Introduction
Synthetic vascular grafts are widely required in a
variety of clinical diseases, including peripheral arterial
disease, coronary artery disease, congenital heart disease and
arteriovenous access (1). Animal
models may be used to simulate the human anatomy, physiology and
pathological processes, and are widely applied in tissue
engineering experiments prior to clinical translation. To enable
long-term vessel substitution studies of animal models in
vivo, a suitable succession imaging monitor modality is
essential.
Polymer grafts have been used and studied widely
since the 1950s (2,3); however, they have demonstrated limited
success in the area of small-diameter vessels (<6 mm) owing to
the occurrence of acute and sub-acute thrombosis (4). Hitherto, modification of the
small-diameter grafts for long-term patency is the primary
objective of several tissue engineering groups (5–7);
however, in vivo observation is challenging due to the small
diameter. Thus, the present study focused on the study of an in
vivo succession monitor imaging modality of animal models
implanted with small-diameter grafts.
The evaluation of the patency of vessels and blood
flow characteristics following vascular graft implantation surgery
in animals is performed using angiography, the gold standard
(8). However, it is an invasive
monitoring method, which may lead to surgical complications,
including hyperanesthesia, bleeding, lameness and possibly early
humane euthanasia related to the complications (9–11).
Therefore, an alternative non-invasive technique that provides
rapid, safe, reproducible and reliable results is essential to
reduce animal sacrifices and to benefit long-term experimental
observations (12,13).
Several non-invasive imaging modalities exist,
including multidetector computed tomography angiography (MDCTA)
(14), magnetic resonance
angiography (MRA) (15) and vascular
Doppler ultrasonography (16).
Doppler ultrasound is a frequently applied method used to assess
the rates of stenosis of main arteries in humans (14). It includes three modalities: B-mode
(grayscale), color Doppler evaluation and velocity measurements.
B-mode allows an optimal two-dimensional (2D) anatomic assessment,
measurements of diameter, and the observation of lumen acoustic
transmission and surrounding soft tissues. Color Doppler allows the
visualization of flow abnormalities, including color filling defect
of the grafts and turbulence related to the presence of stenosis.
Finally, the spectral Doppler measures the blood flow velocity and
is the cornerstone of the functional evaluation of grafts (17). The blood flow velocity is the
quantitative parameter for grading the patency of synthetic
vascular grafts and may provide valuable information on critical
parameters, including i) the morphology and composition of vascular
grafts, ii) the patency of stent and graft materials and iii) the
localization and remodeling of the implant materials over a
particular time period (18). The
present research group speculated that ultrasound would be an
excellent screening method in animal models due to its
non-invasiveness, availability and successful applications in
humans (19).
Currently, the research of the present group is
focused on exploring small-diameter polymer vascular grafts in
vivo. In a previous study by the present research group, a
novel orthogonally functionalizable poly(ester urethane)urea with
disulfide and amino groups (PUSN) was developed for surface
covalent cofunctionalization, which may be electrospun into fibrous
grafts. The scaffolds were successfully modified by heparin (Hep;
PUSN-Hep) and Hep/EPC recruiting peptide (TPS) peptide
(PUSN-Hep/TPS) to increase the anticoagulant properties and effects
of blood compatibility and proliferations (20). Thus, the orthogonally functionalized
PUSN grafts demonstrated potential for application in
small-diameter vascular regeneration (20). However, the translation of such
vascular grafts into clinically useful products that are suitable
for implantation into patients has been slow (21–23). One
of the clinical transformation difficulties involves monitoring the
position and performance of vascular prostheses upon implantation
(24). Thus, an intravital technique
is required for continuous monitoring of the development of
vascular prostheses implanted in animal models without animal
dissection due to long-term patency. Among all animal models, the
carotid artery of the rabbit is a classic model for small-diameter
grafts (25).
Therefore, the present study utilized vascular
ultrasound to monitor the synthetic vascular graft implanted into
the carotid arteries of rabbits every week.
Materials and methods
Animals
A total of 15 male New Zealand white rabbits (8
weeks old, 2.5±0.6 kg) were purchased from Shanghai Chedun
Experimental Animal Raising Farm (Shanghai, China) for use in the
present in vivo study of three kinds of fibrous vascular
grafts [PUSN, PUSN-Hep and PUSN-Hep/TP, which were manufactured by
our group as described previously (20); n=5/group for each graft]. Rabbits
were maintained in a controlled environment (24°C; 45–65% humidity
and were exposed to a 12-h light/dark cycle) and had free access to
food and water. The patency rates of all grafts were analyzed using
Origin 8.0 (Origin Lab Corp., Northampton, MA, USA). All
experimental protocols were approved by the Animal Care and
Experiment Committee of Shanghai Jiao Tong University School of
Medicine (Shanghai, China).
Surgical procedures
Rabbits were anesthetized with 30 mg/kg
pentobarbital sodium (Merck KGaA, Darmstadt, Germany) by
intravenous injection at the edge of the ear, and 100 U/kg Hep
(Shanghai Pharma No. 1 Biochemical & Pharmaceutical Co., Ltd.,
Shanghai, China) was administered by intravenous injection at the
edge of the ear for anticoagulation prior to surgery. A length of
~1.0 cm of the right carotid artery of each rabbit was removed, and
the vascular graft with a 2 mm-diameter and 1.2-cm length was sewn
in an end-to-end fashion using 9-0 monofilament nylon sutures.
