Introduction
Kidney transplantation is a method of treating
patients with end stage renal disease. The development of
immunosuppressive treatments and improvements in transplant surgery
have led to a decrease in acute rejection of transplanted kidneys
in the early stage following kidney transplantation, and the 1-year
survival rate is now ~90% (1).
However, maintaining long-term allograft function remains
challenging and the 10-year survival rate is only 68% in living
donor kidney allograft recipients (1). It has been demonstrated that chronic
renal allograft dysfunction (CRAD) is the primary factor affecting
the long-term survival of patients receiving renal allografts.
Early pathological changes in CRAD include inflammatory cell
infiltration into tubulointerstitial spaces, and smooth muscle cell
(SMC) proliferation and migration (2). Immune and non-immune factors contribute
to the pathogenesis of CRAD, including renal ischemia-reperfusion
(I/R) injury, which inevitably occurs following kidney
transplantation and may cause graft dysfunction. This determines
the short- and long-term outcomes of transplant recipients
(3). Oxidative stress serves a role
in the pathophysiology of renal injury in I/R. During reperfusion,
cellular metabolism increases rapidly, resulting in the increased
generation of reactive oxygen species (ROS), cytokines and
chemokines, which promote inflammatory cell infiltration and lead
to tissue damage and graft dysfunction (4).
Sirtuin 3 (sirt3) is involved in the regulation of
antioxidant pathways. Sirt3 is a member of the sirtuin protein
family and is a nicotinamide adenine dinucleotide (NAD+)
dependent protein deacetylase. It is highly expressed in
well-oxygenated tissues, including the brain, muscles, heart,
kidneys and adipose tissue (5).
Sirt3 is primarily localized in the mitochondrial matrix, and its
overexpression increases the oxidative capacity of mitochondria and
causes a concurrent decrease in ROS levels (6). Increased expression of sirt3 protein
inhibits the nuclear factor-κB (NF-κB) dependent transcriptional
activity of inflammatory genes, reduces malondialdehyde (MDA)
levels and increases superoxide dismutase (SOD) activity.
Subsequently, overexpression of SIRT3 increases the oxidative
capacity of mitochondria and produces a parallel decrease in ROS
levels (7). This may be the
mechanism by which sirt3 exerts antioxidant and anti-inflammatory
effects in proximal tubular cells (7). However, the role of sirt3 in CRAD
remains unknown. Thus, the current study investigated the
expression and role of sirt3 in the early stage of CRAD in a
standardized rat model.
Materials and methods
Animals
A total of 60 naive inbred male Fischer (F344)
(n=30) and Lewis (Lew) rats (n=30) weighing 150–220 g, aged
8–12-week-old were purchased from Charles River Laboratories, Inc.
(Beijing, China). The rats were kept in a specific pathogen free
laboratory at the Animal Experimental Center of the Southern
Medical University (Guangzhou, China). The rats were fed a standard
rat chow diet, had free access to water, were housed under standard
conditions with a controlled temperature (24±2°C), humidity
(40–60%) and a 12-h light/dark cycle. The rats were acclimatized
for 1 week prior to the experiment. The current study was approved
by the Committee on the Ethics of Animal Experiments of the
Southern Medical University (permission no. 00119486) and was
performed in accordance with the Guide for the Care and Use of
Laboratory Animals published by the United States National
Institutes of Health (8).
Study design
Kidneys from Fischer (F344) rats were orthotopically
transplanted into Lewis (LEW) rats. The Fischer-Lewis model has
become the most intensively studied and widely accepted animal
model of chronic renal allograft rejection because of its
similarity to the progress of events following human renal
transplantation, thus the Fischer-Lewis model was established in
the present study (9). Rats were
split into three groups as follows: i) A Lew control group
consisting of uninephrectomized male Lew rats (n=10); ii) a F344
control group consisting of uninephrectomized male F344 rats
(n=10); and iii) an allograft group consisting of Lew rats
receiving transplants (n=10).
