Introduction
Nephrotic syndrome (NS) is one of the most common
kidney diseases in children and adults. Its pathology includes
proteinuria, with consequent hypoalbuminemia and generalized edema.
Proteinuria is considered to be caused by impaired glomerular
function. Podocytes (foot processes) represent an important
structure of the glomerular filtration membrane. Podocyte injury is
considered to be one of the most critical factors in the
development of proteinuria caused by glomerular filtration barrier
dysfunction and continuous injury may cause severe apoptosis,
leading to proteinuria, glomerulosclerosis and renal impairment;
the major reason is that podocytes are highly differentiated cells
with specific structural and biological functions, which are not
renewable after sustained injury (1,2). To
date, a large number of studies have indicated that podocyte
injury, loss and dysfunction have an important role in pathologies
including focal segmental glomerulosclerosis and membranous
nephropathy (3–5). Immunosuppressants (e.g., steroids or
cyclosporine A) have been used based on anecdotal evidence to treat
NS (6). Unless a contraindication
exists, glucocorticoids (GC) continue to be the first-line therapy
(7). However, chronic exposure to GC
results in significant toxicity, including obesity, growth
retardation, hypertension, poor bone health and cosmetic effects,
particularly for children. Therefore, novel therapeutic strategies
for NS are required.
Chinese medicinal herbs are considered to be a
promising source of potential treatments, as the large variety of
species contains pharmaceutically active components with broad
medicinal applications. Radix Astragali (Huangqi), the root of
Astragalus membranaceus Bunge, is widely used in Traditional
Chinese Medicine due to its anti-inflammatory, anti-oxidative,
immunoregulatory and neuroprotective activities (8,9), and is
also commonly used in the treatment of NS (10–12). The
principal bioactive components extracted from Radix Astragali are
known as astragalosides (AST). The results of a previous study
suggested that intravenous infusion of AST was safe and well
tolerated in healthy Chinese volunteers, and the adverse events,
including raised total bilirubin and rash, were mild and
spontaneously resolved (13). A
study by Wang et al (14)
confirmed that AST improved the expression of the glomerular
podocyte-associated proteins nephrin and podocin in rats with
adriamycin (ADR)-induced nephropathy. However, the effects of AST
on podocytes, their signaling transduction pathways and the
internal regulatory mechanisms require further study. ADR-induced
NS is a classical NS model, which was first reported by Bertani
et al (15). ADR induces
thinning of the glomerular endothelium and podocyte effacement
associated with the loss of the size- and charge-specific barrier
for the filtration of plasma proteins. Therefore, the present study
used this ADM-induced podocyte injury model, which was treated with
different doses of AST and oxidative stress, proliferation and
migration were assessed to explore its effect on injured podocytes,
as well as the underlying mechanisms.
Materials and methods
Instruments and materials
AST was purchased from Shanghai Jingdu Biotechnology
Co., Ltd. (Shanghai, China). The mouse podocyte clone 5 (MPC5) cell
line was obtained from Tong Pai Bio-tech Co., Ltd. (Shanghai,
China). ADR was purchased from Wuhan Xinwei Ye Chemical Co., Ltd.
(Wuhan, China). Hoechst 33342 staining solution and fluorescein
isothiocyanate (FITC)-labeled phalloidin was obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The reagent kits
for lactate dehydrogenase (LDH; cat. no. A020-1), malondialdehyde
(MDA; cat. no. A003-4) and superoxide dismutase (SOD; cat. no.
A001-1-1) were supplied by Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). The ELISA kits for matrix
metalloproteinase (MMP)-2 (cat. no. ml002275889) and MMP-9 (cat.
no. ml037717 were purchased from Shanghai Enzyme-linked
Biotechnology Co., Ltd. (Shanghai, China). In addition, a
SpectraMAX 190 microplate reader (Molecular Devices, Sunnyvale, CA,
USA), a Transwell chamber (Corning Inc., Corning, NY, USA; pore
size, 8 µm), an inverted microscope (Axio Observer A; Zeiss Inc.,
Wetzlar, Germany) and a laser-scanning confocal microscope (Olympus
FluoView™ 1000; Olympus Corp., Tokyo, Japan) were used in the
present study.
