Introduction
Diabetes is a chronic progressive disease that
primarily manifests as an endocrine disorder (1). According to the World Health
Organization, the prevalence of diabetes worldwide will reach 334
million people (2) and therefore it
poses a serious threat to human health. Diabetes typically results
from hyperglycaemia due to insulin resistance and a relative lack
of insulin; the majority of the increased risk of mortality is
attributable to associated macrovascular atherosclerotic diseases
(3,4). Out of all patients with diabetes,
>90% have type 2 diabetes mellitus (T2DM) (5). Currently, the most effective methods of
treating T2DM are medical interventions, including surgical
procedures such as Roux-en-Y gastric bypass (RYGBP) and ileal
transposition (IT), anti-diabetes medication (insulin, metformin)
and undergoing lifestyle changes, such as adhering to a healthy
diet and partaking in regular exercise (6–8).
RYGBP surgery and IT are the most commonly performed
surgical procedures to treat patients with T2DM (9,10).
Pories et al (11)
demonstrated that, following RYGBP, blood glucose levels in
patients with T2DM are more effectively regulated compared with
prior to the operation. Indeed, following RYGBP, 83% of patients
with T2DM exhibit normal glucose and glycated hemoglobin levels,
even following discontinuation of all glucose-lowering agents
(12). It is also worth noting that
overweight patients exhibited normal insulin levels following RYGBP
surgery, which reduced glucose levels (13). RYGBP markedly improves the prognosis
of obese patients with T2DM and reduces the likelihood of
complications occurring, including perioperative mortality,
pulmonary embolism and dysrhythmias (11). However, a significant proportion of
patients with T2DM are not obese and it remains unknown whether
they can benefit from RYGBP. Previous studies have demonstrated
that IT is able to induce a hypoglycemic effect in a non-obese rat
model of T2DM (10,14). However, the effectiveness, safety and
clinical applications of IT in humans still require further
clarification.
It has been reported that the expression of
phosphoenolpyruvate carboxykinase (PEPCK) is upregulated in a rat
model of diabetes and these rats are either completely lacking in
insulin or exhibit increased plasma glucocorticoid levels (15,16).
Glucose-6-phosphatase (G-6-Pase) is the final gatekeeper of glucose
efflux from cells and catalyzes the last step of gluconeogenesis
(17). Previous studies have
suggested that G-6-Pase contributes to the development of diabetes
and its promoter has several elements in common with PEPCK
(18–20). Insulin receptor substrate (IRS)
proteins, including IRS-1, IRS-2 and IRS-4 also serve a critical
role in the signal transduction of insulin (21). In humans, a number of polymorphisms
have been identified in the IRS2 gene and these polymorphisms may
increase the risk of T2DM in various populations where they are
more prevalent (22).
In the present study, a T2DM rat model was used to
evaluate the effects of RYGBP combined with IT on T2DM, compared
with RYGBP or IT alone. Levels of diabetes-related factors,
including triglyceride (TG), total cholesterol (TC), fasting blood
glucose (FBG), gastric inhibitory polypeptide (GIP), glucagon-like
peptide (GLP-1), PEPCK1, G-6-Pase and IRS-2, were measured and used
as evaluation indicators. The primary aim of the present study was
to assess the effects of RYGBP combined with IT in the treatment of
non-obese T2DM, with the aim of developing a novel effective
treatment of T2DM that induces T2DM remission and reduces the risk
of complications occurring.
Materials and methods
Animals
A total of 40 male Goto-Kakizaki (GK) rats (age, 8
weeks; weight, 270±30 g) with non-obese T2DM and 8 healthy male
Sprague Dawley (SD) rats (age, 8 weeks; weight, 270±30 g), were
allowed to acclimate to the facility in cages for 1 week prior to
experiments under a 12 h dark/light cycle at 22±2°C. All rats were
obtained from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai,
China). The 8 healthy rats formed the control group and the 40 GK
rats were randomly divided into the following groups (all n=8): An
untreated T2DM group (DM group), a sham group, a RYGBP surgery
group (RYGBP group), an IT surgery group (IT group) and an RYGBP
and IT combination group (RYGBP+IT group). All protocols involving
animals were approved by the Ethical Committee of the Fifth
Hospital of Wuhan, Wuhan University (Wuhan, China; ethical approval
no. 20160311). All dissections were performed according to
recommendations proposed by the European Commission, and all
efforts were made to minimize suffering in our animals.
Surgical intervention
All rats were fasted overnight for ≥12 h and then
intraperitoneally anesthetized with 350 mg/kg 10% chloral hydrate
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). For RYGBP, the
gastric bypass model was established following a previously
described protocol (23). For IT
surgery, Treitz's ligament was identified, the jejunum was divided
5 cm aborally and the ileal loop was the interpositioned in an
isoperistaltic fashion, forming two end-to-end anastomoses
(24). For the combination of RYGBP
and IT surgery, Treitz's ligament was identified, the jejunum was
divided 5 cm aborally and anastomoses were formed in the distal
jejunum and proximal. A total of 8 male GK rats were selected to
undergo sham surgery. A division of the small intestine with
subsequent anastomoses was performed at the distal jejunum and
proximal and ileum without IT. The sham procedure involved
gastrotomy, enterotomy and repair. All rats were maintained on a
standardized post-operative protocol during which a liquid diet was
provided from day 2 post-surgery. During the experiment, 1 rat from
the sham group, 1 rat from the RYGBP group and 2 rats from the
RYGBP+IL group succumbed. This is in line with the mortality rates
of rats in previous studies (25–27).