Following this, the skin was closed with 3-0 nylon sutures under
stringent aseptic conditions. Aspirin (AstraZeneca, Wuxi, China)
was administrated daily (2 mg/kg) by oral administration as an
anticoagulant for 1 week.
Ultrasound examinations
Ultrasound examinations of the carotid arteries of
the rabbits were performed at the Laboratory at Shanghai Children's
Medical Center, Shanghai Jiao Tong University School of Medicine by
an experienced sonographer. The patency of the implanted grafts was
monitored by a color Doppler ultrasound diagnostic system (GE LOGIQ
9; GE Healthcare, Chicago, IL, USA) 4 and 8 weeks after
implantation using an M12L multi high-frequency linear transducer
(GE Healthcare, Chicago, IL, USA).
For the examination, the rabbit's head was in the
supine position and turned away from the examination side. The
right common carotid artery with the implanted graft and its
forward and distal carotid vessel region constituted the region of
interest, and the left common carotid represented the control.
Firstly, in the grayscale mode, the sound beam was
adjusted perpendicular to the wall of the graft, focusing on the
acquisition of two parallel echogenic lines corresponding to the
lumen interfaces. In order to optimize the image and reduce
artefacts, the parameters were preset as follows: The highest
frequency was set as 12 MHz; the lowest possible depth was set as
2.0 cm so that the artery of interest was at ~two thirsds of the
display; the optimum dynamic range was set as 69 to increase the
contrast of the image; and the operator adjusted the precise gain
setting to 38 in order to visualize 2D images for the proximal and
distal walls.
Following this, the hemodynamic assessment was
conducted using the color flow mapping and pulse-Doppler technique.
The color Doppler used the highest frequency as 7.5 MHz, the lowest
possible pulse repetition frequency (PRF) of 1.4 kHz to avoid
aliasing, and the maximum color gain was set as 46 with minimum
possible background noise. The pulse-Doppler examination used the
highest frequency as 5.6 MHz and the maximum power gain as 41. It
was performed in the longitudinal view. Sample gate was placed
within the lumen without touching the wall with an ultrasound
incident angle <60°.
Dissection and pathological
histology
The patency of implanted grafts was monitored 4 and
8 weeks after implantation. Subsequently, the animals were
sacrificed. The vascular grafts with native tissue segments above
and below were explanted, rinsed with saline and fixed in a 4%
paraformaldehyde solution overnight at 4°C. The samples were
embedded in paraffin, and then sectioned into 5-µm sections. They
were stained with hematoxylin for 8–10 min and then eosin for 30–60
sec at room temperature. Subsequently histological analysis was
performed under a fluorescence microscope (Nikon Eclipse 80i; Nikon
Corporation, Tokyo, Japan) at a magnification of ×5.
Statistical analysis
PUSN was used as a control group and PUSN-Hep and
PUSN-Hep/TPS were used as the experimental groups. Kaplan-Meier
analysis was used to compare the patency rates of the groups and A
log rank test also performed to compare the patency rates of the
different groups by GraphPad Prism 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA). A total of five repeats were performed.
The data were analyzed by one-way analysis of variance. P<0.05
was considered to indicate a statistically significant
difference.
Results
Ultrasonography of the carotid artery
without transplantation
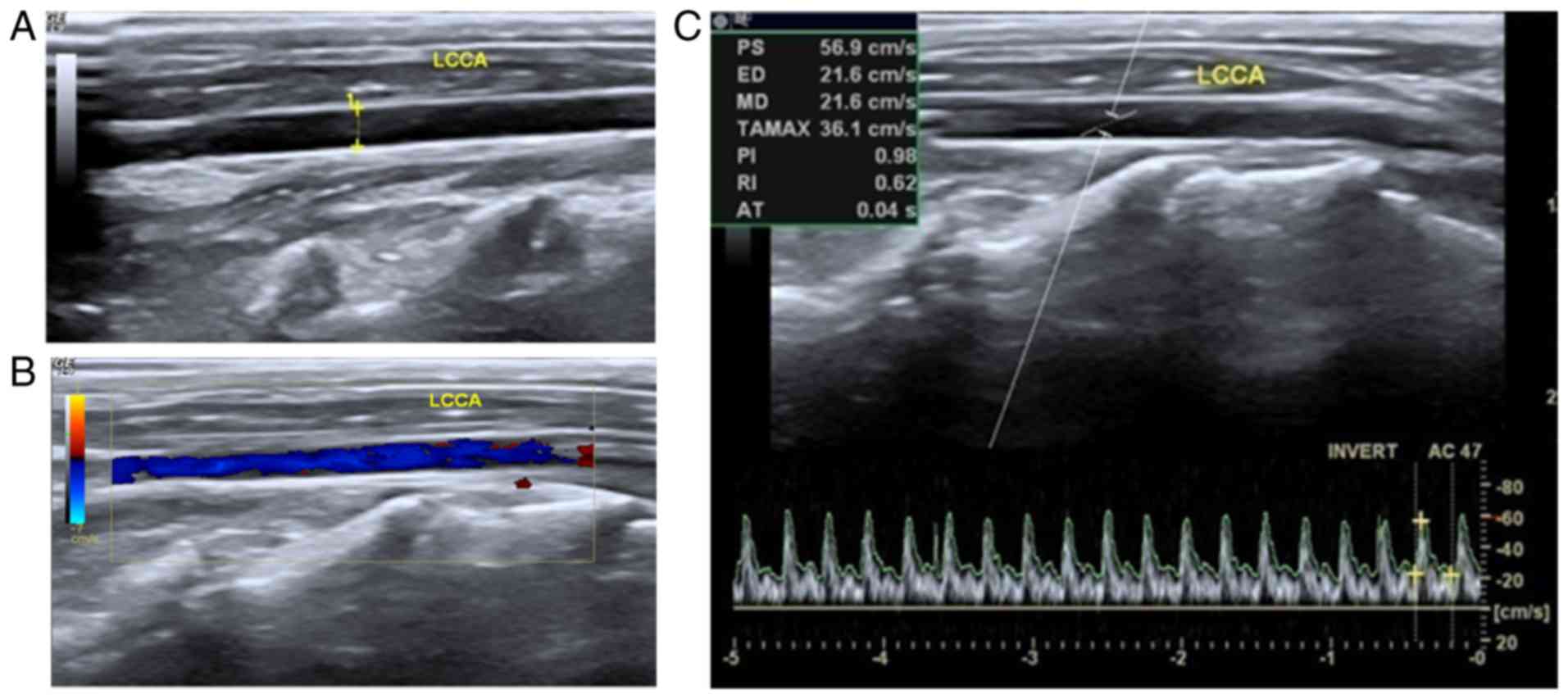
As demonstrated in Fig.