Cell culture and treatment
The MPC5 mouse podocytes (Tong Pai Bio-tech Co.,
Ltd, Shanghai, China) were recovered from freezing and maintained
in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% heat-inactivated fetal calf serum
(HyClone; GE Healthcare, Little Chalfont, UK). The MPC5 cells were
cultured and expanded by growth in a medium containing 10 U/ml
interferon-γ (Peprotech, Rocky Hill, NJ, USA) at 37°C with 100%
relative humidity and 5% CO2, When cells reached 70%
confluence, they were subcultured and treated in groups.
Grouping
MPC5 podocytes were divided into a control group
(complete medium), podocyte injury group (0.5 µmol/l ADR),
low-concentration AST treatment group (ASTL group; 0.5 µmol/l ADR +
50 µg/ml AST), medium-concentration AST treatment group (ASTM
group; 0.5 µmol/l ADR + 100 µg/ml AST) and high-concentration AST
treatment group (ASTH group; 0.5 µmol/l ADR + 200 µg/ml AST). Each
cell group was incubated at 37°C for 24 h, digested with pancreatin
(0.25%; Lanzhou Lihe Biotechnology Co., Ltd., Lanzhou, China) for
subculture (at 37°C for 24 h) and used for further
experimentation.
Cell proliferation assay
The differentiated and mature podocytes were seeded
into 96-well plates at a density of 2.0×103 per well.
After full adherence, cells were treated as aforementioned and each
experimental condition was performed in six wells. After 24 h of
incubation, MTT reagent (5 mg/ml, 20 µl) was added and the cells
were incubated for another 4 h. After the supernatant was
discarded, 150 µl dimethyl sulfoxide (DMSO) was added to each well,
followed by agitation for 15 min. The optical density of every well
at 570 nm (OD570) was read with an ELISA plate reader and the
survival rate was calculated according to the following formula:
Survival rate=OD570treatment group/OD570control
group ×100%. The experiment was repeated three times.
Detection of hypoxic damage and
oxidative stress indices
After the above mentioned treatments, cells were
washed three times with PBS, followed by radioimmunoprecipitation
assay buffer with repeated freezing and thawing. Subsequently, the
lysates were analysed with the LDH, MDA and SOD kits following the
manufacturer's instructions, and the OD was measured at 490, 532
and 550 nm, respectively, to calculate the production or activity
of LDH, MDA and SOD.
Observation of the cytoskeleton
Cells in each group were fixed with 4%
paraformaldehyde at room temperature for 10 min, washed once with
PBS, treated with 0.1% Triton X-100 for 10 min and blocked with 5%
bovine serum albumin (cat. no. A8010-10g; Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) for 30 min. After
washing twice with PBS, 10 µg/ml FITC-phalloidin was added to the
central cell surface, followed by incubation away from light at
37°C for 2 h and washing for three times with PBS for 5 min each
time. Hoechst 33342 staining solution was then added, followed by
incubation away from light at room temperature for 10 min.
Subsequent to washing for 3 times with PBS for 5 min each time, the
cells were mounted with anti-fade fluorescence mounting medium,
observed with the Olympus FluoView™ 1000 laser confocal scanning
microscope and images were captured.
Cell migration assay
Each group of MPC5 podocytes was pre-processed by
starvation treatment for 6 h, digested with 0.25% pancreatin
(Lanzhou Lihe Biotechnology Co., Ltd., Lanzhou, China) at room
temperature for 2 min, the cell density was adjusted and
2×105 cells were inoculated to each compartment of a
Transwell chamber. A total of 500 µl medium containing 10% serum
was added to the bottom wells of the 24-well plate and the plate
was cultured at 37°C for 12 h, followed by fixing with methanol at
4°C for 10 min and staining with 0.1% crystal violet for 30 min at
room temperature. The cells were observed and images were captured
under an inverted microscope after wiping off the cells in the
upper chamber with cotton swabs. Subsequently, the crystal violet
was extracted by rinsing each well with 33% acetic acid. The eluent
was added to a 96-well plate and the absorbance was read with a
microplate reader at a wavelength 570 nm, which indirectly reflects
the number of migrating cells.
