Introduction
Ulcerative colitis is a chronic nonspecific
inflammatory disease occurring in the colon and rectum,
specifically between the colon mucosa and submucosa (1). The causes of ulcerative colitis remain
unknown, but the pathogenesis of inflammatory ulcerative colitis
has been demonstrated to be associated with exogenous substances,
genetics and the immune response (2–4).
Symptoms of ulcerative colitis include diarrhea, stomachache,
hematochezia and weight loss and may lead to arthritis,
iridocyclitis, liver dysfunction and skin lesions (5–7).
Importantly, a systematic review and meta-analysis indicated that
patients with ulcerative colitis are at a higher risk of developing
colorectal cancer (8). Therefore,
efficient treatments for ulcerative colitis are crucial to patients
for the prevention of ulcerative colitis-induced complications.
Inflammatory responses are one of the most common
symptoms that patients with ulcerative colitis have (9). A previous study indicated that
inflammation and oxidative stress serve roles in the perpetuation
of the inflammatory process and subsequent DNA damage associated
with ulcerative colitis (10).
Okayasu (11) reviewed the mechanism
underlying the development of ulcerative colitis and ulcerative
colitis-associated carcinoma, referring mainly to summarized data,
revealing the importance of inflammation in the progression of
ulcerative colitis. Another study revealed the proteomic
inflammation profile of patients with ulcerative colitis, as
determined by a comparative analysis of inflamed and non-inflamed
colon biopsies (12). These studies
suggest that inhibition of inflammatory responses contributes to
clinical symptom remission in patients with ulcerative colitis.
Costus root is a type of traditional Chinese
medicine that has been regarded as a multifunctional drug for the
treatment of metabolic diseases (13,14). A
previous study demonstrated that Costus root had antinociceptive
and anti-inflammatory properties in experimental animals (15). In addition, the anti-inflammatory and
antipyretic properties of the rhizome of Costus root were also
demonstrated in carrageenan-induced paw edema and cotton
pellet-induced granuloma formation (16). Furthermore, Anyasor et al
(17) studied the properties of
Costus root in rat models of arthritis and the results indicated
that Costus root hexane leaf fractions possessed substantial
anti-inflammatory and antioxidant properties against inflammatory
diseases, particularly arthritis. These studies suggest that Costus
root may be beneficial as an anti-inflammatory agent to prevent the
progression of metabolism-associated diseases.
In the present study, a complex formula of Costus
root granules was produced and its therapeutic effects in a rat
model of ulcerative colitis were investigated. The extract of
Costus root, Ingredient dissolution into a traditional water
decoction (IDTWD), was used as the control to identify the
therapeutic effects of Costus root. Different analyses indicated
that Costus root granules exhibited the same results as IDTWD. The
molecular mechanism underlying the anti-inflammatory effect of
Costus root granule on colonic epithelial cells in experimental
rats was examined. It has been demonstrated that the
phosphoinositide 3-kinase/RAC-α serine/threonine-protein kinase
(PI3K/AKT) pathway is involved in the regulation and release of
pro-inflammatory cytokines, including tumor necrosis factor (TNF)-α
and serves important roles in the development and progression of
ulcerative colitis (18). The
findings of the present study suggest that Costus root granules
significantly ameliorate inflammation and apoptosis in the colonic
epithelium through regulation of transforming growth factor (TGF)-β
mediation of the PI3K/AKT signaling pathway. Furthermore, Costus
root granule treatment improved stomachache, hematochezia and aided
weight gain in rats with ulcerative colitis.
Materials and methods
Animal study
A total of 20 male Sprague Dawley
(specific-pathogen-free) rats (6 weeks old; weighing 280–320 g)
were purchased from Shanghai SLAC Experimental Animals Co., Ltd.
(Shanghai, China). All rats were housed under controlled
temperatures (23±2°C) and humidities (55±5%) in a 12 h light/dark
cycle with ad libitum access to food and water. An ulcerative
colitis rat model was generated by induction with
2,4,6-trinitrobenzene sulphonic acid (TNBS). TNBS (80 mg/kg) was
intrarectally administered to the rat colon. TNBS-induced
inflammation and alterations in colon morphology were observed,
with features similar to those identified in chronic inflammatory
diseases in humans. Rats were divided into three groups, which were
treated with Costus root, IDTWD (cat. no. 16022921) (both 1,000
mg/kg; Tianjin Red Sun Kang Rentang Pharmaceutical Sales Co., Ltd.,
Tianjin, China) or the same volume of PBS by gavage once daily. The
treatments continued for 30 days. Symptoms of ulcerative colitis in
rats were observed on days 0 and 30 as described previously
(19). The present study was
performed in accordance with the recommendations in the Guide for
the Care and Use of Laboratory Animals of China (20). All surgical procedures were approved
by the Committee on the Ethics of Tianjin Medical University
General Hospital (Tianjin, China).
