Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a
liver disorder, which occurs predominantly during the third
trimester of pregnancy (1). ICP is
characterized by pruritus, jaundice, high serum bile acid levels
and abnormal liver function, which adversely affects fetal health
(1). Although the etiology of ICP is
not fully understood, previous results have suggested that female
hormones may have a causative role (2).
Previous findings have indicated an etiologic role
for estrogens in the initiation of ICP (3). ICP typically occurs in the last
trimester, when estrogen levels are highest (4). In addition, ICP is more common in
multiple pregnancies compared with single pregnancies (5). The estrogen oral contraceptive used
among women with a personal or family history of ICP may result in
clinical features of ICP, including pruritus, elevated serum
aminotransferases and bile acid levels, particularly when former
high-dose preparations were used (6).
The synthetic estrogen 17α-ethinylestradiol (EE) has
been demonstrated to reduce bile flow formation in experimental
animals (7). In vitro studies
have reported that EE impairs the expression and function of
hepatocyte transporters, including multidrug resistance-associated
protein 2 (MRP2) and the bile salt export pump (BSEP), which have
important roles in bile salt-dependent component of bile flow
(BSDF) and bile salt-independent flow (BSIF) (8–10).
Furthermore, ICP induced by EE in rodents is an established animal
model to assess the mechanisms of estrogen-induced cholestasis
(11).
Herb decoction of Chinese medicine is an effective
method and has been used in the clinic to treat ICP (12,13). Yin
Huang Mixture (YHHJ; patent no. 200910031240.7) is a clinical
experiential decoction that was formulated in the Maternal and
Child Health Medical Institute (Obstetrics and Gynecology Hospital,
Nanjing, China) and was predominantly composed of Artemisia
capillaries Thunb, Hypericum japonicum Thunb,
Eucommia ulmoides Oliver, Rheum officinale Baill,
Gardenia jasminoides Ellis, Poria cocos Wolf and
Dictamnus dasycarpus Turcz. YHHJ has been used to treat ICP
in clinic for decades. YHHJ has previously been revealed to
ameliorate itching and reduce serum bile acid levels, is well
tolerated by pregnant women and has no adverse effects in mothers
or newborns (14,15). However, the molecular mechanism of
YHHJ on ICP remains to be elucidated.
The present study focused on the action of YHHJ in
regulating membrane transporters, particularly MRP2, which
transports lipophilic substances and contributes to the formation
of BSDF (16) and the BSEP, which
mediates the concentrative transport of monovalent bile salts into
the canaliculus and generates the BSIF (17), using rats and primary isolated rat
hepatocytes with EE-induced cholestasis.
Materials and methods
Preparation of YHHJ
The aforementioned herbs (Department of Pharmacy,
Obstetrics and Gynecology Hospital Affiliated to Nanjing Medical
University, Nanjing, China) were soaked in water for 0.5 h at room
temperature, followed by two cycles of reflux extraction. The first
round of reflux extraction used 10 l of water and was performed for
2 h at 80–100°C, whereas the second round used 8 l of water and was
performed for 1 h at 80–100°C. The decoction was subsequently
filtered through double gauze and the filtrate was concentrated
under reduced pressure (0.1–0.5 kgf/cm2) using a rotary
evaporator to a final concentration that was equivalent to 1.8 g
crude drug/ml of solution. The residue was stored at 4°C until
use.
Animals and treatment
In the present study, 50 male Sprague-Dawley rats
(age, 6–8 weeks) were purchased from Shanghai Laboratory Animal
Centre (SLAC) Laboratory Animal Co., Ltd. (Shanghai, China) with an
initial body weight of 180–200 g. Rats had ad libitum access
to a standard laboratory diet and water and were housed at room
temperature with a humidity of 50% and a 12 h light/dark cycle.