ELISA
The levels of MMP-2 and MMP-9 in the supernatants of
the cells were determined by ELISA. The MPC5 culture medium was
collected and centrifuged at a speed of 13,000 × g for 15 min at
4°C to pelletize the debris. The levels of the two proteins were
determined according to the manufacturer's protocols for the kits.
The colorimetric reaction was measured at 450 nm.
Statistical analysis
The experiment was repeated at least three times and
values are expressed as the mean ± standard deviation. Statistical
analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY,
USA). A Student's t-test was used to compare the means between two
groups and comparisons between multiple groups were made by one-way
analysis of variance, after that a post-hoc Tukey's tests was
conducted. P<0.05 was considered to indicate a statistically
significant difference.
Results
AST improves the survival rate of
podocytes with ADR-induced injury
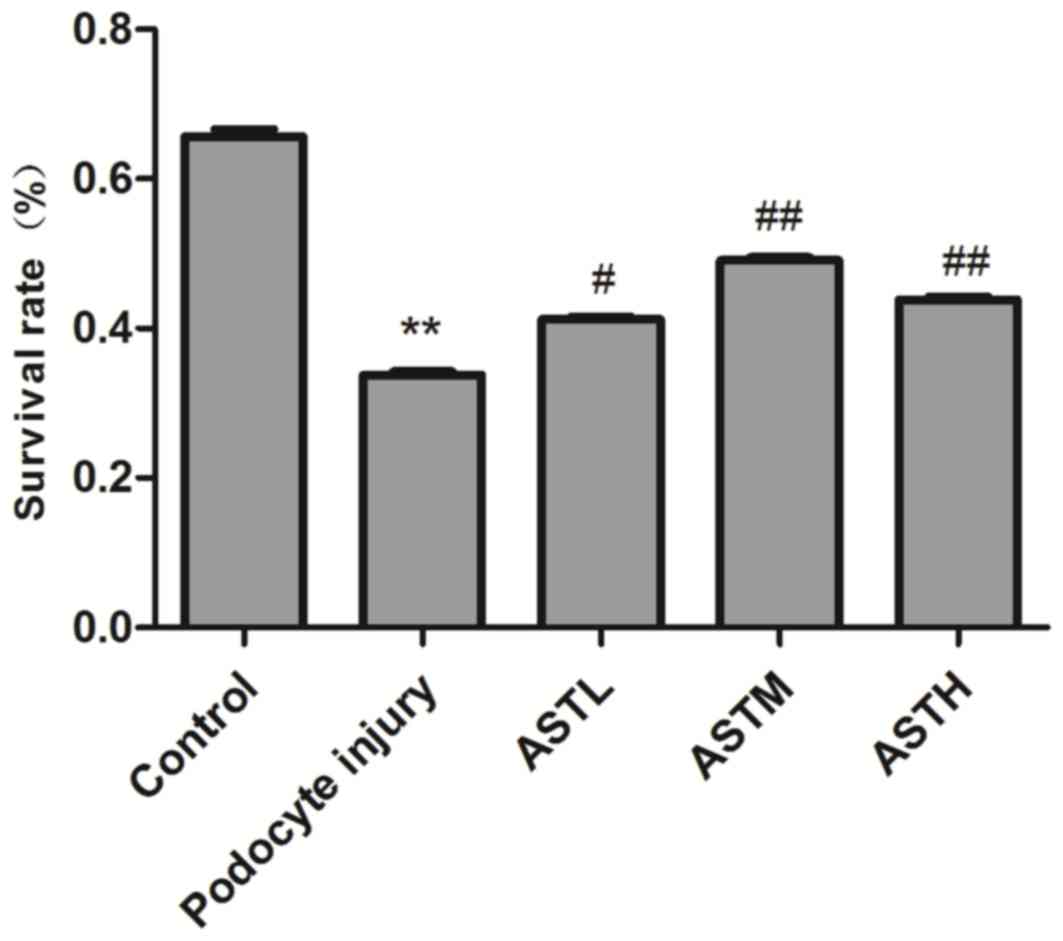
The results of the MTT assay indicated that the
survival rate of MPC5 podocytes was 52±2.4% after treatment with
0.5 µmol/l ADR for 24 h, which was significantly reduced compared
with that in the control group (P<0.01; Fig. 1). The survival rates in the ASTL,
ASTM and ASTH groups were 63±3.4, 75±3.2 and 67±3.0, respectively,
which were higher than those in the podocyte injury group
(P<0.05). However, no dose-dependent effect was observed among
the groups treated with different AST concentrations.