Uninephrectomy surgery
Surgery was performed under general anesthesia with
3% sodium pentobarbital (30 mg/kg; Hangzhou Zhongmei Huadong
Pharmaceutical Co., Ltd., Hangzhou, China) on the F344 rats and the
LEW rats. A half-inch incision was made on the left flank portion
of abdomen and the kidney was pulled out of the abdomen by holding
the perirenal fat at the lower pole with blunt forceps, which
separated the kidney from the surrounding fat and supra renal
gland. Furthermore, ligation of the renal artery and vein and
ureter ~0.5 cm below the level of hilum with a non-absorbable
surgical suturing thread was performed and the kidney was snap
resected. The uninephrectomized rats received ceftriaxone via i.p.
injection for 3 days.
Kidney transplantation
A total of 15 male Fischer (F344) weighing 150–220
g, aged 8–12-week-old were used for this procedure. A surgical
ASX-2 microscope was purchased from Shanghai Anxin Optical
Instrument Manufacture Co., Ltd. (Shanghai, China). Orthotopic
kidney transplantation was performed as previously described
(9). Surgery was performed under
general anesthesia with 3% sodium pentobarbital (30 mg/kg)
administered via intraperitoneal (i.p.) injection. The left kidney
from the recipient Lew rat was removed and the left renal vessels
were clamped. The left donor kidney from a F344 rat was then
removed, cooled and positioned orthotopically in the recipient Lew
rat. The renal arteries, veins and ureters of the donor and
recipient were then anastomosed end-to-end with 10-0 prolene
(Ningbo Lingqiao Biological Technology, Ningbo, China). No ureteral
stents were used. A low dose of cyclosporine A (1.5 mg/kg/day;
Novartis International AG, Basel, Switzerland) was administered for
10 days following transplantation to suppress acute rejection. To
prevent infection, recipients received ceftriaxone (Rocephin; 20
mg/kg/day) via i.p. injection for 3 days. The right native kidney
of each recipient was removed 10 days following surgery. Rats
exhibiting overt signs of unsuccessful surgeries were excluded from
the current study. Subsequently there were 10 rats per group
following exclusion.
Tissue harvesting
All rats were anesthetized with i.p. injection of 60
mg/kg sodium pentobarbital and subjected to cardiac exsanguination
12 weeks following surgery. Kidneys were harvested from rats in all
groups. However, only kidneys with no visible grafting
complications, including pyelonephritis or hydronephrosis, were
selected for subsequent experimentation. The kidney of each rat was
cut into two sections: One section was stored at −80°C in liquid
nitrogen for western blot analysis. Rat serum samples (from 2 ml
blood) were used for the evaluation of blood urea nitrogen, as well
as creatinine with an automatic biochemical analyzer (AU5400;
Olympus, Tokyo, Japan). Urine protein excretion was measured via an
enzymatic kinetic method using commercial kits purchased from
Nanjing Jiancheng Biological Engineering Research Institute
(Nanjing, China) and 1 ml 24-h urine samples that were taken prior
to sacrifice. The second part of the kidney was fixed in 4%
formalin for the histopathological examination and
immunohistochemistry.
Renal histopathology
Kidney tissues were fixed in 4% paraformaldehyde at
room temperature for 24 h and embedded in paraffin. Paraffin
sections with a thickness of ~2 µm were stained with hematoxylin
and eosin or periodic acid-Schiff (PAS) at room temperature for 30
min to assess the extent of renal pathological changes and sections
were observed with a light microscope. There were ≥100 glomeruli
counted in each kidney section. Using the Banff 07 Classification
(10), interstitial cellular
infiltration, tubulopathy/interstitial fibrosis, glomerulopathy and
arterial intimal fibroplasia were scored on a scale from 0 to 3+,
and the sum of these scores (range, 0–12+) was calculated for each
sample.
Immunohistochemistry
Immunohistochemistry was used to detect the
expression of sirt3 protein in the renal allograft tissue. Antigens
were retrieved using the citric acid buffer (pH 6.0) microwave
antigen retrieval method and detected via PV-9001 staining (Golden
Bridge Biotechnology Co., Beijing, China). Tissues were boiled for
15 min at a temperature ranging 95–100°C and staining with PV-9001
was performed at room temperature for 60 min. The paraffin sections
4 µm thick were hydrated in an alcohol gradient (75, 85, 95, and
100% at 37°C for 3 min per concentration) and 3% hydrogen peroxide
was used to block the endogenous peroxidase. Sections were then
incubated with rabbit polyclonal anti-sirt3 antibodies (cat. no.