Rats were divided into the model group (n=30) and a control group
(n=10). In the model group, rats received 5 mg/kg/day EE
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) via intraperitoneal
injection for 10 consecutive days to induce cholestasis, which was
confirmed by measuring serum alanine transaminase (ALT), aspartate
aminotransferase (AST), serum total bile acid (TBA) and total
bilirubin (TBil). In the control group (N), rats received similar
volumes of propylene glycol (2.5 ml/kg/day; Panreac Quimica SLU,
Barcelona, Spain; solvent vehicle). On day 11 following EE
initiation, the model group was further divided into three groups
(n=10 each): Model (M), low-dose YHHJ (YL) and high-dose YHHJ (YH)
groups. The YL and YH groups were gavaged with YHHJ (9 and 36
g/kg/day, respectively) continuously for a total of 14 days. In
parallel experiments, the N and M groups were gavaged with
distilled water.
Sample collection
On the 10th day following EE administration, rats
from each group were anesthetized with pentobarbital sodium, (50
mg/kg via intraperitoneal injection; Panreac Quimica SLU) and
plasma samples were extracted from the retro-orbital plexus. Rats
were sacrificed and the liver was weighed prior to being aliquoted
for mRNA and protein extraction.
Biochemical analyses
TBA levels were determined from plasma samples using
a radioimmunoassay kit (cat. no. 50004463; Diasys Diagnostic
Systems GmbH, Holzheim, Germany). Serum concentrations of TBil
(cat. no. CH0101003; Maccura Biotechnology Co., Ltd., Chengdu,
China), ALT (cat. no. 1.02.1203; Shanghai Fuxing Changzheng Medical
Science Co., Ltd., Shanghai, China) and AST (cat. no. 1.02.1003;
Shanghai Fuxing Changzheng Medical Science Co., Ltd.) were
determined using kits and measured using an automated biochemical
analyzer (Beckman Coulter, Inc., Brea, CA, USA). All kits were
performed according to the manufacturer's instructions.
Hepatocyte isolation
Hepatocytes were harvested from the normal livers of
an additional 10 male Sprague-Dawley rats according to the Seglen
method with certain modifications, including the insertion of the
liver perfusion catheter through the right atrium and suprahepatic
vena cava (18). Cell viability was
determined using the trypan blue exclusion test (18). Freshly isolated hepatocytes were
seeded onto collagen-coated 6-well plates at a density of
2×105 cells/ml in Williams medium E (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
penicillin (100 IU/ml), streptomycin (100 µg/ml), insulin (4
µg/ml), dexamethasone (10 nmol/l) and 10% (v/v) fetal bovine serum
(Invitrogen). Cells were incubated at 37°C in a humidified
atmosphere containing 5% CO2.
Cytotoxic assay
The cytotoxic effect of YHHJ on hepatocyte cells was
evaluated using the XTT assay. Cells were seeded onto 96-well
culture plates at 1×104 cells/well. Following a 4 h
incubation at 37°C, various concentrations of YHHJ (0, 100, 200,
300, 400, 500, 600, 700, 800, 900 and 1,000 µg/ml) were added into
each well. The plate was subsequently incubated at 37°C in an
atmosphere containing 5% CO2 for 72 h. The medium (YHHJ
water extract) was discarded and 50 µl (1 mg/ml) XTT reagent
(Sigma-Aldrich; Merck KGaA) was added and the plates were
re-incubated at 37°C for an additional 3 h to allow the development
of formazan. The optical densities were subsequently measured with
an ELISA reader (PerkinElmer, Inc., Waltham, MA, USA) at a
wavelength of 450 nm. The 50% cytotoxic concentration
(CC50) was defined as the concentration of YHHJ extract
that reduced the cell viability by 50% when compared with untreated
controls.
Hepatocyte culture and treatment
Following a 12 h incubation at 37°C, the Williams
medium E was refreshed. When hepatocytes reached a density of
2×105 cells/well, they were incubated in the presence or
absence of 1 µmol/l EE for 12 h at 37°C. Subsequently, the medium
was aspirated and the hepatocytes were treated with (YH group) or
without (KB group) YHHJ (300 µg/ml as determined by XTT assay) in
Williams medium E containing 0.2% bovine serum albumin (Invitrogen)
for 24 h at 37°C.
Extraction of membrane fraction
Ground liver tissues were centrifuged at a speed of
1,100 × g for 15 min at 4°C. Hepatocyte pellets were then collected
and homogenized in radioimmunoprecipitation assay extraction buffer
containing protease inhibitors (25 µg/ml leupeptin and 1 mmol/l
phenylmethylsulfonyl fluoride; Beyotime Institute of Biotechnology,
Haimen, China) and centrifuged at 1,100 × g for 15 min at 4°C. The
supernatant containing the membrane fraction was collected and
stored at −70°C prior to analyses. Protein concentrations of the
membrane fractions were determined using the BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's insructions (19).