AST reduces the oxidative stress
levels of podocytes with ADR-induced injury
Quantification of LDH released from each group of
MPC5 podocytes with kits indicated that treatment of MPC5 cells
with 0.5 µmol/l ADR for 24 h resulted in a ~2-fold increase
compared with the control group (P<0.05). The low, medium and
high concentrations of AST reduced the ADR-induced increase of LDH
generation to 1.64±0.07-, 1.33±0.09- and 1.46±0.11-fold of that in
the control group, respectively, which was significantly lower than
that in the ADR injury group (P<0.05; Table I). Furthermore, compared with that in
the control group, the 24-h treatment of MPR5 podocytes with 0.5
µmol/l ADR significantly increased the relative content of MDA in
podocytes to 2.71±0.40-fold (P<0.05), while decreasing the SOD
activity to 0.78±0.01-fold (P<0.05). The low, medium and high
concentrations of AST inhibited the ADR-induced increase of
relative MDA generation to 2.06±0.23, 1.70±0.19 and 1.98±0.26,
respectively, which was significantly different from that in the
ADR injury group (P<0.05). Furthermore, AST at the low, medium
and high concentrations significantly increased the SOD activities
relative to those in the control group from 0.77±0.01 in the ADR
group to 0.86±0.03, 0.93±0.02 and 0.87±0.03, respectively
(P<0.05; Table I).
 | Table I.Levels of LDH, MDA and SOD in
podocytes in different groups. |
Table I.
Levels of LDH, MDA and SOD in
podocytes in different groups.
| Group | LDH | MDA | SOD |
|---|
| Control |
1.00±0.00 |
1.00±0.00 |
1.00±0.00 |
| Podocyte injury |
1.95±0.11a |
2.71±0.40a |
0.77±0.01a |
| ASTL |
1.64±0.07b |