2627, 1:100; Cell Signaling Technology, Inc., Danvers, MA, USA)
overnight at 4°C. Sections were washed with PBS, incubated with the
goat anti-rabbit secondary antibody (cat. no. PV-9001, diluted
1:100; Golden Bridge Biotechnology Co., Ltd., Beijing, China) for
20 min at room temperature and washed a further three times with
PBS. Sections were then incubated in a 3,3′-diaminobenzidine
chromogen solution (OriGene Technologies, Inc., Rockville, MD, USA)
and re-stained with hematoxylin at 37°C for 1 min. The intensity of
positive staining for sirt3 was quantified using Image-Pro Plus
software version 7.0.1.658, Media Cybernetics, Inc. (Rockville, MD,
USA).
Western blot analysis
Kidney tissues stored in the −80°C freezer were
homogenized on ice in a lysis buffer consisting of 0.1 mol/l Tris
buffer (pH 7.4), 0.1 mmol/l EDTA, 1 mmol/l dithiothreitol, 1 mmol/l
phenylmethylsulfonyl fluoride, a protease inhibitor cocktail (Roche
Applied Science, Madison, WI, USA) and a protein phosphatase
inhibitor. The protein concentrations of the samples were measured
using a BCA Protein assay kit (Beyotime Institute of Biotechnology,
Shanghai, China). Protein samples (50 µg/lane) were loaded on to a
10% SDS gel and SDS PAGE was performed. Subsequently, samples were
transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA),
which were then blocked with 5% bovine serum albumin (cat. no.
B2064; Sigma-Aldrich; Merck KGaA) in TBST for 1 h at 4°C. The
membranes were then washed three times with TBST and incubated with
anti-sirt3 antibodies (cat. no. 2627, 1:2,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) overnight at 4°C. Membranes
were washed and then incubated with a goat anti-rabbit IgG,
horseradish peroxidase conjugate secondary antibody (cat. no.
CW0156S, 1:5,000; CWBiotech, Beijing, China) for 1 h at room
temperature. The target bands were observed using the SuperSignal
West Pico enhanced chemiluminescent substrate (Pierce; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). All analyses were
repeated in triplicate. Protein band intensities were quantified
using Image J software (version 1.46, National Institutes of
Health, Bethesda, MD, USA).
Measurement of SOD activity and MDA
levels
The SOD and MDA levels in the renal tissues were
assessed using commercial Total Superoxide Dismutase and
Malondialdehyde assay kits. (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) according to the manufacturer's
protocol. SOD activity was detected using a xanthine oxidase-based
technique and MDA content was assayed using the thiobarbituric acid
method.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
for Windows (IBM Corp., Armonk, NY, USA). Data are presented as the
mean ± standard error of the mean (SE). A one-way analysis of
variance followed by the LSD test was used to determine the
significance of differences in multiple-group comparisons.
Spearman's rank correlation analysis was used to assess the
association between different factors in the groups. P<0.05 was
determined to indicate statistically significant difference.
Results
Renal functional changes
Orthotopic kidney transplantation in rats had been
successfully established (Fig. 1)
The levels of serum creatinine, blood urea nitrogen and 24 h
urinary protein excretion were significantly increased in the
allograft group compared with the F344 and Lew rat controls
(P<0.05; Table I).
 | Table I.Comparison of renal function indexes
and histopathological evaluations among the allograft group and the
F344 and Lewis control groups. |
Table I.
Comparison of renal function indexes
and histopathological evaluations among the allograft group and the
F344 and Lewis control groups.
| Group | n | Serum creatinine
(µmol/l) | Blood urea nitrogen
(mmol/l) | 24 h urine protein
excretion (mg/day) | Quantity of
infiltrated interstitial mononuclear cells | Quantity of SMCs in
the vascular wall | Interstitial
fibrosis | Summed Banff
score |
|---|
| F344 controls | 10 | 49.33±2.94 | 7.65±0.63 | 9.21±6.13 | 7.99±6.71 | 5.52±1.90 | 0.07±0.01 | 1.42±0.73 |
| Lewis controls | 10 | 46.50±6.44 | 7.35±0.86 | 11.16±3.24 | 8.67±5.50 | 5.70±1.83 | 0.06±0.03 | 1.12±0.41 |
| Allograft rats | 10 |
57.00±4.23a |
10.49±0.42a |
28.53±50.48a |
34.52±7.94a |
15.65±2.50a | 0.09±0.03 |
4.64±1.94a |
Histological changes
Kidney sections from the F344 and Lew control groups
did not exhibit marked histological changes or SMC migration.