Western blot analysis
An aliquot of the membrane fraction (30 µg protein)
was mixed with an equal volume of sample buffer. Following
heat-treating at 95°C for 10 min, 20 µg protein samples were loaded
per lane and separated using 10% SDS-PAGE and transferred to
polyvinylidene fluoride (PVDF) membranes. Following incubation,
membranes were blocked with 5% non-fat dried milk at room
temperature for 2 h. The PVDF membranes were then washed three
times using Tris-buffered saline with 0.1% Tween-20 for 10 min and
incubated overnight at 4°C with anti-MRP2 monoclonal antibody
(1:500; cat. no. F0410; Abcam, Cambridge, UK) and anti-BSEP
polyclonal antibody (1:1,000; cat. no. 927043; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Bound antibodies were
incubated at room temperature for 2 h with horseradish
peroxidase-conjugated anti-rabbit antibodies (1:5,000; cat. no.
GR44808-4; Cell Signaling Technology, Inc., Danvers, MA, USA) and
detected using an electrochemiluminescence kit (Pierce; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions and exposed to X-ray film for 1 min. β-actin was the
loading control (1:1,000; cat. no. H2906; Cell Signaling
Technology, Inc.). The intensity of selected bands were captured
and analysed using ImageJ software (version 1.46; National
Institutes of Health, Bethesda, MD, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from rat livers and cultured hepatocytes
was extracted using TRIzol reagent (Invitrogen). In each
experiment, 1 µg total RNA was reverse-transcribed using
PrimeScript RT Reagent kit with gDNA Eraser (Takara Bio, Inc.,
Otsu, Japan) according to the manufacturer's instructions. PCR
reactions, which contained SYBR PCR supermix (Invitrogen), cDNA and
primers (Table I), were run on a
LightCycler 1.5. The thermal cycling conditions consisted of 95°C
for 15 sec, followed by 45 cycles at 95°C for 15 sec, 58°C for 10
sec and 72°C for 15 sec. Quantification of gene expression relative
to GAPDH (the housekeeping gene) was performed using
the2−ΔΔCq method (20).
All experiments were performed in triplicate.
 | Table I.Primers for quantitative reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for quantitative reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward sequence | Reverse sequence |
|---|
| Rat MRP2 |
5′-CCAATGTTTTGAATGCGGAG-3′ |
5′-GTACCACTGGAGTAGCTAGGA-3′ |
| Rat BSEP |
5′-CACTGGCCTTCTGGTATGGT-3′ |
5′-CAGTCCTCTGCCGATGTTCG-3′ |
| Rat GAPDH |
5′-AATGTATCCGTTGTGGATCTGA-3′ |
5′-TCTTCCACCACTTCGTCCG-3′ |
Statistical analyses
Data were expressed as the mean ± standard deviation
Statistical analysis was performed using SPSS 11.0 software (SPSS,
Inc., Chicago, IL, USA) one-way analysis of variance followed by
Student-Newman-Keuls multiple range test. P<0.01 was considered
to indicate a statistically significant difference.
Results
Effect of YHHJ on serum cholestatic
indices in EE-induced cholestatic rats
The administration of EE for 10 days resulted in a
significant increase in serum TBA and TBil levels compared with the
N group (P<0.01; Table II). The
protective effect of YHHJ on cholestasis was observed in YL and YH
groups and the results revealed a significant reduction in serum
TBA and TBil levels compared with the M group (P<0.01; Table II). Furthermore, compared with the N
group, the M group exhibited increased serum ALT and AST levels;
however, the difference was not statistically significant and YHHJ
did not significantly decrease serum ALT and AST levels in
EE-induced cholestatic rats (Table
II).
 | Table II.Effect of YHHJ on serum cholestatic
indices in EE induced cholestatic rats. |
Table II.