2.06±0.21b |
0.86±0.03b |
| ASTM |
1.33±0.09c |
1.70±0.19c |
0.93±0.02c |
| ASTH |
1.46±0.11c |
1.96±0.21c |
0.89±0.03c |
AST attenuates ADR-induced decreases
in the migratory capacity of podocytes
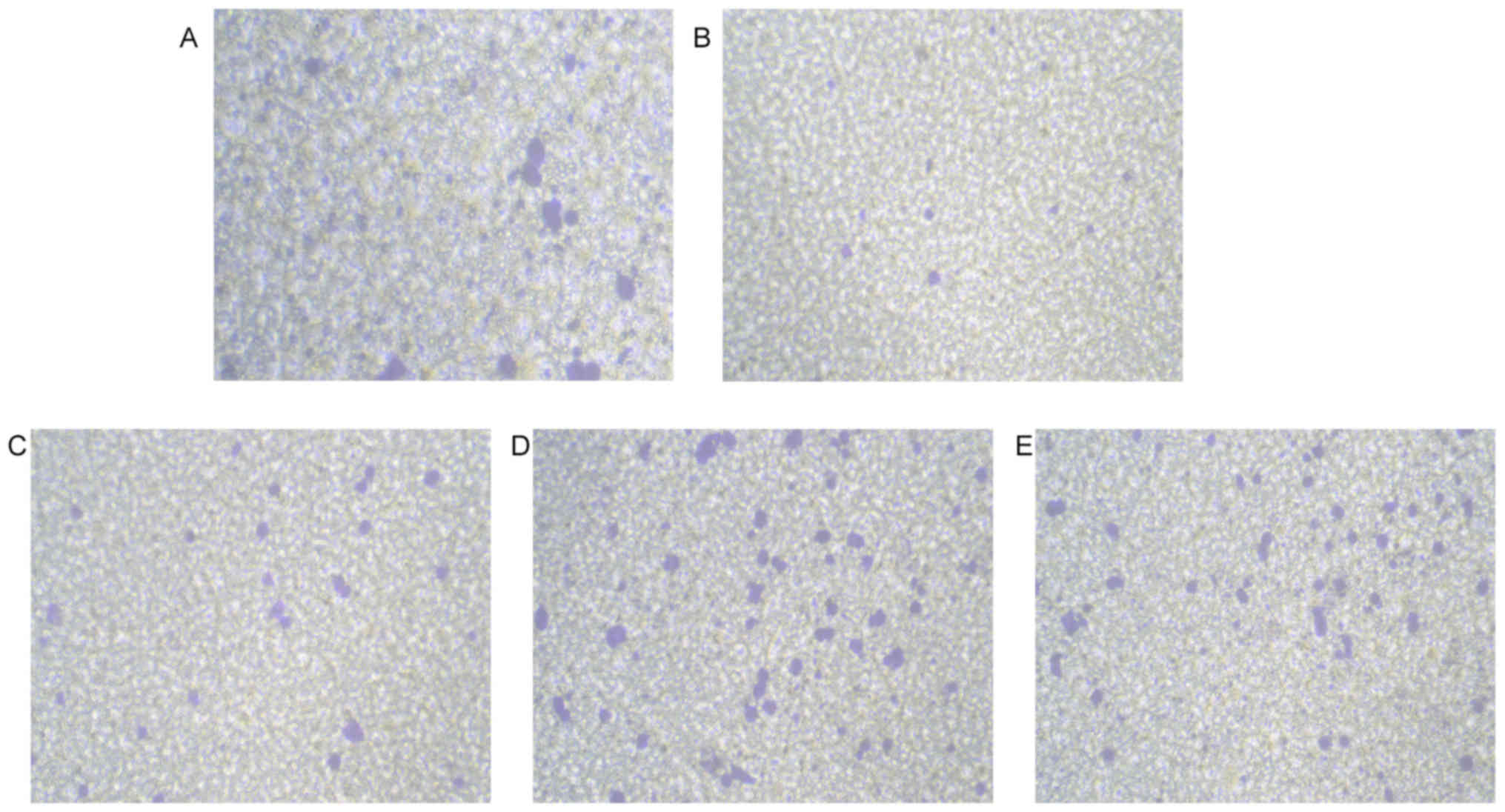
The results of the Transwell cell migration assay
indicated that compared with that in the control group, the
mobility of podocytes in each experimental group changed
significantly. The relative mobility of podocytes in the ADR injury
group was 35±2.1%, of that of the normal MPC5 cells (P<0.05).
AST at the low, medium and high concentration inhibited the
ADR-induced decline of podocyte migration ability (P<0.05 vs.
control group). However, no statistically significant difference
was present between the ASTM and ASTH groups (P>0.05; Figs. 2 and 3).
AST inhibits ADR-induced decreases of
MMP-2 and MMP-9 expression in podocytes
The results of the ELISA indicated that ADR
treatment significantly downregulated the protein expression of
MMP-2 and MMP-9 (P<0.05), while the low, medium and high
concentrations of AST significantly inhibited these declines in the
podocyte injury group (P<0.05; Table
II).
 | Table II.Expression levels of MMP2 and MMP9
proteins in different groups. |
Table II.
Expression levels of MMP2 and MMP9
proteins in different groups.
| Group | MMP-2 | MMP-9 |
|---|
| Control |
1.00±0.00 |
1.00±0.00 |
| Podocyte injury |
0.44±0.05a |
0.36±0.03a |
| ASTL |
0.56±0.04b |
0.52±0.02b |
| ASTM |
0.77±0.05c |
0.80±0.03c |
| ASTH |
0.78±0.07c |
0.73±0.03c |
Effects of AST on the cytoskeleton of
podocytes with ADR-induced injury
As presented in Fig.