Blood and tissue collection
Blood was collected from rat tail veins prior to and
4, 8, 12 and 16 weeks following surgery. FBG levels were measured
using a glucometer. Blood was then centrifuged at 2,000 × g for 10
min to separate the serum at room temperature. ELISA was then used
evaluated the levels of GLP-1 and GIP and an automatic biochemical
analyzer used to measure the levels of TG and TC (BS-450; Mindray,
Nanshan, Shenzhen, China). All rats were euthanized via
intraperitoneal administration of 150 mg/kg sodium pentobarbital
(Sigma-Aldrich; Merck KGaA) 16 weeks following surgery. Liver
tissue was collected and stored at −80°C.
ELISA
GLP-1 and GIP levels in the sera of rats were
evaluated by GLP-1 (cat. no. RA20061) and GIP (cat. no. RA20389)
ELISA kits obtained from Bio-Swamp Life Science (Wuhan, China) and
the assay was performed in accordance with the manufacturer's
protocols.
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from liver tissue using
TRIzol reagent (Takara Bio Inc., Dalian, China) and assessed using
an ultraviolet spectrophotometer and 1% agarose electrophoresis.
For each sample, 1 µg RNA was reverse transcribed to obtain
first-strand cDNA using the PrimeScript® RT reagent kit
with gDNA Eraser (Takara Bio, Inc.) following the manufacturer's
instructions. The reaction mixture (20 µl total volume) contained
10 µl 2X SYBR Premix Ex Taq™ (Takara Bio, Inc.), 0.5
µmol/l each primer and 0.2±0.02 µg cDNA template. The following
three-step qPCR reaction was performed: Pre-denaturation at 95°C
for 30 sec, followed by 40 cycles, including denaturation at 95°C
for 3 min and annealing at 60°C for 20 sec and elongation at 72°C
for 20 sec. The primes used were as follows: PEPCK forward,
5′-TCAAGTGCCTCCACTCCG-3′ and reverse, 5′-GAACAAGCCCGTGTAGTCCTT-3′;
G-6-Pase forward, 5′-GAAAGAATGAACGTGCTCC-3′ and reverse,
5′-CAGTATCCCAACCACAAGAC-3′; β-actin forward,
5′-AGAGGGAAATCGTGCGTGAC-3′ and reverse,
5′-CAATAGTGATGACCTGGCCGT-3′. The threshold cycle (Cq) was
determined for each reaction and the Cq values for each gene of
interest were normalized to that of β-actin, which acted as a
control. Levels of gene expression were then calculated using the
2−ΔΔCq method (28). For
each group, three samples were measured and three technical
replicates of each measurement were obtained.
Western blot analysis
Protein expression was analyzed by western blot
analysis. Tissue samples were washed with PBS and homogenized at
4°C in radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China) containing protease
inhibitor. Subsequently, tissues were centrifuged at 12,000 × g for
15 min at 4°C. Protein concentration was determined using a BCA kit
(Bio-Swamp Life Science). Equal amounts of protein (30 µg) were
separated by 10% SDS-polyacrylamide gel and then transferred onto a
PVDF membrane (EMD Millipore, Billerica, MA, USA). Membranes were
blocked for 2 h at room temperature with 5% skim milk in
Tris-buffered saline (20 mmol/l Tris, 500 mmol/l NaCl and 0.05%
Tween-20). Subsequently, the membrane was incubated with primary
antibodies against IRS-2 (cat. no. ab13410), Akt (cat. no. ab8805),
phosphorylated (p)-Akt (cat. no. ab38449) (all dilution 1:500) and
β-actin (cat. no. ab6276; dilution, 1:4,000) overnight at 4°C (all
Abcam, Cambridge, UK). β-actin was used as an internal reference.
Membranes were subsequently washed with Tris-buffered saline and
incubated with goat anti-rabbit secondary antibody conjugated to
horseradish peroxidase (cat. no. W4011; dilution, 1:3,000; Promega
Corporation, Madison, WI, USA) for 2 h at room temperature.
Immunoreactivity was visualized via a colorimetric reaction using
enhanced chemiluminscent substrate buffer (EMD Millipore).
Membranes were analyzed using a Gel Doc EZ imager and bans were
quantified using Quantity One 5.0 (Bio-Rad Laboratories, Hercules,
CA, USA).
Statistical analysis
SPSS 19.0 software (IBM Corp, Armonk, NY, USA) was
used for data analysis and statistical differences were detected
using one-way analysis of variance followed by Dunnett's post hoc
test. All values were expressed as the mean ± standard error mean.
Differences were considered to be statistically significant at
P<0.05.
Results
Surgical treatment regulates
hyperglycemia and decreases TC and TG levels in the blood of T2DM
rats
FGB, TC and TG were evaluated in each of the groups
prior to and 4, 8, 12 and 16 weeks following surgery. FBG, TC and
TG levels in all treatment groups were higher than those of the
control group prior to surgery (Fig.