Effect of YHHJ on serum cholestatic
indices in EE induced cholestatic rats.
| Group | TBA (µmol/l) | Tbil (µmol/l) | ALT (U/l) | AST (U/l) |
|---|
| N | 1.85±0.58 | 1.72±0.33 | 132.61±6.42 | 150.73±6.08 |
| M |
3.55±0.89a |
3.65±0.42a | 133.63±3.41 | 155.70±4.81 |
| YL |
2.12±0.72b |
1.92±0.23b | 126.12±5.49 | 156.41±5.18 |
| YH |
2.18±0.76b | 1.95±0.3b | 126.81±3.57 | 156.00±5.69 |
Effect of YHHJ on the protein
expression levels of MRP2 and BSEP in EE-induced cholestatic
rats
Consistent with previous findings (8–10), the
present western blot analysis also revealed that MRP2 and BSEP
protein expression levels were significantly reduced following EE
treatment compared with the N group (P<0.01; Fig. 1). However, these protein expression
levels were significantly increased following YHHJ administration
(P<0.01; Fig. 1). Results
indicated that here were no significant differences in MRP2 and
BSEP protein expression levels between YL and YH groups.
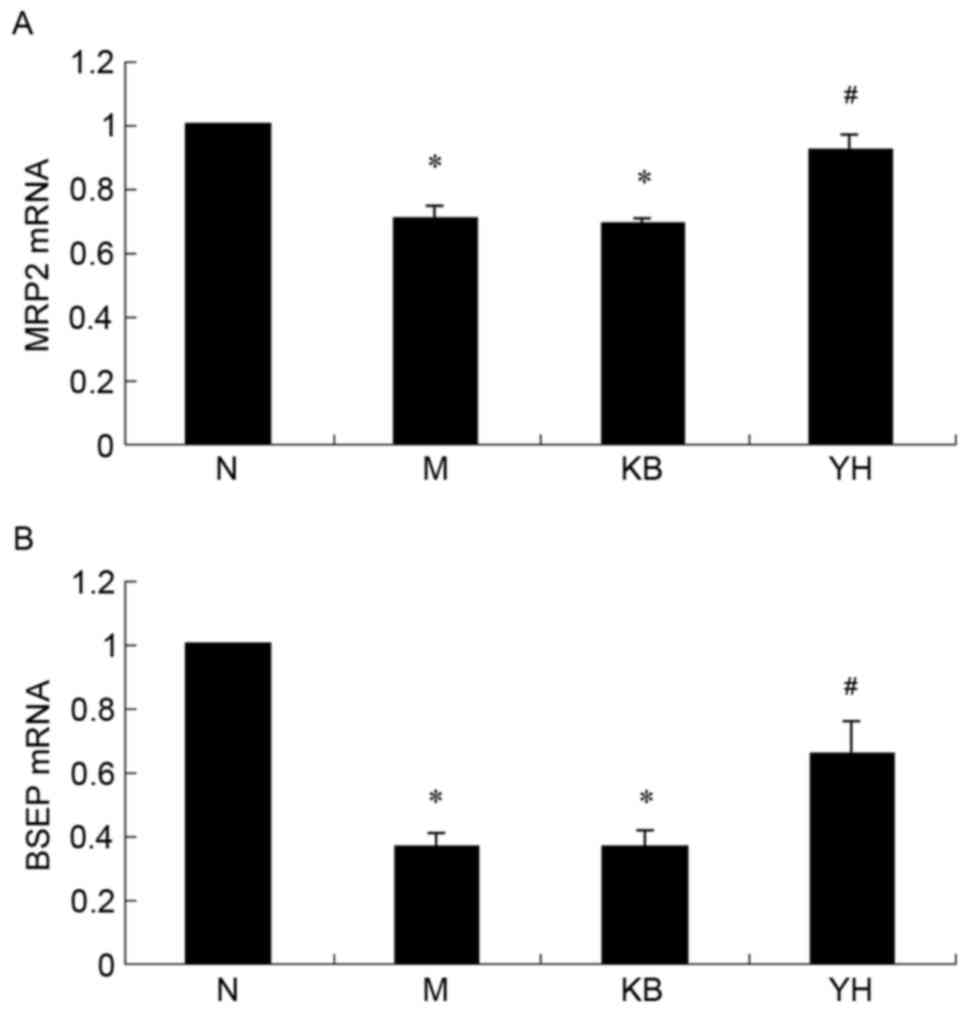
Effect of YHHJ on the mRNA expression
levels of MRP2 and BSEP in EE-induced cholestatic rats
To assess the effect of YHHJ on ICP on a molecular
level, mRNA expression levels of MRP2 and BSEP were determined
using RT-qPCR. As indicated in Fig.