However, kidney sections from the allograft group exhibited
interstitial mononuclear cell infiltration, intimal proliferation
and SMC migration (Fig. 2). The
Banff scores of the allograft group were significantly higher
compared with those of the two control groups (P<0.05; Table I). Early pathology change of CRAD was
indicated by infiltration of mononuclear cells during
tubulointerstitial inflammation and the proliferation and migration
of smooth muscular cells (SMCs). The late change was observed by
sclerosis of renal blood vessel and tubulointerstitial fibrosis.
These findings suggested that after 12 weeks, kidney sections from
the allograft group exhibited interstitial mononuclear cell
infiltration. The difference in interstitial fibrosis among groups
was not significant, indicating that a rat kidney transplantation
model at the early stage of CRAD was successfully established.
SOD and MDA levels in renal
tissues
SOD activity was decreased while the levels of MDA
were increased in the renal tissues in the allograft group compared
with the F344 and Lew controls (P<0.05; Table II). These results suggest that
oxidative stress was present in the kidneys at the early stage of
CRAD in the model established in the current study.
 | Table II.Comparisons of SOD and MDA activity in
renal tissue among the allograft group and the F344 and Lewis
control groups. |
Table II.
Comparisons of SOD and MDA activity in
renal tissue among the allograft group and the F344 and Lewis
control groups.
| Group | n | SOD (U/mg) | MDA (ng/mg) |
|---|
| F344 controls | 10 | 80.74±5.22 | 1.06±0.13 |
| Lewis controls | 10 | 77.54±3.30 | 1.29±0.19 |
| Allograft rats | 10 |
61.15±2.74a |
4.13±1.81a |
Sirt3 expression
Immunohistochemical analysis demonstrated that sirt3
was strongly expressed in the cytoplasm of tubular epithelia cells
and the renal arterioles of the kidneys from control rats. However,
the expression of sirt3 was significantly decreased in the kidneys
from the allograft group (P<0.05; Fig. 3). Western blotting also demonstrated
that the expression of sirt3 was significantly decreased in the