4, after 24 h of incubation, the podocyte skeleton in the
control group exhibited bright green filaments, arranged in
parallel along the cell polarity with the cell microfilament
structure being intact and clear. The nuclei had regular and clear
shapes with uniform blue staining, and were located in the cell
center. However, in the podocytes in the ADR injury group, F-actin
was accumulated with irregular shapes, faint color and uneven
distribution. The microfilament structure was not intact, the
nuclear shape was irregular, the cytoplasm and nuclear areas
appeared non-specifically stained and apoptotic bodies and
micronuclei were visible. The podocyte microfilaments co-treated
with a low concentration of AST presented with clearly bundled
fibers without polar arrangement characteristics and were loose.
However, non-specific staining and apoptotic bodies in the
cytoplasm and nuclear areas were still observed. Compared with the
control group, the microfilament structure of podocytes co-treated
with the medium and high concentration of AST was not clear, but
appeared more intact than that in the ADR injury group and the ASTL
group.
Discussion
ADR is an antibiotic that is commonly used as an
anti-tumor drug in the clinic. However, the oxidation of
semiquinone free radical products formed by its metabolism in the
kidneys results in the generation of large amounts of reactive
oxygen species, which cause irreversible damage to the glomerular
ultrafiltration membrane (15). To
investigate this, the ADR-induced podocyte injury model is widely
used (16–20), as this method is simple and likely to
cause significant typical manifestations of podocyte injury and the
changes in cell morphology, viability and podocyte-specific
proteins conform to the typical manifestations of human glomerular
podocyte injury, which may therefore be used to study this
condition. In the present study, the experimental results indicated
that ADR significantly reduced the podocyte survival rate and
intervention with different concentrations of AST effectively
inhibited the reduction of the podocyte survival rate. Therefore,
AST protected podocytes from the damaging effect of ADR. However,
no dose-dependent effect was observed among the AST treatment
groups (ASTL, ASTM and ASTL), where the protective effect of the
medium dose AST against podocyte injury was highest.
The podocyte cytoskeleton mainly consists of
F-actin, the changes of which may lead to foot process effacement,
alter the cell phenotype and induce podocyte apoptosis (21). The results of the laser confocal
microscopic analysis performed in the present study indicated that
the structural integrity of the nucleus and cytoskeleton of
podocytes with ADR-induced injury was severely damaged. It was
indicated that the ADR-induced podocyte injury model was
successfully established in the present study. However,
intervention with the medium and high concentration of AST
maintained the structural integrity of the cytoskeleton. In
addition, compared with the cell migration ability in the podocyte
injury group, the migration rates of podocytes co-treated with the
medium and high concentration of AST were significantly higher,
which indicated that the podocytes treated by AST retained a better
cytoskeletal integrity. While it was demonstrated that treatment
with AST had a protective effect against podocyte injury, the
precise underlying mechanism remains elusive.
MMPs are a specific group of enzymes that perform
extracellular matrix degradation by zinc-dependent protein
hydrolysis. It has been indicated that MMPs, particularly MMP-2 and
MMP-9, have a key role in podocyte injury (22). Previous studies have also
demonstrated that ADR-induced inhibition of podocyte migration and
cell injury may be associated with the downregulation of MMP-2 and
MMP-9 at the mRNA and protein level (22,23),
which was consistent with the results of the present study.
Furthermore, the present study indicated that AST increased the
expression of MMP-2 and MMP-9 compared with that in the podocyte
injury group. It was demonstrated that the protective effect of AST
on the proliferation and migration ability of ADR-injured podocytes
may be associated with the upregulation of the expression of MMP-2
and MMP-9. In future studies, the molecular signaling of the MMP
pathway should be further assessed, which may reveal the underlying
mechanisms.