2, mRNA expression levels of MRP2 (Fig. 2A) and BSEP (Fig. 2B) were significantly downregulated in
EE-induced cholestatic rats compared with the N group (P<0.01).
YL and YH groups indicated that YHHJ administration significantly
increased MRP2 and BSEP mRNA expression levels compared with the M
group (P<0.01; Fig. 2). However,
the difference in MRP2 and BSEP mRNA expression levels between the
YL and YH groups were not statistically significant.
Cytotoxic effects of YHHJ water
extract on cell viability
The cytotoxic effect of YHHJ water extract towards
rat hepatocytes was evaluated using the XTT assay. Results
indicated that YHHJ displayed cytotoxic effects only at high
concentrations (data not shown). The survival rate of the cells was
58.7% at a concentration of 700 µg/ml YHHJ water extract and
decreased to 41.0% when treated with 1,000 µg/ml. YHHJ demonstrated
CC50 at a concentration of 847.5 µg/ml (Fig. 3). YHHJ water extract, however, was
indicated to be less toxic toward rat hepatocytes at concentrations
of ≤300 µg/ml, with the survival rate of ~80%. Therefore, only
concentrations of 300 µg/ml were selected for the subsequent
experiments.
Effect of YHHJ water extract on the
protein expression levels of MRP2 and BSEP in EE-induced
cholestatic hepatocytes
To assess whether YHHJ water extract affected the
protein expression levels in EE-induced cholestatic rat
hepatocytes, western blot analysis was performed. Consistent with
the changes observed in mRNA expression levels in vivo, EE
significantly decreased the protein levels of MRP2 and BSEP
(Fig. 4) in the M group at 12 h
post-administration compared with the N group (P<0.01).
Furthermore, YHHJ water extract significantly increased MRP2 and
BSEP (Fig. 4) protein expression
levels in EE-induced cholestasis hepatocytes compared with the KB
group (P<0.01).
Effect of YHHJ water extract on the
mRNA expression levels of MRP2 and BSEP in EE-induced cholestatic
hepatocytes
To assess the effect of the YHHJ water extract on
mRNA expression levels of MRP2 and BSEP in EE-induced cholestatic
hepatocytes, an in vitro experiment in rat hepatocytes was
performed. In agreement with the in vivo studies, the mRNA
expression levels of MRP2 (Fig. 5A)
and BSEP (Fig. 5B) were
significantly downregulated by EE treatment compared with the N
group (P<0.01). Compared with the KB group, the YHHJ water
extract significantly reversed the EE-induced downregulation of
MRP2 and BSEP (P<0.01; Fig.
5).
Discussion
ICP typically occurs during the last trimester, when
estrogen and progesterone reach their maximum levels (1). Previous studies have reported that
reproductive hormones have a role in the etiology of ICP (21,22). EE
has been demonstrated to reduce bile flow formation in experimental
animals, thus representing a useful model to study estrogen
cholestasis (11).
All hormones are metabolized by the liver and an
excess of metabolites influences the activity of biliary
canalicular transporters (23).
Studies have indicated that decreased expression and function of
canalicular MRP2 and BSEP are major factors of deteriorated bile
flow formation in EE-induced cholestasis (24,25). As
reported in the present study, the canalicular transport proteins
MRP2 and BSEP are downregulated in EE-induced hepatic rats and
primary isolated rat hepatocytes.
YHHJ is derived from Yin Chen Hao Tang, which is one
of the most frequently used prescriptions in traditional Chinese
medicine practice and has been recognized as a hepatoprotective
agent for various types of liver diseases, including cholestasis
(26,27).
The present results revealed that YHHJ water extract
significantly decreased the levels of serum TBA and TBil in
EE-induced cholestasis in rats. However, no significant increase in
serum ALT and AST levels were detected in the M group. Similarly,
no significant changes were detected in ALT and AST levels when
YHHJ was administered following EE injection. These differences in
liver function tests between the animal model used in the present
study and patients with ICP may be due to the estrogen metabolites
themselves and the time of the disease formation (28).