1). However 16 weeks following surgery, FBG, TC and TG levels
in the RYGBP, IT and RYGBP+IT groups were significantly decreased
compared with the sham and DM groups. Notably, TC and TG levels in
the RYGBP+IT group were significantly lower than those in the RYGBP
and IT groups 16 weeks following surgery.
 | Figure 1.Levels of FBG, TC and TG of rats in
each group prior to and 4, 8, 12 and 16 weeks following surgery.
(A) Changes in FBG following surgery. (B) Changes in TC following
surgery. (C) Changes in TG following surgery. Data are presented as
the mean ± standard error of the mean (n=3); *P<0.05 and
**P<0.01 vs. CON; ##P<0.01 vs. SO;
▲P<0.05 and ▲▲P<0.01 vs. IT group. CON,
control group; DM, diabetes model group; SO, sham operation group;
RYGBP, RYGBP surgery group; IT, ileal transposition group;
RYGBP+IT, RYGBP combined with IT surgery group; RYGBP, Roux-en-Y
gastric bypass; FBG, fasting blood glucose; TC, total cholesterol;
TG, triglyceride. |
Surgical treatment decreases GIP and
GLP-1 levels in the serum of T2DM rats
GIP and GLP-1 levels in the serum of rats were
determined using ELISA (Fig. 2). GIP
levels in all rats with T2DM were increased significantly compared
with the control group prior to surgery. However, 16 weeks
following surgery, GIP levels in the RYGBP, IT and RYGBP+IT groups
were significantly lower than those of the DM and sham groups
(Fig. 2A). GLP-1 levels in the rats
with T2DM were significantly decreased compared with control group
prior to surgery. However, 16 weeks following surgery, GLP-1 levels
in the RYGBP, IT and RYGBP+IT groups were significantly higher than
those of the DM, sham and control groups (Fig. 2B). GLP-1 levels in the sham and DM
groups remained significantly lower than those of the control.
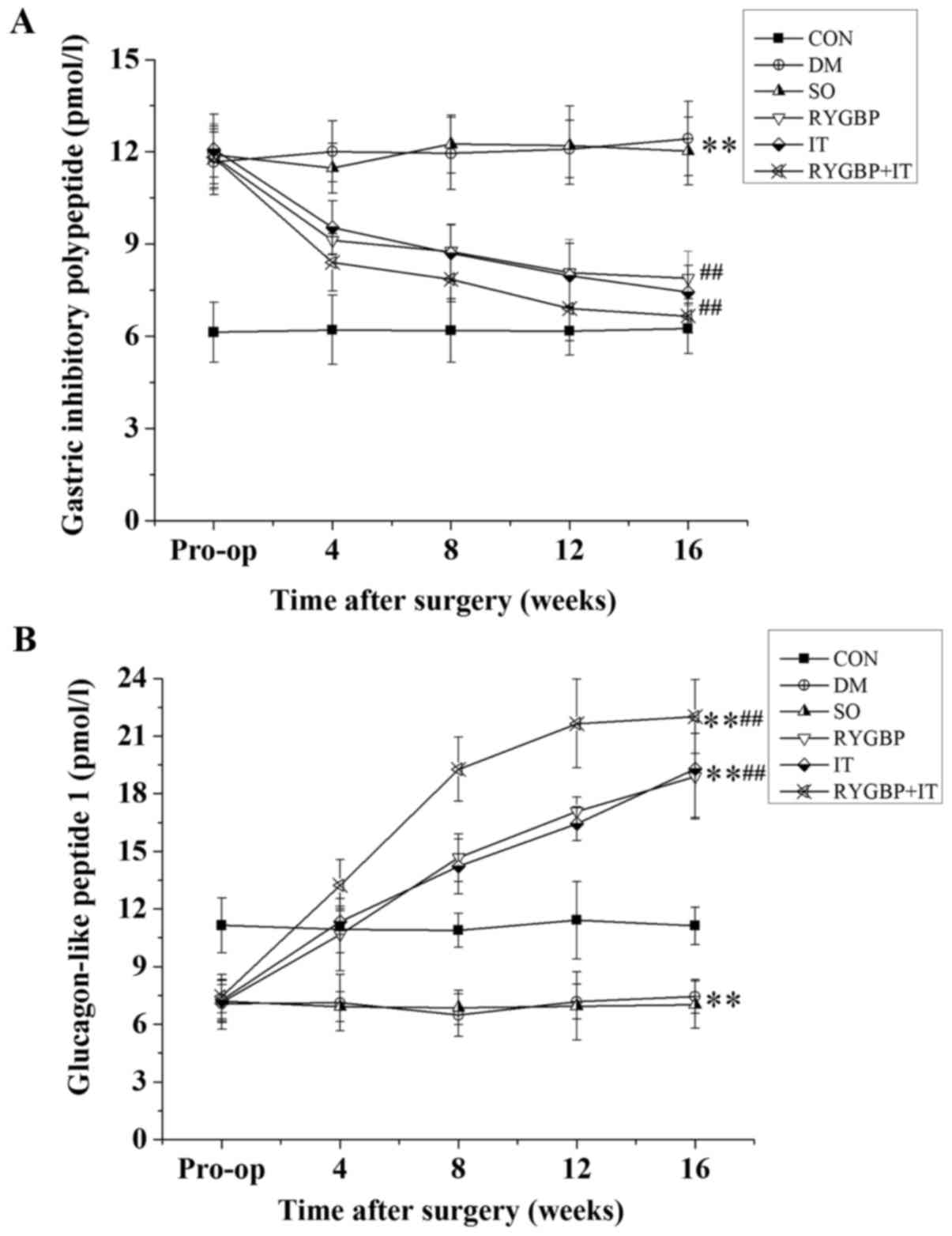 | Figure 2.GIP and GLP-1 levels in rats from
each group prior to and 4, 8, 12 and 16 weeks following surgery.