allograft group compared with the F344 and Lew controls (P<0.05;
Fig. 4).
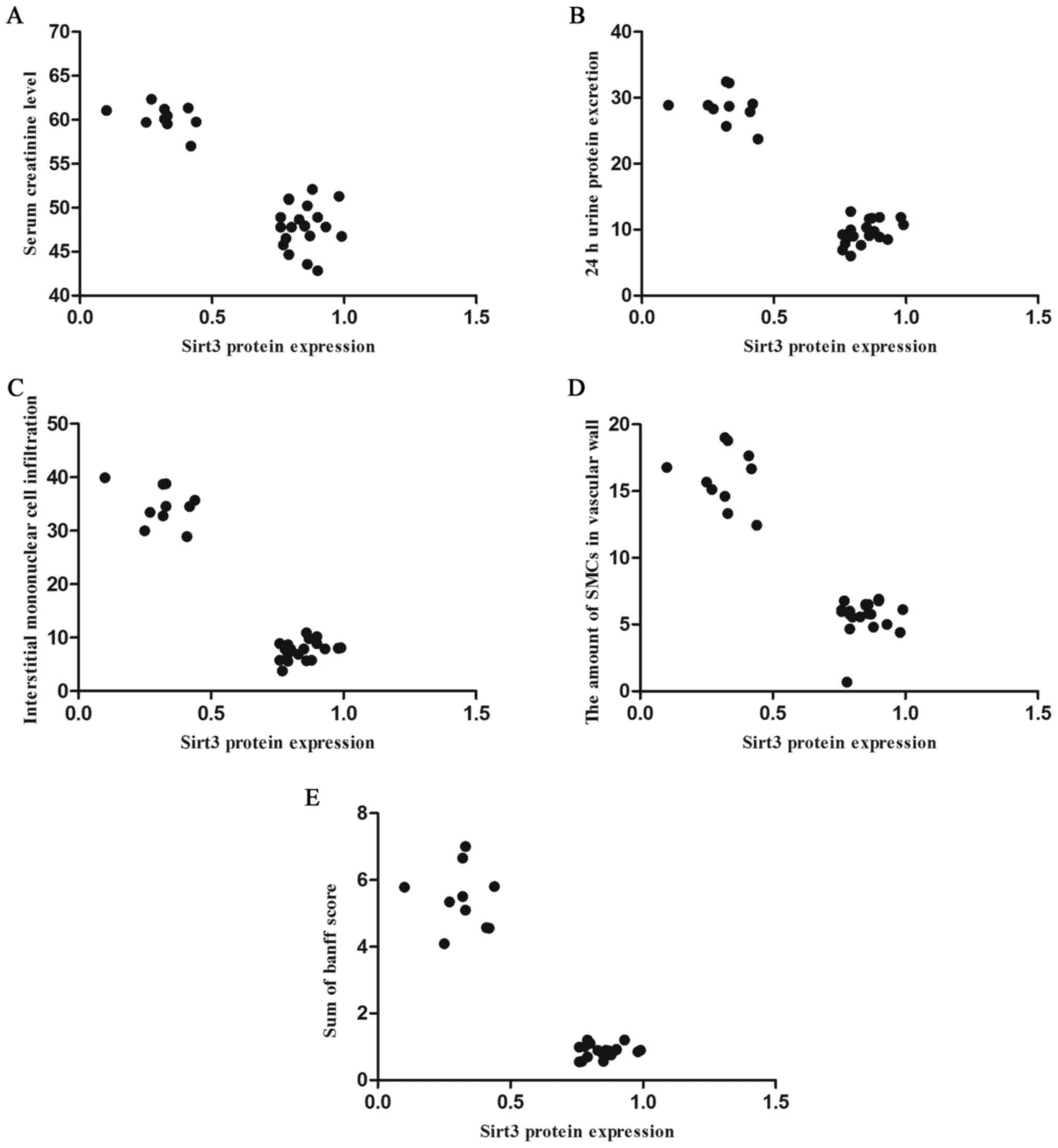
Correlation analysis
Spearman's rank correlation analysis identified that
there were negative correlations between the expression of sirt3
and serum creatinine levels (r=−0.656; P<0.05; Fig. 5A), 24 h urinary protein excretion
(r=−0.925; P=0.002; Fig. 5B),
tubulointerstitial mononuclear cell infiltration (r=−0.564;
P<0.05; Fig. 5C), the number of
SMCs in the vascular wall (r=−0.669; P<0.05; Fig. 5D) and the sum of BANFF scores in
allografts (r=−0.637, P<0.05; Fig.
5E).
Discussion
The current study determined the relevance of the
expression of sirt3 in the pathogenesis of CRAD in a rat model.
Sirt3 expression was significantly decreased in rats with CRAD at
week 12. The present study demonstrated that the early changes in
the pathology of rats with CRAD were tubulointerstitial mononuclear
cell infiltration and SMC proliferation and migration. The
decreased expression of sirt3 was correlated with the pathological
hallmarks of CRAD, including urinary protein excretion, serum
creatinine levels, tubulointerstitial mononuclear cell infiltration
and SMC migration in the vascular wall. These results suggest that
sirt3 may serve a role as an endogenous protective factor in the
early stage of CRAD.
CRAD is the primary factor affecting the long-term
survival of patients receiving renal allografts (1). Transplanted kidneys are prone to
oxidative stress-mediated injury caused by I/R, which generates
large amounts of ROS (11).
Reperfusion injury and reactive oxygen compounds serve an important
role in the pathophysiology of acute allograft rejection and kidney
function in the early post-transplantation phase but also affect
long-term kidney function (11).
Levels of oxidative stress are increased in patients who have
undergone transplantation, suggesting that supplementary
antioxidants may be beneficial. However, the molecular mechanisms
of oxidative stress in CRAD remain unclear and further studies are
required.
Oxidative stress promotes inflammation by activating
NF-κB, which may then aggravate oxidative stress-mediated injury.