In order to study the potential molecular mechanism
of YHHJ in the treatment of ICP, the effect of YHHJ on the
expression of hepatobiliary transporters MRP2 and BSEP in
EE-induced cholestasis in rats and in primary cultured rat
hepatocytes was assessed. The present results demonstrated that
YHHJ administration significantly increased the protein expression
levels of MRP2 and BSEP in EE-induced cholestasis.
To investigate the gene expression of MRP2 and BSEP
and validate the upregulation of MRP2 and BSEP proteins, MRP2 and
BSEP mRNA expression levels were determined. The data indicated
that YHHJ water extract significantly increased MRP2 and BSEP mRNA
expression levels at 24 h after YHHJ water extract treatment.
The present findings determined that, using
EE-induced cholestasis in rats and in primary cultured rat
hepatocytes, water extract of YHHJ may be a potential therapeutic
agent for the treatment of ICP, as indicated in vitro and
in vivo. One of the molecular mechanisms associated with the
anti-cholestasis effects of YHHJ may involve upregulation of bile
acids transporters, MRP2 and BSEP. However, the possible signaling
pathways have not been determined.
Based on a recent study, the phosphoinositide
3-kinase/protein kinase B signaling pathway is associated with
estrogen-induced cholestasis in isolated rat hepatocyte couplets
and may be responsible for the abnormal function and localization
of MRP2 and BSEP (29). In future
studies, the effect of YHHJ on the phosphoinositide
3-kinase/protein kinase B signaling pathway will be
investigated.
In conclusion, the present study may enhance the
understanding of the fundamental aspects of the role of YHHJ in
hepatoprotection on EE-induced cholestasis and provides potential
insights that may impact medical practice.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81102611).
References
|
1
|
Geenes V and Williamson C: Intrahepatic
cholestasis of pregnancy. World J Gastroenterol. 15:2049–2066.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lammert F, Marschall HU, Glantz A and
Matern S: Intrahepatic cholestasis of pregnancy: Molecular
pathogenesis, diagnosis and management. J Hepatol. 33:1012–1021.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Williamson C and Geenes V: Intrahepatic
cholestasis of pregnancy. Obstet Gynecol. 124:120–133. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Germain AM, Carvajal JA, Glasinovic JC,
Kato CS and Williamson C: Intrahepatic cholestasis of pregnancy: An
intriguing pregnancy-specific disorder. J Soc Gynecol Investig.
9:10–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Palmer DG and Eads J: Intrahepatic
cholestasis of pregnancy: A critical review. J Perinat Neonatal
Nurs. 14:39–51. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williamson C, Hems LM, Goulis DG, Walker
I, Chambers J, Donaldson O, Swiet M and Johnston DG: Clinical
outcome in a series of cases of obstetric cholestasis identified
via a patient support group. BJOG. 111:676–681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marrone J, Soria LR, Danielli M, Lehmann
GL, Larocca MC and Marinelli RA: Hepatic gene transfer of human
aquaporin-1 improves bile salt secretory failure in rats with
estrogen-induced cholestasis. Hepatology. 64:535–548. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JM, Trauner M, Soroka CJ, Stieger B,
Meier PJ and Boyer JL: Expression of the bile salt export pump is
maintained after chronic cholestasis in the rat. Gastroenterology.
118:163–172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kullark-Ublick GA and Meier PJ: Mechanism
of cholestasis. Clin Liver Dis. 4:357–385. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fickert P, Zollner G, Fuchsbichler A,
Stumptner C, Pojer C, Zenz R, Lammert F, Stieger B, Meier PJ,
Zatloukal K, et al: Effects of ursodeoxycholic and cholic acid
feeding on hepatocellular transporter expression in mouse liver.