(A) Changes in GIP levels in rats following surgery. (B) Changes in
GLP-1 levels in rats following surgery. Data are presented as the
mean ± standard error of the mean (n=3); **P<0.01 vs. CON group,
##P<0.01 vs. SO group. CON, control group; DM,
diabetes model group; SO, sham operation group; RYGBP, RYGBP
surgery group; IT, ileal transposition group; RYGBP+IT, RYGBP
combined with IT surgery group; RYGBP, Roux-en-Y gastric bypass;
GIP, gastric inhibitory polypeptide; GLP-1, glucagon-like
peptide. |
Surgical treatment reduces the mRNA
expression levels of PEPCK and G-6-Pase in the liver of T2DM
rats
The mRNA expression levels of PEPCK and G-6-Pase in
the liver of rats were evaluated using RT-qPCR. Compared with the
control group, mRNA levels of PEPCK and G-6-Pase in the DM, sham,
RYGBP and IT groups were significantly increased (Fig. 3). However, compared with the sham
group, the mRNA levels of PEPCK and G-6-Pase in the RYGBP and IT
groups were decreased significantly. Furthermore, compared with the
IT group, the mRNA levels of PEPCK and G-6-Pase in the RYGBP+IT
group were significantly decreased and were similar to those of the
control group.
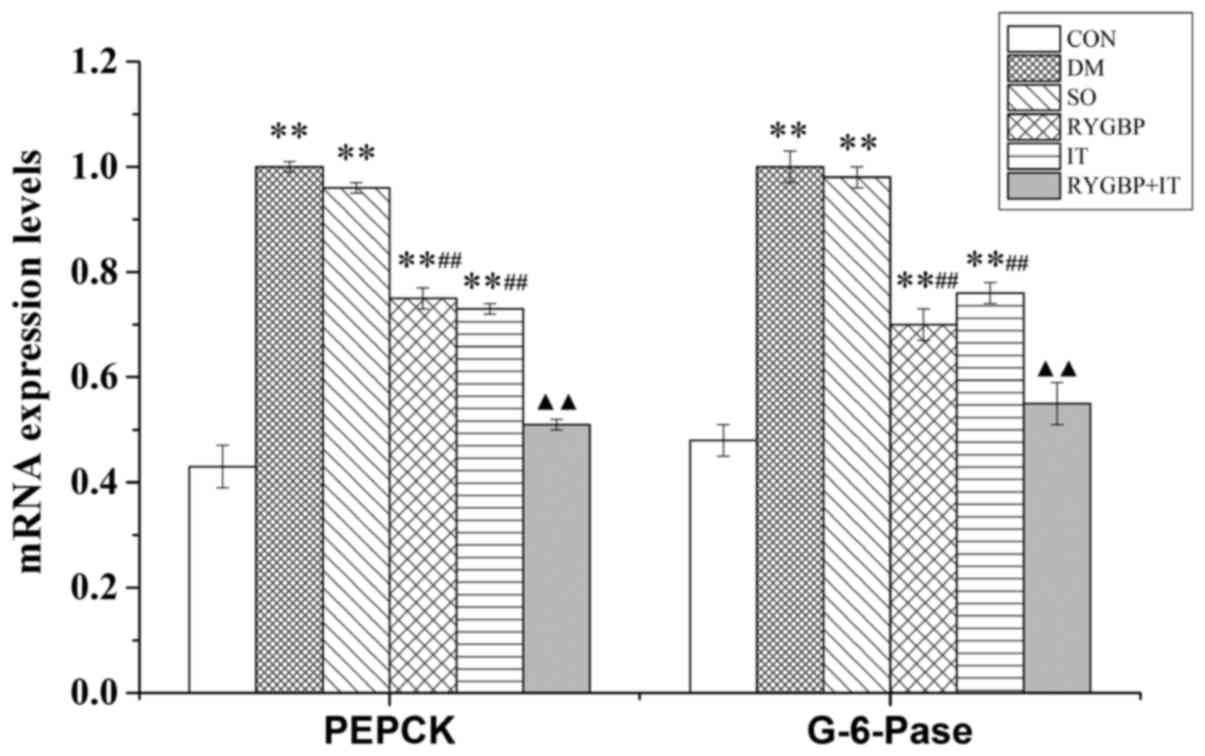 | Figure 3.The mRNA expression of PEPCK and
G-6-Pase of rats from each group 16 weeks following surgery. Data
are presented as the mean ± standard error of the mean (n=3).