Oxidative stress has a key role in the pathogenesis
of various diseases. Antioxidants protect cells and tissues from
oxidative stress by scavenging free radicals and reactive oxygen
species. The reactive oxygen species generated during oxidative
stress-associated processes reduce the expression of α3β1, promote
lipid peroxidation and affect cellular signaling cascades, thereby
damaging podocytes (20). In the
present study, the cell damage index LDH and the oxidative stress
parameter MDA were significantly increased, while the activity of
SOD, an antioxidant index, was significantly decreased in
ADR-induced podocytes compared with those in the normal control
group. However, AST decreased LDH and MDA levels and increased SOD
activity compared with those in the podocyte injury group, and the
medium concentration of AST was most effective. Glomerular
podocytes are a highly differentiated end-stage cell type, whose
proliferation ability is limited and they are difficult to
regenerate once damaged. In the present study, intervention with
AST was likely associated with the reduction of the oxidative
stress response of podocytes.
In conclusion, co-treatment with AST maintained a
balance of the oxidative stress environment in MPC5 podocytes with
ADR injury to improve their migration ability and inhibit the
rearrangement and destruction of the cytoskeleton. As a possible
mechanism, AST inhibits ADR-induced decreases in the expression of
MMP-2, MMP-9 in order to protect against podocyte injury.
Acknowledgements
Not applicable.
Funding
This study was funded by the Gansu Provincial
Administration of Traditional Chinese Medicine Research (Gansu
Province Administration of Traditional Chinese Medicine, grant no.
G2K-2014-59).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
YPS and YCS designed the present study, analyzed the
data and wrote the manuscript; XXC and XL established the podocyte
injury model; JL and WJC assisted with technical performance and
contributed to writing the manuscript. All authors read and
approved the final manuscript. YPS and YCS contributed equally to
the present study as first authors. The final version of the
manuscript has been read and approved by all authors and each
author believes that the manuscript represents honest work.
Ethical approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NS
|
nephrotic syndrome
|
|
AST
|
astragalosides
|
|
ADR
|
adriamycin
|
|
MPC5
|
mouse podocyte clone 5
|
|
ASTL
|
low-concentration AST
|
|
ASTM
|
medium-concentration AST
|
|
ASTH
|
high-concentration AST
|
|
LDH
|
lactate dehydrogenase
|
|
MDA
|
malonaldehyde
|
|
SOD
|
superoxide dismutase
|
|
MMP
|
matrix metalloproteinase
|
|
MT1
|
metallothionein 1
|
References
|
1
|
Pavenstadt H, Kriz W and Kretzler M: Cell
biology of the glomerular podocyte. Physiol Rev. 83:253–307. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shankland SJ and Al'Douahji M: Cell cycle
regulatory proteins in glomerular disease. Exp Nephrol. 7:207–211.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu C, Xuan X, Che R, Ding G, Zhao M, Bai
M, Jia Z, Huang S and Zhang A: Dysfunction of the
PGC-1α-mitochondria axis confers adriamycin-induced podocyte
injury. Am J Physiol Renal Physiol. 306:F1410–F1417. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maezawa Y, Onay T, Scott RP, Keir LS,
Dimke H, Li C, Eremina V, Maezawa Y, Jeansson M, Shan J, et al:
Loss of the podocyte expressed transcription factor Tcf21/Pod1
results in podocyte differentiation defects and FSGS. J Am Soc
Nephrol. 25:2459–2470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanamura K, Tojo A and Fujita T: Urinary
and glomerular podocytes in patients with chronic kidney diseases.