1, clear images of the carotid arteries in the rabbits were
generated by the use of transcutaneous ultrasound. The location and
echo character, wall structure, as well as the caliber were
bilaterally analyzed. As demonstrated in Fig. 1A, calipers and the dotted line
indicated that the caliber was 0.2 cm, and the carotid artery's
blood flow pattern could be distinguished. Thus, the left-side
artery without vascular prosthesis implantation was utilized as a
control. Within a normal patent artery, a grayscale sonogram
demonstrated the anechoic lumen and the hyperechoic linear wall of
the vasculature (Fig. 1A). Color
flow mapping indicated the pure color blood flow filling the
vascular lumen (Fig. 1B).
Furthermore, the regular arterial spectrum was detected based on
pulse-Doppler sonogram examination. The pulsing patterns were tall
and sharp systolic peaks with low and broad end diastolic velocity
(Fig. 1C).
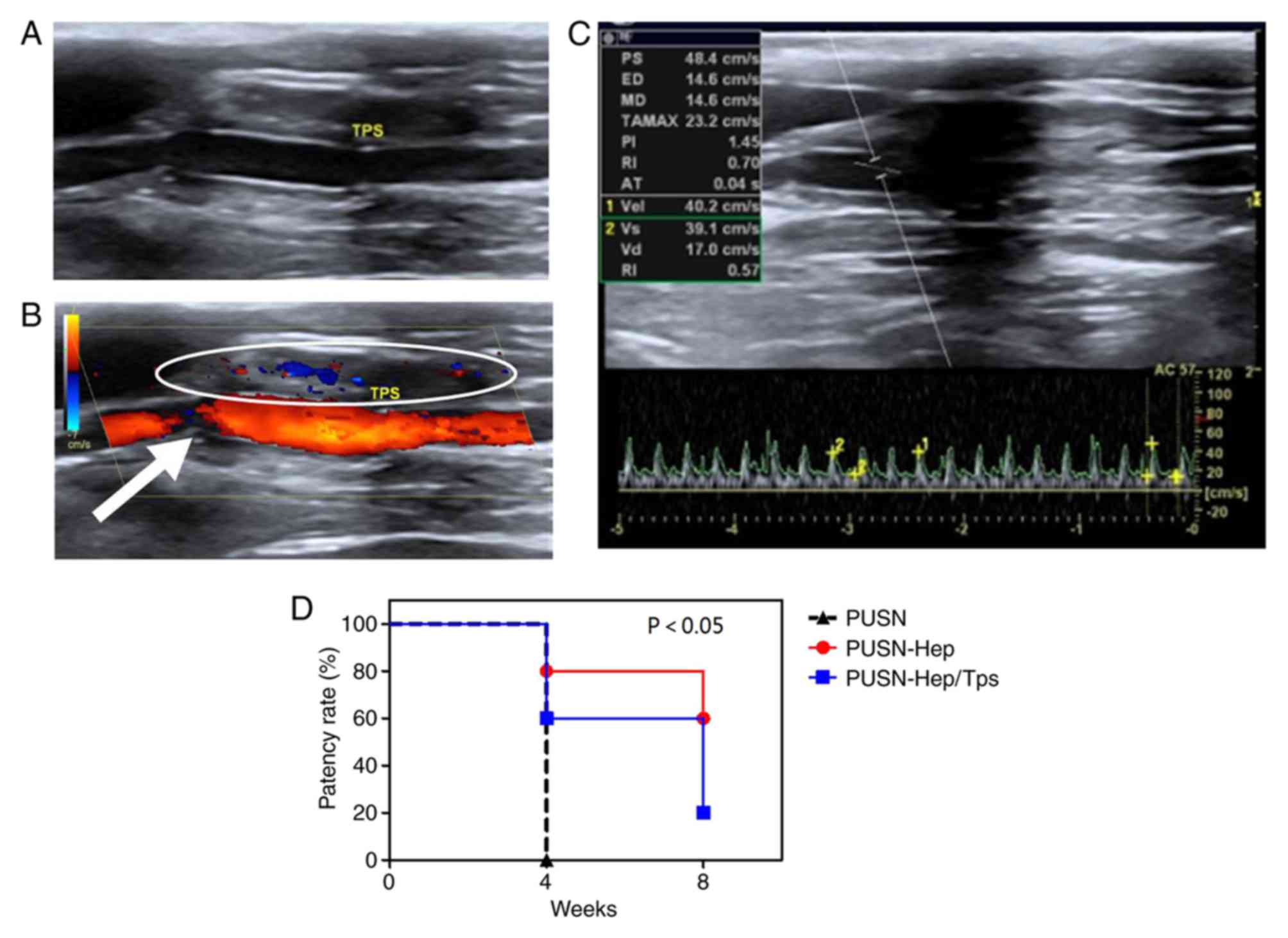
Distinguishing blood flow in grafts
following transplantation in situ
Patency
Grayscale ultrasound demonstrated the clear anechoic
within the graft of experimental group PUSN-Hep/TPS, similar to the
forward and the distal carotid vessels (Fig. 2A), which were filled with bright and
pure blood flow signals during the color Doppler examination
(Fig. 2B). In addition, sustained
and steady artery blood flow spectrums were observed in the
pulse-wave Doppler examination (Fig.
