SPSS 19.0 (IBM SPSS,
Armonk, NY, USA). P<0.05 was considered as statistically
significant difference.
Results
MiR-216a-5p is upregulated in RCC
tissues and cell lines
The expression levels of miR-216a-5p in 24 paired
RCC tissues and cell lines were explored by RT-qPCR. As shown in
Fig. 1A, miR-216a-5p was upregulated
in the RCC tissues. Relative expression of miR-216a-5p is shown in
Fig. 1B. The mean relative
expression of RCC tissues was 2.07 (P<0.05). As demonstrated in
Fig. 1C, the expression levels of
miR-216a-5p in 293T cell line was significantly lower when compared
with RCC cell lines ACHN (P<0.05) and 786-O (P<0.05).
Validation of cell transfection
efficiency
The transfection efficiency of miR-216a-5p mimic
group and inhibitor group was explored by RT-qPCR. As shown in
Fig. 1D, the expression levels of
miR-216a-5p were 538.68-fold higher in ACHN cells (P<0.01) after
transfection with miR-216a-5p mimic and 75.93-fold higher in 786-O
cells (P<0.01) when compared with NC group. The expression
levels of miR-216a-5p was 0.09 times in ACHN cells (P<0.001)
transfected with miR-216a-5p inhibitor and 0.35 times in 786-O
cells (P<0.01) when compared with the inhibitor NC group.
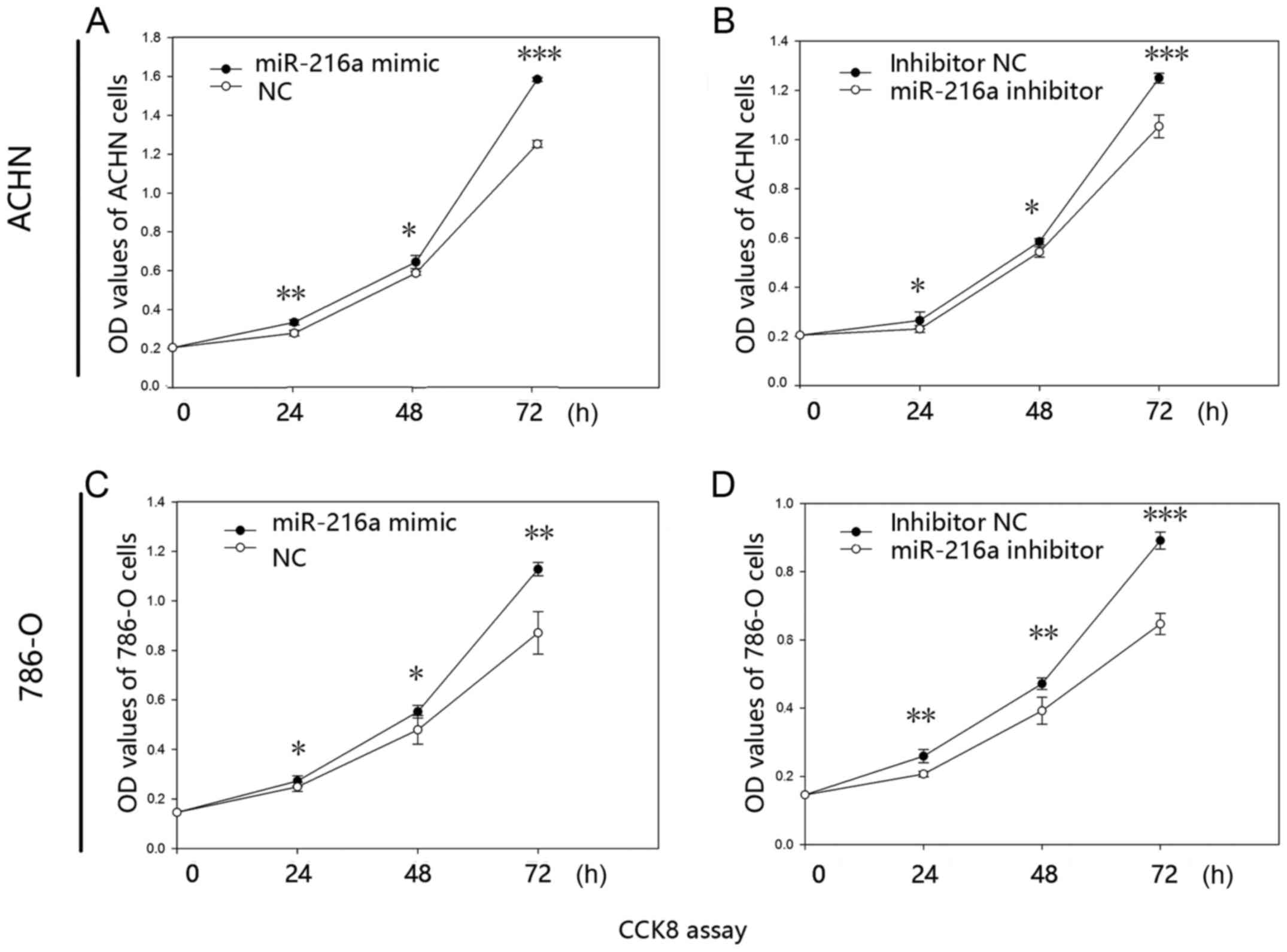
MiR-216-5p promotes RCC cell
proliferation
The proliferative ability of miR-216a-5p was
validated by CCK-8 assay in vitro. The results revealed that
the proliferative ability of mimic group was increased by 19.99%
(24 h; P<0.01), 9.72% (48 h; P<0.05) and 26.50% (72 h;
P<0.001) in the ACHN cells (Fig.