Clin Exp Nephrol. 18:95–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takeda A, Ohgushi H, Niimura F and
Matsutani H: Long-term effects of immunosuppressants in
steroid-dependent nephrotic syndrome. Pediatr Nephrol. 12:746–750.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hogan J and Radhakrishnan J: The treatment
of minimal change disease in adults. J Am Soc Nephrol. 24:702–711.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shahzad M, Shabbir A, Wojcikowski K,
Wohlmuth H and Gobe GC: The Antioxidant effects of radix astragali
(astragalus membranaceus and related species) in protecting tissues
from injury and disease. Curr Drug Targets. 17:1331–1340. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu G, Song J, Guo Y, Wang T and Zhou Z:
Astragalus injection protects cerebral ischemic injury by
inhibiting neuronal apoptosis and the expression of JNK3 after
cerebral ischemic reperfusion in rats. Behav Brain Funct. 9:362013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng M, Yuan W, Zhang R, Fu P and Wu T:
Chinese herbal medicine Huangqi type formulations for nephrotic
syndrome. Cochrane Database Syst Rev. 6:Cd0063352013.
|
|
11
|
Korbet SM: Treatment of primary FSGS in
adults. J Am Soc Nephrol. 23:1769–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan W, Wang J and Wu T: Chinese herbal
medicine Huangqi type formulations for nephrotic syndrome. Cochrane
Database Syst Rev. 2:Cd0063352008.
|
|
13
|
Xu M, Yin J, Xie L, Zhang J, Zou C, Zou J,
Liu F, Ju W and Li P: Pharmacokinetics and tolerance of toal
astragalosides after intravenous infusion of astragalosides
injection in healthy Chinese volunteers. Phytomedicine.
20:1105–1111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang N, Wei RB, Li QP, Yang X and Chen X:
Protective effects of astragaloside in rats with adriamycin
nephropathy and underlying mechanism. Chin J Nat Med. 14:270–277.
2016.PubMed/NCBI
|
|
15
|
Bertani T, Poggi A, Pozzoni R, Delaini F,
Sacchi G, Thoua Y, Mecca G, Remuzzi G and Donati MB:
Adriamycin-induced nephrotic syndrome in rats: Sequence of
pathologic events. Lab Invest. 46:16–23. 1982.PubMed/NCBI
|
|
16
|
Yi M, Zhang L, Liu Y, Livingston MJ, Chen
JK, Nahman NS Jr, Liu F and Dong Z: Autophagy is activated to
protect against podocyte injury in adriamycin-induced nephropathy.
Am J Physiol Renal Physiol. 313:F74–F84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saito Y, Okamura M, Nakajima S, Hayakawa
K, Huang T, Yao J and Kitamura M: Suppression of nephrin expression
by TNF-alpha via interfering with the cAMP-retinoic acid receptor
pathway. Am J Physiol Renal Physiol. 298:1436–1444. 2010.
View Article : Google Scholar
|
|
18
|
Zhang HT, Wang WW, Ren LH, Zhao XX, Wang
ZH, Zhuang DL and Bai YN: The mTORC2/Akt/NFκB pathway-mediated
activation of TRPC6 participates in adriamycin-induced podocyte
apoptosis. Cell Physiol Biochem. 40:1079–1093. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang N, Wei RB, Li P, Li QP, Yang X, Yang
Y, Huang MJ, Wang R, Yin Z, Lv Y and Chen XM: Treatment with
irbesatan may improve slit diaphragm alterations in rats with
adriamycin-inducednephropathy. J Renin Angiotensin Aldosterone
Syst. 17:14703203166468842016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Susztak K, Raff AC, Schiffer M and
Böttinger EP: Glucose-induced reactive oxygen species cause
apoptosis of podocytes and podocyte depletion at the onset of
diabetic nephropathy. Diabetes. 55:225–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asanuma K, Yanagida-Asanuma E, Faul C,
Tomino Y, Kim K and Mundel P: Synaptopodin orchestrates actin
organization and cell motility via regulation of RhoA signaling.
Nat Cell Biol. 8:485–491. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei FY, Zhou TB, Qin YH, Chen XP and Li
ZY: Potential signal pathway of all-trans retinoic acid for MMP-2
and MMP-9 expression in injury podocyte induced by adriamycin. J
Recept Signal Transduct Res. 34:378–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Merkle M, Ribeiro A, Köppel S and Wörnle
M: TNF-α enhances TLR3-dependent effects on MMP-9 expression in
human mesangial cells. Cell Biol Int. 36:1155–1160. 2012.
View Article : Google Scholar : PubMed/NCBI
|