2C). The patency rate of all grafts was reflected clearly in
the present study (Fig. 2D). PUSN
grafts were all blocked (0/5) at 4 weeks, while PUSN-Hep grafts
demonstrated 80% patency (4/5) at week 4 and 60% (3/5) at week 8.
The PUSN-Hep/TPS grafts indicated a patency of 60% (3/5) at 4
weeks, which was decreased to 20% (1/5) at 8 weeks. The differences
between the control group (PUSN) and experimental groups (PUSN-Hep
and PUSN-Hep/TPS) were statistically significant.
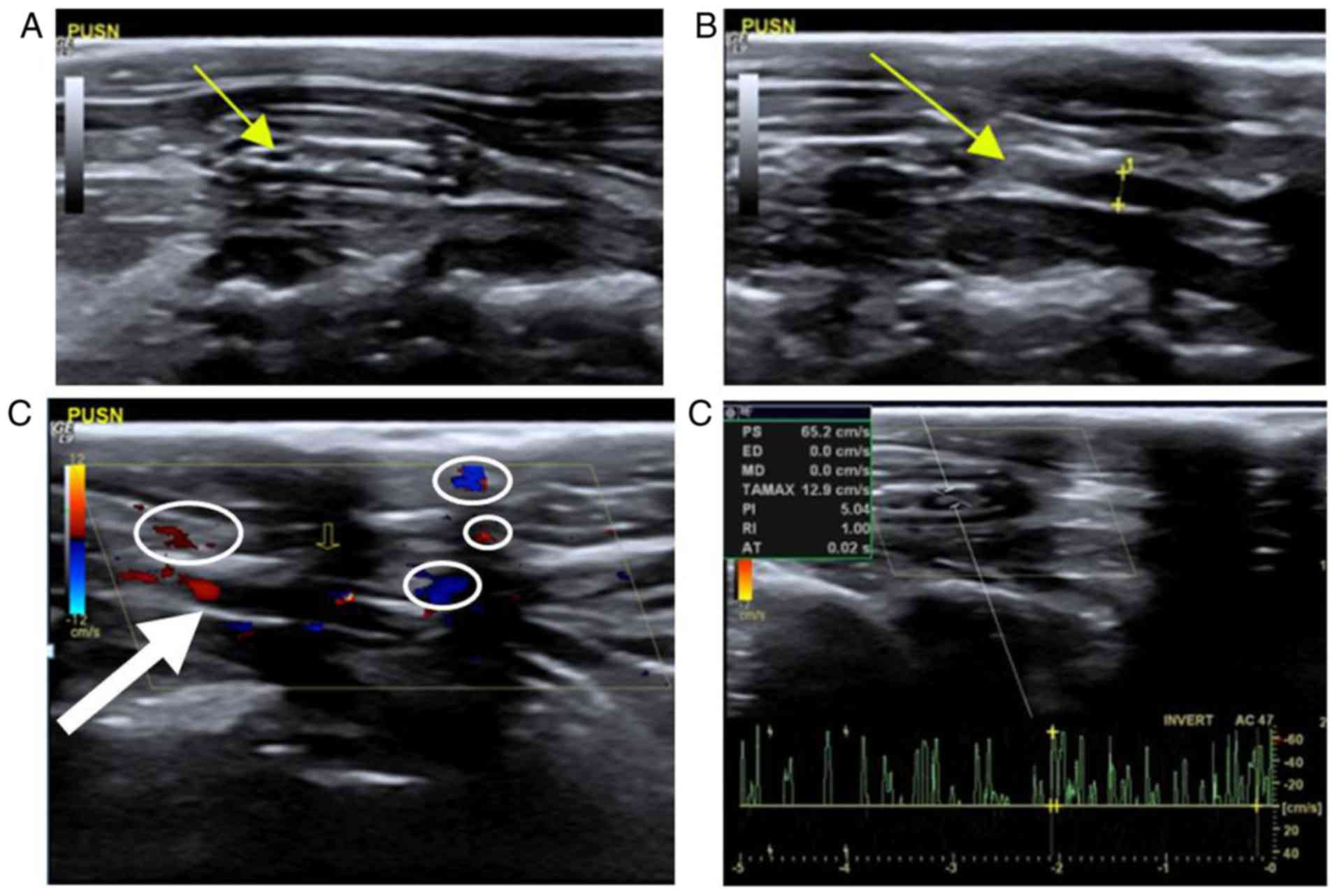
Stenosis
When the graft of control group PUSN was stenosed,
heterogeneity was observed in iso- and hyper-echo within the lumen
in grayscale sonography, albeit with varying anechoic areas
(Fig. 3A). The forward and the
distal carotid vessel cavity could be observed; however, their
cavities were narrower than the normal carotid artery to varying
degrees, as indicated in Fig. 3B.