2A), while the proliferative ability was reduced in the
inhibitor group by 12.99% (24 h; P<0.05), 7.13% (48 h;
P<0.05) and 15.68% (72 h; P<0.001) (Fig. 2B). Similarly, proliferative ability
of mimic group was significantly increased by 9.50% (24 h;
P<0.05), 15.20% (48 h; P<0.05) and 29.48% (72 h; P<0.01)
in the 786-O cells (Fig. 2C), while
the inhibitor group was reduced by 20.19% (24 h; P<0.01), 16.85%
(48 h; P<0.01) and 27.42% (72 h; P<0.001) (Fig. 2D).
miR-216-5p increases the RCC cell
viability
The viability of miR-216a-5p on RCC cell was
validated by MTT assay in vitro. As shown in Fig. 3, cell viability after transfection
with the miR-216a-5p mimic increased by 35.08% (P<0.01) in ACHN
cells and by 20.56% (P<0.01) in 786-O cells. Inversely,
viability of cells transfected with the miR-216a-5p inhibitor was
reduced by 39.82% (P<0.001) in ACHN cells and by 26.19%
(P<0.01) in 786-O cells.
MiR-216-5p promotes RCC cell
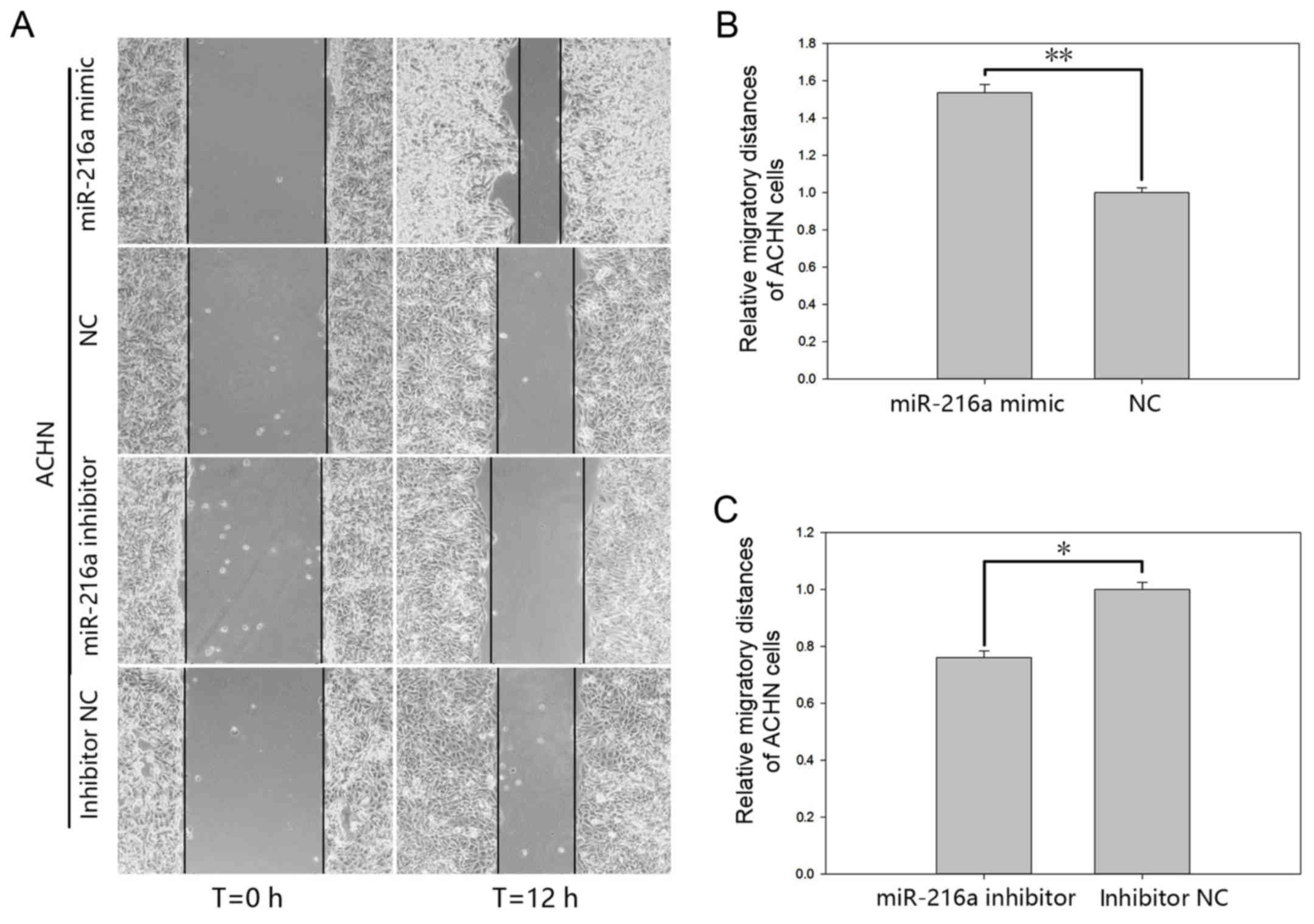
motility
The effect of miR-216a-5p on cell motility of RCC
cells was validated by a wound scratch assay and the Transwell
assay (Figs. 4 and 5). The results of the wound scratch assay
demonstrated that the distance migrated by the cells transfected
with miR-216a-5p mimic was increased by 53.49% (P<0.01; Fig. 4B) in ACHN cells and by 36.84%
(P<0.05; Fig. 5B) in 786-O cells
at 12 h post-transfection. In contrast, the distance migrated by
cells transfected with miR-216a-5p inhibitor was reduced by 23.93%