**P<0.01 vs. CON group, ##P<0.01 vs. SO group,
▲▲P<0.01 vs. IT group. CON, control group; DM,
diabetes model group; SO, sham operation group; RYGBP, RYGBP
surgery group; IT, ileal transposition group; RYGBP+IT, RYGBP
combined with IT surgery group; PEPCK, phosphoenolpyruvate
carboxykinase; G-6-pase, Glucose-6-phosphatase. |
Surgical treatment reduces the protein
levels of IRS-2 and p-Akt in the liver of T2DM rats
Levels of IRS2, Akt and p-Akt protein in the liver
of rats were evaluated by western blotting (Fig. 4A). Compared with control group, the
expression of IRS-2 in the DM, sham, RYGBP and IT groups were
increased significantly (Fig. 4B).
However, compared with the DM group, IRS-2 expression in the RYGBP
and IT groups were significantly decreased. Furthermore, IRS-2
expression in the RYBGP+IT group was significantly lower than that
of the RYBGP and IT groups. p-Akt expression in the DM, sham, RYGBP
and IT groups was significantly lower than in the control group;
however, p-Akt expression in the RYGBP and IT groups was
significantly increased compared with the sham group. In addition,
p-Akt expression in the RYGBP+IT group was significantly higher
than that of the RYBGP or IT groups. Akt expression did not differ
significantly among any of the groups.
 | Figure 4.The expression of IRS-2, Akt and
p-Akt proteins in rats from each group 16 weeks following surgery.
(A) Representative western blots and (B) quantification of western
blotting results. Data are presented as the mean ± standard error
of the mean (n=3); *P<0.05 or **P<0.01 vs. CON group,
#P<0.05 vs. SO group, ▲P<0.05 or
▲▲P<0.01 vs. IT group. CON, control group; DM,
diabetes model group; SO, sham operation group; RYGBP, RYGBP
surgery group; IT, ileal transposition group; RYGBP+IT, RYGBP
combined with IT surgery group; RYGBP, Roux-en-Y gastric bypass;
IRS-2, insulin receptor substrate 2. |
Discussion
Diabetes is chronic metabolic disease characterized
by hyperglycemia that is induced by defects in insulin secretion or
activity (1). T2DM is the most
common type of clinical diabetes. At present, the pathogenesis of
T2DM has not been fully elucidated; however its primary clinical
features, which are insulin resistance and β cell dysfunction, have
been identified (29). Medication to
treat patients with diabetes aims to achieve glycemic control and
reduce the risk of associated complications arising; this differs
from the desired end-point following metabolic surgery, which is
euglycemia (30). It has been
demonstrated that metabolic surgery is able to effectively regulate
T2DM and obesity (31). Although
innovative procedures to treat diabetes are being investigated,
including the novel anti-diabetic drugs, DDP-4 inhibitor and SGLT-2
inhibitor, as well as surgical treatment by laparoscopic sleeve
gastrectomy, RYGBP and IT, their precise mechanisms of action
remain to be elucidated (32,33). In
the present study, the effects of three surgical treatments on a
rat model of T2DM were investigated. Levels of insulin
metabolism-related genes and proteins were used to assess the
effect of surgery on T2DM.
Buchwald et al (12) reported that RYGBP surgery can
regulate glycosylated hemoglobin and FBG in obese patients and may
also alleviate symptoms of the disease, including hypertension,
hyperlipidemia and gastroesophageal reflux. Furthermore, Pories
et al (11) demonstrated that
the treatment efficiency of RYGBP on T2DM may reach 99%. The
results of the present study demonstrated that RYGBP and IT
significantly decrease levels of FBG, TC and TG in rats with T2DM
and indicated that these decreases were more significant in rats
that underwent RYGBP combined with IT. GIP is a member of the
glucagon peptide superfamily, which is secreted by K cells that are
distributed throughout the proximal digestive tract. The main roles
of GIP are to promote the secretion of insulin, increase
sensitivity to insulin and induce secretion of GLP-1 (34). Previous studies have reported that
GIP receptor gene knockout reduces the efficacy of GIP and
therefore promotes insulin resistance, indicating that GIP may
serve an important role in the development of T2DM (35,36). The
results of the current study indicated that surgical treatment
significantly decreased GIP levels in rats with T2DM and also
reduced blood glucose levels. This indicates that the low secretion
of GIP caused by the digestion of food without the involvement of
the proximal small intestine may serve an important role in the
surgical treatment of T2DM.
GLP-1 is a core mediator in the intestine-islet axis
regulation of T2DM (37). RYGBP
surgery induces a decrease in FBG, which is accompanied by an
increase in GLP-1 and a decrease in insulin resistance (38). In the present study, GLP-1 levels in
T2DM rats were significantly decreased compared with the control
group. However, following surgical treatment, GLP-1 levels were
significantly increased and GLP-1 levels in the RYGBP+IT group were
significantly higher than in the RYGBP and IT groups. This
indicates that surgical treatment can induce GLP-1 release to exert
a hypoglycemic effect and the RYGBP+IT surgery is the most
effective method of achieving this. The results of the present
study are in accordance with a study by Patriti et al
(39), which reported that IT
surgery significantly improved glucose tolerance and reduced
insulin resistance in GK rats.
T2DM primarily manifests as abnormally elevated FBG,
caused by glucagon regulation of liver gluconeogenesis (40,41).