Calipers and a dotted line measured the caliber as 0.15 cm, which
was narrower than that of the control side (Fig. 1A). In addition, some hyperecho may
have occurred in the anastomotic area (Fig. 3B). In the color Doppler map, some
blood signals were observed within the graft; however, the blood
signal could not be detected in the anechoic areas within the lumen
(Fig. 3C). The pulse-Doppler
sonogram examination revealed disorderly and unsystematic signals
and noise, although some spectrums could still be obtained
(Fig. 3D).
Occlusion
In grayscale sonography, heterogeneous hyperecho
occurred within the lumen when the graft of control group PUSN was
occluded. The forward and the distal carotid vessel cavity could
not be clearly observed (Fig. 4A).
In the color Doppler map, no blood flow signals were detected
within the lumen; however, a few dotted blood flow signals around
the occluded cavity could be speculated as small lateral branches
(Fig. 4B). Furthermore, few and
irregular wavelet spectra, or even total lack of signals, were
detected in the pulse-Doppler examination (Fig. 4C).
Continuous monitoring of the flow changes
in a graft
Patency
For the patent vessels, there were few changes of
echo charater within the lumen of grafts during the experimental
period. After 4 weeks, a weak echo followed by iso-echo appeared
gradually in the lumen of grafts, which indicated that their clear
lumens gradually transformed to blurred lumens. Furthermore, as
demonstrated in Fig. 5, the forward
and/or the distal carotid vessels of experimental group
PUSN-Hep/TPS became narrower over time (Fig. 5A and C). Fig. 5B represents the matching histological
staining image of Fig. 5A,
demonstrating the smooth inner membrane, while Fig. 5D represents the matching histological
staining image of Fig. 5C,
displaying intimal hyperplasia in the lumen, corresponding to the
stenosis trend detected by the ultrasound.
Severe stenosis
As demonstrated in Fig.
6, for the vessels of control group PUSN with severe stenosis,
the heterogeneity iso- and hyperecho of the graft (Fig. 6A) gradually transformed to
homogeneous hyperecho (Fig. 6C).
Notably, the narrow inner diameter of its forward vessel was
measured by calipers and dotted line 2 as 0.16 cm, and the
expansion inner diameter of the distal stenosis was measured by
calipers and dotted line 1 as 0.23 cm (Fig. 6E). Fig. 6B
and D represent the matching histological staining images of
Fig. 6A and C, respectively, as
substantiated by the stenosis condition of grafts that severely
induced intraluminal thrombus and nearly resulted in total
occlusion after 8 weeks of implantation.
Clear location of the grafts
High-frequency grayscale ultrasound has sufficient
spatial resolution to distinguish the location of the graft
conveniently on the surface. In B-mode sonography, it was possible
to distinguish all of the anatomical layers of implanted grafts,
including the lumen, graft wall, and the forward and distal carotid
vessels (Fig. 2A). It was also
possible to distinguish the location of anastomotic stenosis
(Fig. 6E).
Discussion
Tissue engineering vascular grafts are a promising
vascular substitute for the surgical treatment of diseased
small-diameter vessels, particularly in young patients, with a wide
prospect for clinical application (26). To assist in the clinical translation
of tissue-engineered vascular grafts, angiography has been employed
for assessing the patency of grafts (27). However, this invasive procedure is
associated with the potential risks of vascular complications,
including dissection, bleeding and pseudoaneurysm, and cannot
monitor the longitudinal localization and function of grafts
(28). The applicable non-invasive
imaging modality is a superior choice in the tissue engineering
experimental stage (29,30). Vascular ultrasound techniques have
been validated and are widely used in human subjects (31). Other imaging methods of carotid
arteries may include digital subtraction angiography (DSA), MDCTA
or MRA. DSA is considered the gold standard and is an indivisible
part of the carotid stenting procedure; however, it is invasive
(32). A study by Savic et al
(14) demonstrated a significant
agreement between MDCTA and ultrasound in the measurement of the
degree and extent of carotid artery stenosis. However, the most
notable disadvantages of MDCTA are the use of iodine contrast
medium intravenously and radiation (32). MRA has refined the diagnostic
capacity of non-invasive vascular imaging, and has progressively
become competitive with DSA (15).
MRA is more expensive than carotid US and computed tomography
angiography (CTA) and is also less readily available. Furthermore,
MRA is inferior in terms of spatial resolution compared with
ultrasound and CTA due to its voxel view, and it may therefore not
be possible to use it for direct visualization of small-diameter
grafts (33). The general approach
for diagnosis in patients with suspected carotid artery stenosis is
to first perform US and then other non-invasive methods, including
CTA and MRA (14,32). Thus, the present research group
aspire to introduce this useful tool in animal models under
experimental conditions owing to its potential benefits.