(P<0.05; Fig. 4C) in ACHN cells
and by 38.42% (P<0.05; Fig. 5C)
in 786-O cells at 12 h post-transfection.
As demonstrated in Fig.
6, the results of the Transwell assay indicated that the cell
migration for the mimic group was increased significantly by 48.57%
(P<0.05) in the 786-O cells (Fig.
6B) and by 75.81% (P<0.05) in ACHN cells (Fig. 6C), while the cell migration in the
inhibitor group was reduced by 44.75% (P<0.05) in 786-O cells
(Fig. 6B) and by 64.45% (P<0.01)
in ACHN cells (Fig. 6C). Similarly,
the invasive ability of mimic group was increased significantly by
154.14% (P<0.01) in the 786-O cells (Fig. 6B) and by 92.8% (P<0.05) in ACHN
cells (Fig. 6C), while the
invasiveness in the inhibitor group was reduced by 45.61%
(P<0.05) in the 786-O cells (Fig.
6B) and by 49.24% (P<0.01) in ACHN cells (Fig. 6C).
MiR-216-5p suppresses apoptosis. The results of the
flow cytometry (Figs. 7 and 8), showed that the early apoptotic rate of
the mimic group was 4.14%±0.44 vs. 11.8%±0.40 (P<0.01) in ACHN
cells (Fig. 7B) and 4.15%±0.26 vs.
16.46%±0.78 (P<0.01) in 786-O cells (Fig. 8B), while the apoptotic rate in the
inhibitor group was 21.46%±0.75 vs. 11.76%±0.31 (P<0.05) in ACHN
cells (Fig. 7C) and was 27.66%±0.93
vs. 13.7%±0.58 (P<0.05) in 786-O cells (Fig. 8C).
Discussion
miRNAs play a significant role in tumorigenesis and
have been associated with proliferation, differentiation and
apoptosis by activating cancer-promoting genes or dysfunction of
tumor suppressor genes (21).