Research has demonstrated that glucagon is able to promote liver
gluconeogenesis by regulating the expression of key enzymes,
including PEPCK and G-6-Pase; dysregulation of these enzymes may
stimulate the development of glucose metabolism disorders (17). In the present study, PEPCK and
G-6-Pase mRNA levels were significantly increased in the liver of
rats with T2DM, in contrast to previous studies, which demonstrated
that the expression of G-6-Pase and PEPCK is not increased in rats
with T2DM (17,42). Notably, in the current study, PEPCK
and G-6-Pase mRNA levels decreased significantly in rats following
surgical treatment. Furthermore, PEPCK and G-6-Pase mRNA levels in
rats treated with RYGBP combined with IT were significantly lower
than in rats treated with RYGBP or IT alone. In G-6-Pase-deficient
mice, adenoviral rescue with the G-6-Pase gene suggests that even a
fraction of phosphatase activity allows mice to maintain normal
plasma glucose levels (43). This
suggests that surgical treatment may promote plasma glucose levels
by regulating PEPCK and G-6-Pase.
The current study also demonstrated that RYGBP
combined with IT treatment significantly decreased IRS-2 expression
and increased p-Akt expression in rats with T2DM. IRS-2 is highly
expressed in insulin sensitive tissues and serves a critical role
in insulin-signaling pathway (44).
At the molecular level, IRS-2 deficiency results in impaired
insulin-mediated Akt phosphorylation, despite intact IR and IRS-1
tyrosine phosphorylation responses (45). Akt activation may promote the
inhibitory effect of insulin resistance on T2DM by stimulating
glucose transfer, glycogen synthesis, fat deposition and protein
synthesis (46). IRS-2 knockout mice
exhibit characteristics of T2DM and a reduction in IRS-2
phosphorylation may also induce insulin resistance (47,48).
These results demonstrate that IRS-2 serves an important role in
the development of insulin resistance and T2DM. Previous studies
have also demonstrated that Akt activation is decreased in Lepr
(db/+) mice with spontaneous gestational diabetes mellitus, leading
to an increase in the expression G-6-Pase and the inhibition of
hepatic glycogen production in the offspring of these mice
(49,50). The results of the current study
indicate that RYGBP and IT surgical treatment decrease the
expression of PEPECK, G-6-Pase and IRS-2, and increase the
expression of p-Akt in the liver of T2DM rats. Furthermore, the
regulation of insulin signaling pathway related factors was most
effective in rats treated with RYGBP and IT. This indicates that
treatment with RYGBP and IT may stimulate T2DM remission by
regulating the insulin signaling pathway to inhibit insulin
resistance.
In conclusion, the present study used RYGBP combined
with IT surgery to treat mice with T2DM and the results
demonstrated that it more effectively promoted the remission of
T2DM compared with RYGBP or IT surgery alone. Additionally, RYGBP
combined with IT may mediate the insulin-signaling pathway by
regulating the expression of associated factors to induce T2DM
remission.
Acknowledgements
Not applicable.
Funding
The current study was supported by a grant from the
Health and Family Planning Commission of Wuhan, China (grant no.
WX11B12).
Availability of data and materials
All datasets used during the current study are
available from the corresponding author on reasonable request.
Author contributions
ZG designed the experiment, gave final approval of
the version to be published and was responsible for the acquisition
of funding. BW collected the blood and tissue samples, performed
the ELISA assay and critically revised the manuscript. XG analyzed
the data regarding blood index. CY measured the expression of
associated mRNA using RT-qPCR. DR detected the expression levels of
associated proteins using western blotting. LS contributed to the
design of the present study and was a major contributor in writing
the manuscript. YP analyzed the data regarding the expression of
associated mRNA and proteins. JL was involved in drafting the
manuscript and performed the surgical intervention for rats. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethical
Committee of the Fifth Hospital of Wuhan, Wuhan University (Wuhan,
China; ethical approval no. 20160311).
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Zhang P, Zhang H, Han X, Di J, Zhou Y, Li
K and Zheng QI: Effectiveness and safety of laparoscopic Roux-en-Y
gastric bypass for the treatment of type 2 diabetes mellitus. Exp
Ther Med. 11:827–831. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cunningham CM, Larzelere ED and Arar I:
Conventional microscopy vs. computer imagery in chiropractic
education. J Chiropr Educ. 22:138–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mocanu AO, Mulya A, Huang H, Dan O,
Shimizu H, Batayyah E, Brethauer SA, Dinischiotu A and Kirwan JP:
Effect of Roux-en-Y gastric bypass on the NLRP3 inflammasome in
adipose tissue from obese rats. PLoS One. 10:e01397642015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boza C, Muñoz R, Salinas J, Gamboa C,
Klaassen J, Escalona A, Pérez G, Ibañez L and Guzmán S: Safety and
efficacy of Roux-en-Y gastric bypass to treat type 2 diabetes
mellitus in non-severely obese patients. Obes Surg. 21:1330–1336.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gloy VL, Briel M, Bhatt DL, Kashyap SR,
Schauer PR, Mingrone G, Bucher HC and Nordmann AJ: Bariatric
surgery versus non-surgical treatment for obesity: A systematic
review and meta-analysis of randomised controlled trials. BMJ.