Osorio-da Cruz et al (9) demonstrated that vascular ultrasound is
a reliable non-invasive tool used for the routine assessment of
vascular flow and patency in swine undergoing carotid graft
replacement. However, no systematic study has revealed that
vascular ultrasound may be used to monitor small-diameter (<6
mm) blood vessels, for example the carotid arteries of rabbits. The
present study successfully imaged the carotid arteries of rabbits
and implanted grafts for sufficient spatial resolution in order to
monitor the patency and lumen characteristics of scaffolds.
In the present study, B-mode ultrasonography
demonstrated excellent spatial resolution distinguishing the wall
morphology and vascular composition, the lumen of grafts and their
surrounding tissues. Also, it was possible to measure the diameter
of the vessels for quantitative analysis of whether they were
narrowing or expanding. For the clear double strong echo of the
vascular graft wall, it was easy to identify the location of
grafts, and hence, perform the body surface localization of the
graft guided by vascular ultrasound. The experimental animals
receiving graft implantation procedures require a structured
surveillance strategy for follow-up care (34). The experimental rabbits in the
present study exhibited an optimal wound healing ability. The
surgical scars on their skin faded in ~2 weeks. After the skin
scars faded, it was very difficult to label the exact location of
implanted graft. The non-invasive skin localization may increase
the probability of identification of an injury or severe
inflammation (35). Specific types
of radiolucent stents render difficulty in the localization of lost
devices. Furthermore, the stent may be visualized by digital X-ray
fluoroscopy; however, conventional fluoroscopy of the whole body
involves considerable exposure to radiation designating the
fluoroscopy as unrewarding (36).
Presumably, it may be possible to attempt the localization of a
misplaced stent with sonographic guidance in future clinical
practice. We speculate that the non-invasive localization of grafts
may be valuable in tissue engineering experiments, and subsequently
clinical application.
Ultrasound is being used with increasing regularity
in integrative human physiology research to determine the blood
flow and changes in the blood vessel diameter in extracranial
carotid arteries in response to experimental interventions
(18). Thus, this imaging modality
may also be used in laboratory animal studies to assess the
physiological variables and hemodynamic changes of the
stent-implanted arteries. The present study demonstrated that
ultrasound has a high diagnostic accuracy in the characterization
of graft stenosis or occlusions. The shift from B-mode image to
velocity and hemodynamic parameters with increasing severity of an
artery stent stenosis makes it possible to describe the entities
with real physiological differences. The brighter the hue of red or
blue, the higher the velocity of blood flow through the arteries
(37). Based on the received Doppler
frequency shift, spectral Doppler may be used to calculate the
velocity of moving reflectors, such as red blood cells (38). In addition, the patency vessels with
a vascular prosthesis may be analyzed quantitatively. When the
vessels are obstructed, the spectrum curve becomes disorderly and
unsystematic, and may not be detected.
In order to conduct the assessment, the animals
should be placed in a supine position fixed on the examination
couch with the neck fully exposed and hair shaved. Turning the head
around 30° towards the contralateral side elongates the neck
sufficiently. However, applying excessive pressure on the neck with
the transducer is avoided. Thus, the following criteria should be
considered to acquire high-quality images. First, for the
small-diameter arteries of rabbits, a linear transducer with high
frequency should be used to increase the axial resolution. The
parameters in B-mode should be preset as follows: Highest
frequency; appropriate depth so that the artery of interest is at
~two thirds of the display; the focus should be adjusted to the
point of interest; sufficient gain to clearly delineate the vessel
wall from the lumen; decrease the dynamic range to increase the
contrast of the image; and increase the filter noise rejection to
eliminate the acoustic noise from weak echoes. Second, in the color
or spectrum mode, the color box or the sample volume should be
placed over the vessel of interest, the box steered to an adequate
angle of <60° between the beam direction and the vessel, and the
PRF should be set to detect the flow without spuriously
superimposing color over tissue outside the vessel walls. The color
gain is increased to satisfactory filling within the vessel without
bleeding signals into the surrounding tissue. The procedure should
be performed in multiple angles and directions in order to greatly
avoid the interference signals. Finally, the ultrasound approach is
a specialized technique, which is highly operator-dependent,
requiring intensive training (18).
Thus, it is recommended that the same operator performs the
procedure during a lab process for consistent and reliable
results.
In the present study, ultrasound was beneficial to
discriminate near-occlusion from occlusion of the carotid grafts in
rabbits due to the visualization of the residual lumen on the
B-mode image or little blood flow signals on color Doppler.
Presently, the ultrasound B-mode is the most effective method for
demonstrating minor arterial plaques, although color flow imaging
is vital for velocity measurement and essential for differentiating
occlusion from stenosis (39). The
grayscale ultrasonography may present a higher spatial resolution
as compared to color and power Doppler examination; the level of
purity of the graft lumen may serve as a considerable parameter to
judge its patency. During mild-to-severe stenosis, and also
occlusion, gradually increased iso- to hyperecho flocculent masses
occurred in the graft lumens, considering the amount of platelet
deposition for the turbulent blood flow. Furthermore, there may be
some blood flow signals in the lumens, which are ‘mosaic’ colored
for those that are not completely occluded. During total occlusion
of the lumens, few dotted blood flow signals around the cavity were
observed and it was speculated that tiny collateral vessels
formatted around the occluded vascular graft for compensation.
Owing to the non-invasive, fast, non-ionizing and cost-effective
modality, the present research group recommends vascular Doppler
ultrasonography as the superior choice for continuous surveillance
of vascular grafts with almost no contraindications. Additionally,
it may repeatedly be used without animal sacrifice, to provide
valuable information about the grafts, thereby greatly shortening
the experimental period.