Available research literature suggests that higher or lower
expression of miR-216a-5p occurs in a variety of cancers. Some
studies have shown that the expression levels of miR-216a-5p is
downregulated in colorectal cancer (16) and pancreatic cancer (17), while others have reported upregulated
levels of miR-216a-5p in prostate cancer (18) and liver cancer (19). Intracellular environments of
different cells were diversiform, so the signaling pathways of
miR-216-5p were described in certain types of tumor. In the present
study, miR-216a-5p was implicated in RCC, which was observed to be
highly expressed when compared to the expression levels in the
normal tissues.
In colorectal cancer, miR-216a-5p is known to
decrease migration, invasion and inhibit metastasis by targeting
KIAA1199. MiR-216a-5p in colorectal cancer has been shown to be
upregulated and is responsible for maintaining the aggressive
phenotype of tumor cells (16). Hou
et al (22) have demonstrated
that miR-216a-5p significantly inhibits cell growth and promotes
cell apoptosis by targeting the Janus kinase 2 (JAK2) and
transcription 3 (STAT3) in pancreatic cancer (22). In another previous study about
pancreatic cancer, miR-216a-5p has been shown to decrease cell
viability and induce cell apoptosis by silencing MALAT1 expression
(23). In addition, miR-216a-5p is
also reported to be upregulated in prostate cancer and could
inhibit bicalutamide-mediated growth suppression (18). In hepatocellular carcinoma (HCC),
overexpression of miR-216a/217 promotes cell population, migration,
and metastatic ability by targeting PTEN and SMAD7 (19). Chen et al (24) also demonstrated that the three target
sites at its 3′ untranslated region of TSLC1 were targeted by
miR-216a-5p, and miR-216a-5p was observed to be significantly
upregulated in cancerous liver tissues (24).
The functional role of miR-216a-5p in RCC has not
been reported in previous studies and this is the first study to
show that the functional significance of the upregulated
miR-216a-5p, which promotes proliferation, viability, mobility and
suppresses apoptosis in 786-O and ACHN cells. Conversely,
downregulated miR-216a-5p suppressed proliferation, viability,
motility and induced apoptosis in 786-O and ACHN cells. Based on
these results, we postulate that tumorigenesis was promoted by
miR-216a-5p, which acts as an oncogene in RCC.
In addition to being associated with RCC,
miR-216a-5p was found to be a potential biomarker for the early
identification of severe acute pancreatitis (25). Li et al (26) have also reported that that
miR-216a-5p is involved in diabetes and targeted genes including
ANGPTL4, ANXA9, CFH, CHI3L2 and INPP4B (26). Mansego et al (27) found that miR-216a-5p might be
involved in miRNA mediated epigenetic regulation in childhood
obesity and the coding regions of miR-216a-5p were methylated
(27). Furthermore, it was suggested
that miR-216a-5p had an impact on chronic kidney diseases (28), nephrotic syndrome (29) and end-stage failing hearts (30).
In summary, in the present study, we report for the
first time that miR-216a-5p is upregulated in RCC tissues. Also,
our data shows that miR-216a-5p is involved in cellular
proliferation, viability, cell motility and apoptosis. Furthermore,
regulating the expression of miR-216a-5p and further research on
the targets of miR-216a-5p may pave the way for development of
novel therapeutics against renal cancer in the future. It is
important to test the expression of miR-216a-5p in more patients to
get more accurate information about the relationship between
clinicopathological features and the expression of miR-216a-5p. So,
we will explore the clinicopathological features in further
study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101922), Science
and Technology Development Fund Project of Shenzhen (grant nos.
JCYJ20160429090753103 and JCYJ20170307111334308), the fund of
‘San-ming’ Project of Medicine in Shenzhen (grant no.
SZSM201612066), the Fund of Shenzhen Key Laboratory (grant no.
ZDSYS201504301045406) and the fund of Guangdong Key Medical
Subject.
References
|
1
|
White NM and Yousef GM: MicroRNAs:
Exploring a new dimension in the pathogenesis of kidney cancer. BMC
Med. 8:652010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schlesinger-Raab A, Treiber U, Zaak D,
Hölzel D and Engel J: Metastatic renal cell carcinoma: Results of a
population-based study with 25 years follow-up. Eur J Cancer.