Gastroenterology. 121:170–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee J and Boyer JL: Molecular alterations
in hepatocyte transport mechanisms in acquired cholestatic liver
disorders. Semin Liver Dis. 20:373–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheng G and Jun-Ling C: Research progress
of traditional Chinese medicine in the treatment of intrahepatic
cholestasis of pregnancy. Gansu Zhongyi. 16:6–8. 2003.(In
Chinese).
|
|
13
|
Xue-Jun SG: To study thone effect of
intrahepatic cholestasis of pregnancy by chinese medicine. Zhonghua
Zhongyi Yaoxuekan. 32:1404–1406. 2014.(In Chinese).
|
|
14
|
Cui-Ying Z, Jing-Qin C, Hua C, et al:
Clinical research of the effect of YHHJ on the treatment of
intrahepatic cholestasis of pregnancy. Jiangsu Zhongyi. 1:8–9.
2000.(In Chinese).
|
|
15
|
Yue Z: Efficacy comparative study of Yin
huang mixture (YHHJ) and ursodeoxycholic acid on gestational
intrahepatic cholestasis. Heilongjiang Med J. 25:840–842. 2012.
|
|
16
|
Fouassier L, Kinnman N, Lefèvre G, Lasnier
E, Rey C, Poupon R, Elferink RP and Housset C: Contribution of mrp2
in alterations of canalicular bile formation by the endothelin
antagonist bosentan. J Hepatol. 37:184–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stieger B: The role of the
sodium-taurocholatecotransporting polypeptide (NTCP) and of the
bile salt export pump (BSEP) in physiology and pathophysiology of
bile formation. Handb Exp Pharmacol. 201:205–259. 2011. View Article : Google Scholar
|
|
18
|
Garcia F, Kierbel A, Larocca MC, Gradilone
SA, Splinter P, LaRusso NF and Marinelli RA: The water channel
aquaporin-8 is mainly intracellular in rat hepatocytes, and its
plasma membrane insertion is stimulated by cyclic AMP. J Biol Chem.
276:1247–1252. 2001. View Article : Google Scholar
|
|
19
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
20
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stapelbroek JM, van Erpecum KJ, Klomp LW
and Houwen RH: Liver disease associated with canalicular transport
defects: Current and future therapies. J Hepatol. 52:258–271. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shehta A and EI-Agamy DS: Eeffects of
curcumin, resveratrol and ursodeoxycholic acid on ethinylestrdiol
and chlorpromazine induced intrahepatic cholestasis in rats.
Pharmacologyonline. 3:91–100. 2009.
|
|
23
|
Barth A, Klinger G and Rost M: Influence
of ethinyloestradiol propanolsulphonate on serum bile acids in
healthy volunteers. Exp Toxicol Patho. l54:1–386. 2003.
|
|
24
|
Crocenzi FA, Pozzi Sa´nchez EJ, Pellegrino
JM, Favre CO, Rodríguez Garay EA, Mottino AD, Coleman R and Roma
MG: Beneficial effects of silymarin on estrogen-induced cholestasis
in the rat: A study in vivo and in isolated hepatocyte couplets.
Hepatology. 34:329–339. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stieger B, Fattinger K, Madon J,
Kullak-Ublick GA and Meier PJ: Drug- and estrogen-induced
cholestasis through inhibition of the hepatocellular bile salt
export pump (Bsep) of rat liver. Gastroenterology. 118:422–430.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng HY, Lin LT, Huang HH, Yang CM and
Lin CC: Yin Chen Hao Tang, a Chinese prescription, inhibits both
herpes simplex virus type-1 and type-2 infections in vitro.
Antiviral Res. 77:14–19. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee TY, Chang HH, Kuo JJ and Shen JJ:
Changes of hepatic proteome in bile duct ligated rats with hepatic
fibrosis following treatment with Yin-Chen-Hao-Tang. Int J Mol Med.
23:477–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poupon R: Intrahepatic cholestasis of
pregnancy: From bedside to bench to bebside. Liver Int. 25:467–468.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boaglio AC, Zucchetti AE, Sánchez Pozzi
EJ, Pellegrino JM, Ochoa JE, Mottino AD, Vore M, Crocenzi FA and
Roma MG: Phosphoinositide 3-kinase/protein kinase B signaling
pathway is involved in estradiol 17β-D-glucuronide-induced
cholestasis: Complementarity with classical protein kinase C.
Hepatology. 52:1465–1476. 2010. View Article : Google Scholar : PubMed/NCBI
|