The most notable limitation of the present study was
that the measurement of the diameter in different blood flow
situations in vivo was not quantified, which suggests that
further study is required. There were also some background noises
in the color map, which may have disturbed the evaluation of the
blood flow pattern to some degree. Doppler color flow image quality
is controlled by multi-factor parameter settings, including gain,
time gain control, ensemble length, Nyquist limit (PRF), baseline
shift, wall filter, color window angle, location and size (40). The check and balance of these
complicated and numerous parameter settings will generate some
background noise, which was also a limitation of the present study.
Further work should be performed in the selection of the
appropriate instrument models and the most effective combination of
parameter settings.
Acknowledgements
The authors would like to thank Mr. Jun Fang, an the
important member in our synthetic vascular graft experimental group
of Shanghai Children's Medical Center, for his constant
encouragement and guidance.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81671833) and the
Collaborative Innovation Center for Translational Medicine at
Shanghai Jiao Tong University School of Medicine (grant no.
TM201504).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JS, JLZ and JD designed the study. JS, JLZ and JD
performed the experiments, while QW and MY analyzed data. JS and
JLZ wrote the manuscript, MY assisted in preparing the manuscript.
JD and QW reviewed and edited the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Animal Care and Experiment Committee of Shanghai Jiao Tong
University School of Medicine (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dahl SL, Kypson AP, Lawson JH, Blum JL,
Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, et
al: Readily available tissue-engineered vascular grafts. Sci Transl
Med. 68:68ra92011.
|
|
2
|
Wu T, Zhang J, Wang Y, Li D, Sun B,
El-Hamshary H, Yin M and Mo X: Fabrication and preliminary study of
a biomimetic tri-layer tubular graft based on fibers and fiber
yarns for vascular tissue engineering. Mater Sci Eng C Mater Biol
Appl. 82:121–129. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Du J, Xia D, Liu J, Wu T, Shi J,
Song W, Jin D, Mo X and Yin M: Preliminary study of a novel
nanofiber-based valve integrated tubular graft as an alternative
for a pulmonary valved artery. RSC Adv. 6:84837–84846. 2016.
View Article : Google Scholar
|
|
4
|
Zilla P, Bezuidenhout D and Human P:
Prosthetic vascular grafts: Wrong models, wrong questions and no
healing. Biomaterials. 34:5009–5027. 2007. View Article : Google Scholar
|
|
5
|
Thomas LV, Lekshmi V and Nair PD: Tissue
engineered vascular grafts-preclinical aspects. Int J Cardiol.
167:1091–1100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagaoka Y, Yamada H, Kimura T, Kishida A,
Fujisato T and Takakuda K: Reconstruction of small diameter
arteries using decellularized vascular scaffolds. J Med Dent Sci.
61:33–40. 2014.PubMed/NCBI
|
|
7
|
Kurobe H, Maxfield MW, Tara S, Rocco KA,
Bagi PS, Yi T, Udelsman B, Zhuang ZW, Cleary M, Iwakiri Y, et al:
Development of small diameter nanofiber tissue engineered arterial
grafts. PLoS One. 10:e01203282015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Itoda Y, Panthee N, Tanaka T, Ando T,
Sakuma I and Ono M: Novel anastomotic device for distal coronary
anastomosis: Preclinical results from swine off-pump coronary
artery bypass model. Ann Thorac Surg. 101:736–741. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osorio-da Cruz SM, Aggoun Y, Cikirikcioglu
M, Khabiri E, Djebaili K, Kalangos A and Walpoth B: Vascular
ultrasound studies for the non-invasive assessment of vascular flow
and patency in experimental surgery in the pig. Lab Anim.
43:333–337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Culp BC, Brown AT, Erdem E, Lowery J and
Culp WC: Selective intracranial magnification angiography of the
rabbit: Basic techniques and anatomy. J Vasc Interv Radiol.
18:187–192. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujii T, Fukuyama N, Tanaka C, Ikeya Y,
Shinozaki Y, Kawai T, Atsumi T, Shiraishi T, Sato E, Kuroda R, et
al: Visualization of microvessels by angiography using
inverse-Compton scattering X-rays in animal models. J Synchrotron
Radiat. 21:1327–1332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Towner RA, Smith N, Asano Y, He T, Doblas
S, Saunders D, Silasi-Mansat R, Lupu F and Seeney CE: Molecular
magnetic resonance imaging approaches used to aid in the
understanding of angiogenesis in vivo: Implications for tissue
engineering. Tissue Eng Part A. 16:357–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Wang X, Tan KB, Liu P, Zhuo ZX, Liu
Z, Hua X, Zhuo QQ, Xia HM and Gao YH: Molecular imaging of
vulnerable plaques in rabbits using contrast-enhanced ultrasound
targeting to vascular endothelial growth factor receptor-2. J Clin
Ultrasound. 39:83–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Savic ZN, Davidovic LB, Sagic DZ, Brajovic
MD and Popovic SS: Correlation of color Doppler with multidetector
CT angiography findings in carotid artery stenosis.