Oxidative stress and inflammation cause endothelial injury and
arteriosclerosis, which are histopathological characteristics of
CRAD (12). MDA is the product of
lipid peroxidation inside cells and may be used as a marker to
detect the level of oxidative stress occurring in an organism
(13). SOD is an enzyme that removes
free radicals, leading to a reduction in the production of lipid
peroxides, thereby protecting cells and tissue (14). The current study investigated
oxidative stress in the early stage of CRAD by establishing a rat
model of the condition 12 weeks post-transplantation. SOD levels
were significantly decreased while the levels of MDA were
significantly increased in the renal tissues of rats in the
allograft group compared with F344 and Lew control rats
(P<0.05). These results are consistent with those of previous
studies, which demonstrated that the levels of products from
oxidizing proteins, including MDA, carbonylated proteins and
8-hydroxy-2′-deoxyguanosine are increased and glutathione levels
are decreased 1 year following transplantation. These changes are
also associated with atherosclerosis and high levels of serum
creatinine (15,16). It has also been demonstrated that ROS
are important pro-fibrogenic cytokines in chronic allograft
nephropathy and oxidative stress is increased in the presence of
interstitial fibrosis and tubular atrophy, which generally precede
chronic allograft failure (17). A
previous study conducted on 40 patients who had received kidney
transplants demonstrated that increased MDA levels 1 day following
kidney transplantation may be an early prognostic indicator of
delayed graft function and increased levels on day 7 may be a
useful predictor of 1-year graft function (11). Increased levels of MDA reflecting
lipid peroxidation have been detected in rat models of chronic
allograft tubular atrophy/interstitial fibrosis (18). In the current study, an increase in
MDA levels and a decrease in SOD activity were detected in the
renal tissues of rats in the allograft group, indicating that
transplantation caused oxidative damage in the transplanted kidneys
of rats in the early stage of CRAD. This is consistent with the
results of the aforementioned previous studies.
To the best of our knowledge, there have been no
previous studies focusing on the role served by sirt3 in the early
stage of CRAD in rat models. Sirt3 is a human NAD+-dependent
protein deacetylase in humans that belongs to the sirtuin family,
which contains 7 proteins identified in mammals (sirt1, sirt2,
sirt3, sirt4, sirt5, sirt6, sirt7) (19). Sirt3 localizes primarily in the
mitochondria, regulating respiratory chain function, the
tricarboxylic acid (TCA) cycle and fatty acid β oxidation. It also
exhibits antioxidant activity (20).
The ability of sirt3 to protect cells from oxidative damage has
been demonstrated previously, supporting the idea that sirt3 serves
an important role in regulating ROS homeostasis (21). Sirt3 also reduces cellular ROS levels
via a mechanism dependent on the activation of superoxide dismutase
2, peroxiredoxins 3 and 5, glutathione peroxidases and the TCA
cycle enzyme isocitrate dehydrogenase 2 (22,23).
Sirt3 is highly expressed in renal tissues (24,25) and
the sirt3 protein has been widely studied in relation to kidney
disease. In a murine model of cisplatin-induced acute kidney
injury, it was demonstrated that increased oxidative stress and
mitochondrial damage were associated with reduced levels of sirt3.
Following treatment with the antioxidant agent acetyl-l-carnitine,
which enhanced the expression and activity of sirt3, renal function
was improved and the extent of tubular injuries were decreased in
mice (5). The overexpression of
sirt3 inhibits the NF-κB signaling pathways, decreases the
phosphorylation of ERK1/2 and p38, decreases the expression of
inflammatory cytokines and reduces ROS levels in an experimental
model of free fatty acid-mediated tubulointerstitial inflammation.