347:f59342013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jørgensen CH, Gislason GH, Andersson C,
Ahlehoff O, Charlot M, Schramm TK, Vaag A, Abildstrøm SZ,
Torp-Pedersen C and Hansen PR: Effects of oral glucose-lowering
drugs on long term outcomes in patients with diabetes mellitus
following myocardial infarction not treated with emergent
percutaneous coronary intervention-a retrospective nationwide
cohort study. Cardiovasc Diabetol. 9:542010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buchwald H and Williams SE: Bariatric
surgery worldwide 2003. Obes Surg. 14:1157–1164. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DePaula AL, Macedo AL, Mota BR and
Schraibman V: Laparoscopic ileal interposition associated to a
diverted sleeve gastrectomy is an effective operation for the
treatment of type 2 diabetes mellitus patients with BMI 21–29. Surg
Endosc. 23:1313–1320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pories WJ, Swanson MS, MacDonald KG, Long
SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal
JM, et al: Who would have thought it? An operation proves to be the
most effective therapy for adult-onset diabetes mellitus. Ann Surg.
222:339–352. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Buchwald H, Avidor Y, Braunwald E, Jensen
MD, Pories W, Fahrbach K and Schoelles K: Bariatric surgery: A
systematic review and meta-analysis. JAMA. 292:1724–1737. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schauer PR, Burguera B, Ikramuddin S,
Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R,
Barinas-Mitchel E, et al: Effect of laparoscopic Roux-en Y gastric
bypass on type 2 diabetes mellitus. Ann Surg. 238:467–485.
2003.PubMed/NCBI
|
|
14
|
Wang TT, Hu SY, Gao HD, Zhang GY, Liu CZ,
Feng JB and Frezza EE: Ileal transposition controls diabetes as
well as modified duodenal jejunal bypass with better lipid lowering
in a nonobese rat model of type II diabetes by increasing GLP-1.
Ann Surg. 247:968–975. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robinson SW, Dinulescu DM and Cone RD:
Genetic models of obesity and energy balance in the mouse. Annu Rev
Genet. 34:687–745. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Hou SS, Wang W, Yin M, Cheng N, Ge
LL, Yin JJ and Xu J: Hepatic phosphoenolpyruvate carboxykinase
expression after gastric bypass surgery in rats with type 2
diabetes mellitus. Genet Mol Res. 14:16938–16947. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samuel VT, Beddow SA, Iwasaki T, Zhang XM,
Chu X, Still CD, Gerhard GS and Shulman GI: Fasting hyperglycemia
is not associated with increased expression of PEPCK or G6Pc in
patients with type 2 diabetes. Proc Natl Acad Sci USA.
106:12121–12126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vander Kooi BT, Onuma H, Oeser JK, Svitek
CA, Allen SR, Vander Kooi CW, Chazin WJ and O'Brien RM: The
glucose-6-phosphatase catalytic subunit gene promoter contains both
positive and negative glucocorticoid response elements. Mol
Endocrinol. 19:3001–3022. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haber BA, Chin S, Chuang E, Buikhuisen W,
Naji A and Taub R: High levels of glucose-6-phosphatase gene and
protein expression reflect an adaptive response in proliferating
liver and diabetes. J Clin Invest. 95:832–841. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Z, Barrett EJ, Dalkin AC, Zwart AD and
Chou JY: Effect of acute diabetes on rat hepatic
glucose-6-phosphatase activity and its messenger RNA level. Biochem
Biophys Res Commun. 205:680–686. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sesti G, Federici M, Hribal ML, Lauro D,
Sbraccia P and Lauro R: Defects of the insulin receptor substrate
(IRS) system in human metabolic disorders. FASEB J. 15:2099–2111.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bjornholm M, Kawano Y, Lehtihet M and
Zierath JR: Insulin receptor substrate-1 phosphorylation and
phosphatidylinositol 3-kinase activity in skeletal muscle from
NIDDM subjects after in vivo insulin stimulation. Diabetes.
46:524–527. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gatmaitan P, Huang H, Talarico J,
Moustarah F, Kashyap S, Kirwan JP, Schauer PR and Brethauer SA:
Pancreatic islet isolation after gastric bypass in a rat model:
Technique and initial results for a promising research tool. Surg
Obes Relat Dis. 6:532–537. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stygar D, Sawczyn T, Skrzep-Poloczek B,
Karcz-Socha I, Doleżych B, Zawisza-Raszka A, Augustyniak M,
Żwirska-Korczala K and Karcz WK: Ileal transposition in rats
influenced glucose metabolism and HSP70 levels. Open Life Sci.
10:278–284. 2015.
|
|
25
|
Kodama Y, Zhao CM, Kulseng B and Chen D:
Eating behavior in rats subjected to vagotomy, sleeve gastrectomy,
and duodenal switc. J Gastrointest Surg. 14:1502–1510. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang S, Jiao YB, Wang Y, Zou ZD, Zhang
ZZ, Wang YB and Dai LJ: Long-term efficacy of gastric bypass on
non-obese type 2 diabetes mellitus in Goto-Kakizaki rat. Chin J
Clin. 5:2011.
|
|
27
|
Yin DP, Gao Q, Ma LL, Yan W, Williams PE,
McGuinness OP, Wasserman DH and Abumrad NN: Assessment of different
bariatric surgeries in the treatment of obesity and insulin
resistance in mice. Ann Surg. 254:73–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lupi R and Del Prato S: Beta-cell
apoptosis in type 2 diabetes: Quantitative and functional
consequences. Diabetes Metab. 34 Suppl 2:S56–S64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rubino F, Schauer PR, Kaplan LM and
Cummings DE: Metabolic surgery to treat type 2 diabetes: Clinical
outcomes and mechanisms of action. Annu Rev Med. 61:393–411. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schauer PR, Bhatt DL, Kirwan JP, Wolski K,
Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen
SE, et al: Bariatric surgery versus intensive medical therapy for
diabetes-3-year outcomes. N Engl J Med. 370:2002–2013. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cohen RV, Schiavon CA, Pinheiro JS, Correa
JL and Rubino F: Duodenal-jejunal bypass for the treatment of type
2 diabetes in patients with body mass index of 22–34
kg/m2: A report of 2 cases. Surg Obes Relat Dis.