ScientificWorldJournal. 10:1818–1825. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
D'Onofrio M, Mansueto G, Faccioli N,
Guarise A, Tamellini P, Bogina G and Pozzi Mucelli R: Doppler
ultrasound and contrast-enhanced magnetic resonance angiography in
assessing carotid artery stenosis. Radiol Med. 111:93–103. 2006.(In
English, Italian). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grant EG, Benson CB, Moneta GL, Alexandrov
AV, Baker JD, Bluth EI, Carroll BA, Eliasziw M, Gocke J, Hertzberg
BS, et al: Carotid artery stenosis: Grayscale and Doppler
ultrasound diagnosis-Society of Radiologists in Ultrasound
consensus conference. Ultrasound Q. 19:190–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crişan S: Carotid ultrasound. Med
Ultrason. 13:326–330. 2011.PubMed/NCBI
|
|
18
|
Thomas KN, Lewis NC, Hill BG and Ainslie
PN: Technical recommendations for the use of carotid duplex
ultrasound for the assessment of extracranial blood flow. Am J
Physiol Regul Integr Comp Physiol. 309:R707–R720. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Högberg D, Dellagrammaticas D, Kragsterman
B, Björck M and Wanhainen A: Simplified ultrasound protocol for the
exclusion of clinically significant carotid artery stenosis. Ups J
Med Sci. 121:165–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang J, Zhang J, Du J, Pan Y, Shi J, Peng
Y, Chen W, Yuan L, Ye SH, Wagner WR, et al: Orthogonally
functionalizable polyurethane with subsequent modification with
heparin and endothelium-inducing peptide aiming for vascular
reconstruction. ACS Appl Mater Interfaces. 8:14442–14452. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shastri VP: In vivo engineering of
tissues: Biological considerations, challenges, strategies, and
future directions. Adv Mater. 21:3246–3254. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hollister SJ: Scaffold design and
manufacturing: From concept to clinic. Adv Mater. 21:3330–3342.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pashuck ET and Stevens MM: Designing
regenerative biomaterial therapies for the clinic. Sci Transl Med.
4:160sr42012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Appel AA, Anastasio MA, Larson JC and Brey
EM: Imaging challenges in biomaterials and tissue engineering.
Biomaterials. 34:6615–6630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng W, Wang Z, Song L, Zhao Q, Zhang J,
Li D, Wang S, Han J, Zheng XL, Yang Z and Kong D:
Endothelialization and patency of RGD-functionalized vascular
grafts in a rabbit carotid artery model. Biomaterials.
33:2880–2891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Benrashid E, McCoy CC, Youngwirth LM, Kim
J, Manson RJ, Otto JC and Lawson JH: Tissue engineered vascular
grafts: Origins, development, and current strategies for clinical
application. Methods. 99:13–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan M, Ridley L, Dunn DJ, Tian DH, Liou
K, Ozdirik J, Cheruvu C and Cao C: A systematic review and
meta-analysis of multidetector computed tomography in the
assessment of coronary artery bypass grafts. Int J Cardiol.
221:898–905. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Levine GN, Bates ER, Blankenship JC,
Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA,
Hollenberg SM, et al: 2011 ACCF/AHA/SCAI guideline for percutaneous
coronary intervention: A report of the American College of
cardiology foundation/American heart association task force on
practice guidelines and the society for cardiovascular angiography
and interventions. Circulation. 124:e574–e651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hjortnaes J, Gottlieb D, Figueiredo JL,
Melero-Martin J, Kohler RH, Bischoff J, Weissleder R, Mayer JE and
Aikawa E: Intravital molecular imaging of small-diameter
tissue-engineered vascular grafts in mice: A feasibility study.
Tissue Eng Part C Methods. 16:597–607. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mertens ME, Koch S, Schuster P, Wehner J,
Wu Z, Gremse F, Schulz V, Rongen L, Wolf F, Frese J, et al:
USPIO-labeled textile materials for non-invasive MR imaging of
tissue-engineered vascular grafts. Biomaterials. 39:155–163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rübenthaler J, Reiser M and Clevert DA:
Diagnostic vascular ultrasonography with the help of color Doppler
and contrast-enhanced ultrasonography. Ultrasonography. 35:289–301.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Adla T and Adlova R: Multimodality imaging
of carotid stenosis. Int J Angiol. 24:179–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jaff MR, Goldmakher GV, Lev MH and Romero
JM: Imaging of the carotid arteries: The role of duplex
ultrasonography, magnetic resonance arteriography, and computerized
tomographic arteriography. Vasc Med. 13:281–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thukkani AK and Kinlay S: Endovascular
intervention for peripheral artery disease. Circ Res.
116:1599–1613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Berland TL, Smith SL, Metzger PP, Nelson
KL, Fakhre GP, Chua HK, Burnett OL, Falkensammer J, Hickman HJ and
Hinder RA: Intraoperative gamma probe localization of the ureters:
A novel concept. J Am Coll Surg. 205:608–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mohiaddin RH, Roberts RH, Underwood R and
Rothman M: Localization of a misplaced coronary artery stent by
magnetic resonance imaging. Clin Cardiol. 18:175–177. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Merritt CR: Doppler color imaging.
Introduction. Clin Diagn Ultrasound. 27:1–6. 1992.PubMed/NCBI
|
|
38
|
Browne JE: A review of Doppler ultrasound
quality assurance protocols and test devices. Phys Med. 30:742–751.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
von Reutern GM, Goertler MW, Bornstein NM,
Del Sette M, Evans DH, Hetzel A, Kaps M, Perren F, Razumovky A, von
Reutern M, et al: Grading carotid stenosis using ultrasonic
methods. Stroke. 43:916–921. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kremkau FW: Doppler color imaging.
Principles and instrumentation. Clin Diagn Ultrasound. 27:7–60.
1992.PubMed/NCBI
|