This may be the molecular mechanism by which sirt3 induces
antioxidant and anti-inflammatory effects (25). The results from an in vitro
experiment demonstrated that increasing the expression of sirt3 may
inhibit the mesangial hypertrophy induced by high glucose levels,
primarily by adenosine monophosphate-activated protein
kinase-mammalian target of rapamycin signaling pathways (26). The current study demonstrated that
the expression of sirt3 was increased in the cytoplasm of tubular
epithelia cells and in the renal arterioles of rats in the F344 and
Lew control groups, whereas the expression of sirt3 was decreased
in the allograft group. The mean optical density values for sirt3
in the allograft group were lower than those of the F344 and Lew
control groups (P<0.05). Western blotting also indicated a
decrease in the expression of sirt3 protein in the allograft group
(P<0.05). There was a negative correlation between the
expression of sirt3 and mononuclear cell infiltration and SMC
migration in the vascular wall, indicating that decreased
expression of sirt3 protein serves a significant role at the early
development stage of CRAD pathogenesis. The signaling mechanisms
related to oxidative stress in rat models of CRAD and the role of
sirt3 in transplanted kidneys remain unclear. Sirt3 has antioxidant
and anti-inflammatory effects and the results of the current study
suggest that the sirt3 related signaling pathway may serve an
important role in mediating the important early effects of CRAD,
including mononuclear cell infiltration and vascular SMC
proliferation and migration.
The current study has some limitations. The
experimental grouping design is based on our previous study as
follows: The allograft group consisted of Lew rats orthotopically
transplanted with kidneys from F344 rats and the control groups
consisted of uninephrectomized male Lew and F334 rats (9,27,28).
However, the current study lacked a Lew-Lew syngeneic transplant
model in which the Lew rat received a kidney from another Lew rat.
To ensure the reliability of results, a Lew-Lew syngeneic
transplant model should be used as a control in future studies. In
addition, the pathological changes and renal function following the
up-or down-regulation of sirt3 expression were not investigated due
to limits of the experimental conditions. Further studies are
required to investigate the pathogenic mechanisms that lead to
changes in the expression of sirt3 in renal tissue during chronic
allograft dysfunction.
In conclusion, to the best of our knowledge, this is
the first study measuring sirt3 expression in a rat kidney
transplantation model at the early stage of CRAD. The results
indicate that sirt3 may be involved in pathogenic processes at the
early stage of CRAD. Thus, increasing the expression of sirt3 at
the early stage may be a novel therapeutic approach for attenuating
the development of CRAD.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270840).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Safinia N, Afzali B, Atalar K, Lombardi G
and Lechler RI: T-cell alloimmunity and chronic allograft
dysfunction. Kidney Int Suppl. S2–S12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Racusen LC and Regele H: The pathology of
chronic allograft dysfunction. Kidney Int Suppl. S27–S32. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rintala JM, Savikko J, Palin N, Rintala
SE, Koskinen PK and von Willebrand E: Oral Platelet-derived growth
factor and vascular endothelial growth factor inhibitor sunitinib
prevents chronic allograft injury in experimental kidney
transplantation model. Transplantation. 100:103–110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Favreau F, Thuillier R, Cau J, Milin S,
Manguy E, Mauco G, Zhu X, Lerman LO and Hauet T: Anti-thrombin
therapy during warm ischemia and cold preservation prevents chronic
kidney graft fibrosis in a DCD model. Am J Transplant. 10:30–39.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi T, Wang F, Stieren E and Tong Q:
SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial
function and thermogenesis in brown adipocytes. J Biol Chem.
280:13560–13567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giralt A and Villarroya F: SIRT3, a
pivotal actor in mitochondrial functions: Metabolism, cell death
and aging. Biochem J. 444:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koyama T, Kume S, Koya D, Araki S, Isshiki
K, Chin-Kanasaki M, Sugimoto T, Haneda M, Sugaya T, Kashiwagi A, et
al: SIRT3 attenuates palmitate-induced ROS production and
inflammation in proximal tubular cells. Free Radical Bio Med.
51:1258–1267. 2011. View Article : Google Scholar
|
|
8
|
National Research Council. Guide for the
Care and Use of Laboratory Animals. 8th. The National Academies
Press; Washington, DC: 2011
|
|
9
|
Song E, Zou H, Yao Y, Proudfoot A, Antus
B, Liu S, Jens L and Heemann U: Early application of Met-RANTES
ameliorates chronic allograft nephropathy. Kidney Int. 61:676–685.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Solez K, Colvin RB, Racusen LC, Haas M,
Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, et
al: Banff 07 classification of renal allograft pathology: Updates
and future directions. Am J Transplant. 8:753–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fonseca I, Reguengo H, Almeida M, Dias L,
Martins LS, Pedroso S, Santos J, Lobato L, Henriques AC and
Mendonça D: Oxidative stress in kidney transplantation:
Malondialdehyde is an early predictive marker of graft dysfunction.