44:2485–2495. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moch H: An overview of renal cell cancer:
Pathology and genetics. Semin Cancer Biol. 23:3–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hadoux J, Vignot S and De La Motte Rouge
T: Renal cell carcinoma: Focus on safety and efficacy of
temsirolimus. Clin Med Insights Oncol. 4:143–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naito S, Tomita Y, Rha SY, Uemura H, Oya
M, Song HZ, Zhong LH and Wahid MI: Kidney Cancer Working Group
report. Jpn J Clin Oncol. 40 Suppl 1:i51–i56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharma P and Allison JP: Immune checkpoint
targeting in cancer therapy: Toward combination strategies with
curative potential. Cell. 161:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chow TF, Youssef YM, Lianidou E, Romaschin
AD, Honey RJ, Stewart R, Pace KT and Yousef GM: Differential
expression profiling of microRNAs and their potential involvement
in renal cell carcinoma pathogenesis. Clin Biochem. 43:150–158.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Janzen NK, Kim HL, Figlin RA and
Belldegrun AS: Surveillance after radical or partial nephrectomy
for localized renal cell carcinoma and management of recurrent
disease. Urol Clin North Am. 30:843–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: Past, present, and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Negrini M, Ferracin M, Sabbioni S and
Croce CM: MicroRNAs in human cancer: From research to therapy. J
Cell Sci. 120:1833–1840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang D, Zhao L, Shen Q, Lv Q, Jin M, Ma
H, Nie X, Zheng X, Huang S, Zhou P, et al: Down-regulation of
KIAA1199/CEMIP by miR-216a suppresses tumor invasion and metastasis
in colorectal cancer. Int J Cancer. 140:2298–2309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Link A, Becker V, Goel A, Wex T and
Malfertheiner P: Feasibility of fecal microRNAs as novel biomarkers
for pancreatic cancer. PLoS One. 7:e429332012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyazaki T, Ikeda K, Sato W, Horie-Inoue
K, Okamoto K and Inoue S: MicroRNA library-based functional
screening identified androgen-sensitive miR-216a as a player in
bicalutamide resistance in prostate cancer. J Clin Med.
21:1853–1865. 2015. View Article : Google Scholar
|
|
19
|
Xia H, Ooi LL and Hui KM:
MicroRNA-216a/217-induced epithelial-mesenchymal transition targets
PTEN and SMAD7 to promote drug resistance and recurrence of liver
cancer. Hepatology. 58:629–641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palanichamy JK and Rao DS: miRNA
dysregulation in cancer: Towards a mechanistic understanding. Front
Genet. 5:542014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou BH, Jian ZX, Cui P, Li SJ, Tian RQ and
Ou JR: miR-216a may inhibit pancreatic tumor growth by targeting
JAK2. FEBS Lett. 589:2224–2232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Tang X, Shi M, Wen C and Shen B:
MiR-216a decreases MALAT1 expression, induces G2/M arrest and
apoptosis in pancreatic cancer cells. Biochem Biophys Res Commun.
483:816–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen PJ, Yeh SH, Liu WH, Lin CC, Huang HC,
Chen CL, Chen DS and Chen PJ: Androgen pathway stimulates
microRNA-216a transcription to suppress the tumor suppressor in
lung cancer-1 gene in early hepatocarcinogenesis. Hepatology.
56:632–643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang XX, Deng LH, Chen WW, Shi N, Jin T,
Lin ZQ, Ma Y, Jiang K, Yang XN and Xia Q: Circulating microRNA 216
as a marker for the early identification of severe acute
pancreatitis. Am J Med Sci. 353:178–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, Ma W, Xie C, Zhang M, Yin X, Wang F,
Xu J and Shi B: Identification of genes and signaling pathways
associated with diabetic neuropathy using a weighted correlation
network analysis: A consort study. Medicine (Baltimore).
95:e54432016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mansego ML, Garcia-Lacarte M, Milagro FI,
Marti A and Martinez JA: DNA methylation of miRNA coding sequences
putatively associated with childhood obesity. Pediatr Obes.
12:19–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szeto CC, Ching-Ha KB, Ka-Bik L, Mac-Moune
LF, Cheung-Lung CP, Gang W, Kai-Ming C and Kam-Tao LP: Micro-RNA
expression in the urinary sediment of patients with chronic kidney
diseases. Dis Markers. 33:137–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szeto CC: Urine miRNA in nephrotic
syndrome. Clin Chim Acta. 436:308–313. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barsanti C, Trivella MG, D'Aurizio R, El
Baroudi M, Baumgart M, Groth M, Caruso R, Verde A, Botta L, Cozzi L
and Pitto L: Differential regulation of microRNAs in end-stage
failing hearts is associated with left ventricular assist device
unloading. Biomed Res Int. 2015:5925122015. View Article : Google Scholar : PubMed/NCBI
|