3:195–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Müller-Stich BP, Senft JD, Warschkow R,
Kenngott HG, Billeter AT, Vit G, Helfert S, Diener MK, Fischer L,
Büchler MW and Nawroth PP: Surgical versus medical treatment of
type 2 diabetes mellitus in nonseverely obese patients: A
systematic review and meta-analysis. Ann Surg. 261:421–429. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vilsbøll T: On the role of the incretin
hormones GIP and GLP-1 in the pathogenesis of Type 2 diabetes
mellitus. Dan Med Bull. 51:364–370. 2004.PubMed/NCBI
|
|
35
|
Miyawaki K, Yamada Y, Ban N, Ihara Y,
Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, et al:
Inhibition of gastric inhibitory polypeptide signaling prevents
obesity. Nat Med. 8:738–742. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gault VA, Mcclean PL, Cassidy RS, Irwin N
and Flatt PR: Chemical gastric inhibitory polypeptide receptor
antagonism protects against obesity, insulin resistance, glucose
intolerance and associated disturbances in mice fed high-fat and
cafeteria diets. Diabetologia. 50:1752–1762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aylwin S: Gastrointestinal surgery and gut
hormones. Curr Opin Endocrinol Diabetes Obes. 12:89–98. 2005.
View Article : Google Scholar
|
|
38
|
Meirelles K, Ahmed T, Culnan DM, Lynch CJ,
Lang CH and Cooney RN: Mechanisms of glucose homeostasis after
Roux-en-Y gastric bypass surgery in the obese, insulin-resistant
Zucker rat. Ann Surg. 249:277–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Patriti A, Facchiano E, Annetti C, Aisa
MC, Galli F, Fanelli C and Donini A: Early improvement of glucose
tolerance after ileal transposition in a non-obese type 2 diabetes
rat model. Obes Surg. 15:1258–1264. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hancock AS, Du A, Liu J, Miller M and May
CL: Glucagon deficiency reduces hepatic glucose production and
improves glucose tolerance in adult mice. Mol Endocrinol.
24:1605–1614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jecht M: Leptintherapie bei
Typ-1-diabetes-bedingtem insulinmangel. Der Diabetologe. 6:214–216.
2010. View Article : Google Scholar
|
|
42
|
Muñoz MC, Barberà A, Domínguez J,
Fernàndezalvarez J, Gomis R and Guinovart JJ: Effects of tungstate,
a new potential oral antidiabetic agent, in Zucker diabetic fatty
rats. Diabetes. 50:131–138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zingone A, Hiraiwa H, Pan CJ, Lin B, Chen
H, Ward JM and Chou JY: Correction of glycogen storage disease type
1a in a mouse model by gene therapy. J Biol Chem. 275:828–832.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
White MF: IRS2 integrates insulin/IGF1
signalling with metabolism, neurodegeneration and longevity.
Diabetes Obes Metab. 16 Suppl 1:S4–S15. 2014. View Article : Google Scholar
|
|
45
|
Santamaria B, Marquez E, Lay A, Carew RM,
González-Rodríguez Á, Welsh GI, Ni L, Hale LJ, Ortiz A, Saleem MA,
et al: IRS2 and PTEN are key molecules in controlling insulin
sensitivity in podocytes. Biochim Biophys Acta. 1853:3224–3234.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Patti ME, Houten SM, Bianco AC, Bernier R,
Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC,
Pihlajamaki J, et al: Serum bile acids are higher in humans with
prior gastric bypass: Potential contribution to improved glucose
and lipid metabolism. Obesity (Silver Spring). 17:1671–1677. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Withers DJ, Gutierrez JS, Towery H, Burks
DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, et al:
Disruption of IRS-2 causes type 2 diabetes in mice. Nature.
391:900–904. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kubota N, Tobe K, Terauchi Y, Eto K,
Yamauchi T, Suzuki R, Tsubamoto Y, Komeda K, Nakano R, Miki H, et
al: Disruption of insulin receptor substrate 2 causes type 2
diabetes because of liver insulin resistance and lack of
compensatory beta-cell hyperplasia. Diabetes. 49:1880–1889. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shao J, Yamashita H, Qiao L and Friedman
JE: Decreased Akt kinase activity and insulin resistance in
C57BL/KsJ-Leprdb/db mice. J Endocrinol. 167:107–115. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yamashita H, Shao J, Qiao L, Pagliassotti
M and Friedman JE: Effect of spontaneous gestational diabetes on
fetal and postnatal hepatic insulin resistance in Lepr(db/+) mice.
Pediatr Res. 53:411–418. 2003. View Article : Google Scholar : PubMed/NCBI
|


