Transplantation. 97:1058–1065. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reinders ME, Rabelink TJ and Briscoe DM:
Angiogenesis and endothelial cell repair in renal disease and
allograft rejection. J Am Soc Nephrol. 17:932–942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giera M, Lingeman H and Niessen WM: Recent
advancements in the LC- and GC-based analysis of malondialdehyde
(MDA): A brief overview. Chromatographia. 75:433–440. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quarrie R, Lee DS, Reyes L, Erdahl W,
Pfeiffer DR, Zweier JL and Crestanello JA: Mitochondrial uncoupling
does not decrease reactive oxygen species production after
ischemia-reperfusion. Am J Physiol Heart Circ Physiol.
307:H996–H1004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papazova DA, Friederich-Persson M, Joles
JA and Verhaar MC: Renal transplantation induces mitochondrial
uncoupling, increased kidney oxygen consumption, and decreased
kidney oxygen tension. Am J Physiol Renal Physiol. 308:F22–F28.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yildiz A, Gul CB, Ocak N, Ersoy A, Sag S,
Oruc A, Ayar Y, Dagel T, Dirican M and Gullulu M: Fluvastatin
decreases oxidative stress in kidney transplant patients.
Transplant Proc. 47:2870–2874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zahmatkesh M, Kadkhodaee M, Mahdavi-Mazdeh
M, Ghaznavi R, Hemati M, Seifi B, Golab F, Hasani K,
Lessan-Pezeshki M and Einollahi B: Oxidative stress status in renal
transplant recipients. Exp Clin Transplant. 8:38–44.
2010.PubMed/NCBI
|
|
18
|
Ding R, Chen X, Wu D, Wei R, Hong Q, Shi
S, Yin Z, Ma L and Xie Y: Effects of aging on kidney graft
function, oxidative stress and gene expression after kidney
transplantation. PLoS One. 8:e656132013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rahman M, Nirala NK, Singh A, Zhu LJ,
Taguchi K, Bamba T, Fukusaki E, Shaw LM, Lambright DG, Acharya JK
and Acharya UR: Drosophila Sirt2/mammalian SIRT3
deacetylates ATP synthase β and regulates complex V activity. J
Cell Biol. 206:289–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahn BH, Kim HS, Song S, Lee IH, Liu J,
Vassilopoulos A, Deng CX and Finkel T: A role for the mitochondrial
deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad
Sci USA. 105:14447–14452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bause AS and Haigis MC: SIRT3 regulation
of mitochondrial oxidative stress. Exp Gerontol. 48:634–639. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qiu X, Brown K, Hirschey MD, Verdin E and
Chen D: Calorie restriction reduces oxidative stress by
SIRT3-mediated SOD2 activation. Cell Metab. 12:662–667. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Finley LW and Haigis MC: Metabolic
regulation by SIRT3: Implications for tumorigenesis. Trends Mol
Med. 18:516–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sack MN and Finkel T: Mitochondrial
metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol.
4:pii:a0131022012. View Article : Google Scholar
|
|
25
|
Morigi M, Perico L, Rota C, Longaretti L,
Conti S, Rottoli D, Novelli R, Remuzzi G and Benigni A: Sirtuin
3-dependent mitochondrial dynamic improvements protect against
acute kidney injury. J Clin Invest. 125:715–726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhuo L, Fu B, Bai X, Zhang B, Wu L, Cui J,
Cui S, Wei R, Chen X and Cai G: NAD blocks high glucose induced
mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR
pathway. Cell Physiol Biochem. 27:681–690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han C, Zou H, Li Q, Wang Y, Shi Y, Lv T,
Chen L and Zhou W: Expression of the integrin-linked kinase in a
rat kidney model of chronic allograft nephropathy. Cell Biochem
Biophys. 61:73–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu D, Shi Y, Ding Y, Liu X and Zou H:
Dramatic early event in chronic allograft nephropathy: Increased
but not decreased expression of MMP-9 gene. Diagn Pathol. 8:132013.
View Article : Google Scholar : PubMed/NCBI
|