Cell culture
Colonic epithelial cells were isolated from the rats
with ulcerative colitis according to a previously described method
(21). Colonic epithelial cells were
cultured in 5% CO2 at 37°C with Dulbecco's modified
Eagle's medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), penicillin and streptomycin
(100 µg/ml; Sigma-Aldrich; Merck KGaA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the colonic epithelial
cells using the RNeasy Mini kit (Qiagen Sciences, Inc.,
Gaithersburg, MD, USA) according to the manufacturer's protocol. A
total of 1 µg total RNA was reverse transcribed into cDNA using a
High-Capacity cDNA reverse transcription kit (Qiagen Sciences,
Inc.), according to the manufacturer's protocol and quality was
confirmed using electrophoresis. cDNA (10 ng) was subjected to qPCR
using a SYBR Green Master Mix system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), according to the manufacturer's protocol. The
expression levels of TNF-α and interleukin (IL)-1β, 6 and 10 in
colonic epithelial cells were measured by RT-qPCR with β-actin as
an endogenous control. All forward and reverse primers were
synthesized by Invitrogen (Thermo Fisher Scientific, Inc.; Table I). Following 120 sec incubation at
95°C, PCR was performed under the following conditions: 45 cycle
denaturation at 94°C for 30 sec, annealing at 56°C for 30 sec and
elongation at 72°C for 30 sec. Relative mRNA expression changes
were calculated using the 2−ΔΔCq method (22). The results are expressed as a fold
change compared with the control group.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction analysis.
|
| Primer sequence
(5′-3′) |
|---|
|
|
|
|---|
| Target gene | Forward | Reverse |
|---|
| TNF-α |
CTACTCCCAGGTTCTCTTCAA |
GCAGAGAGGAGGTTGACTTTC |
| IL-1β |
GCAACTGTTCCTGAACTCAACT |
ATCTTTTGGGGTCCGTCAACT |
| IL-6 |
TAGTCCTTCCTACCCCAATTTCC |
TTGGTCCTTAGCCACTCCTTC |
| IL-10 |
CACAAAGCAGCCTTGCAGAA |
AGAGCAGGCAGCATAGCAGT |
| β-actin |
GTGGGCGCCCAGGCACCA |
CTCCTTAATGTCACGCACGATTT |
Western blot analysis
Western blotting was performed as previously
described (23). Cells were
homogenized in lysis buffer containing a protease inhibitor to
perform protein extraction (Sigma-Aldrich; Merck KGaA), after which
cells were centrifuged at 6,000 × g at 4°C for 10 min. Protein
concentration was measured using a BCA protein assay kit (Thermo
Fisher Scientific, Inc.). Protein (10 µg) was separated using 12.5%
SDS-PAGE and transferred to polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA). Proteins were then blocked
with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 2 h at
37°C Monoclonal rabbit antibodies directed against apoptosis
regulator Bcl-2 (Bcl-2; cat. no. ab59348), Bcl-2-associated agonist
of cell death (BAD; cat. no. ab32445), cleaved caspase-3 (cat. no.
ab2302), cellular tumor antigen p53 (p53; cat. no. ab1431), AKT
(cat. no. ab8805), PI3K (cat. no. ab40776), TNF-α (cat. no. ab6671)
and IL-1β (cat. no. ab9722), IL-6 (cat. no. ab9324) and IL-10 (cat.
no. ab33471) (all 1:200; Abcam, Cambridge, UK) were incubated with
the protein samples for 1 h at room temperature. This was followed
by incubation with horseradish peroxidase (HRP)-conjugated
polyclonal anti-rabbit Immunoglobulin G antibodies (IgG; 1:10,000;
cat. no. PI-9400; Vector Laboratories, Inc., Burlingame, CA, USA)
for 1 h at room temperature. Immunoreactive bands were visualized
by enhanced chemiluminescence (substrate ECL Select™ Ventana
Benchmark automated staining system; Sigma-Aldrich; Merck KGaA).
The density of bands was analyzed using Quantity One software
version 4.62 (Bio-Rad Laboratories, Inc.).
Immunohistochemical staining
The colonic tissues were fixed using 10% formalin
solution for 12 h at 4°C. Immunohistochemical staining was
performed using an avidin-biotin-peroxidase-technique on colonic
tissues obtained from the rats on day 30. Paraffin embedded tissue
sections 4-µm-thick were prepared and epitope retrieval was
performed for further analysis. The paraffin sections were
incubated with hydrogen peroxide (3%) for 10–15 min at 37°C and
were subsequently blocked with a regular blocking solution (5% skim
milk powder) for 10–15 min at 37°C. Sections were incubated with
anti-Annexin antibodies (1:2,000; cat. no. ab14196; Abcam) at 4°C
for 12 h following blocking. All sections were washed three times
with PBS at 37°C for 5 min and incubated with HRP-conjugated goat
anti-rabbit IgG monoclonal antibodies (1:2000; cat. no. 1706515;
Bio-Rad Laboratories, Inc.) for 1 h at 37°C and were counterstained
with hematoxylin or DAPI for 1 h at 37°C. Images were captured
using a fluorescent microscope (Olympus BX51; Olympus Corp., Tokyo,
Japan) at magnification, ×400.
Apoptosis assays
A terminal deoxynucleotidyl transferase-mediated
dUTP nick end labeling (TUNEL) assay was used to analyze the level
of apoptosis of colonic epithelial cells. Colonic epithelial cells
were incubated with CoCl2 for 4 h and then placed on
glass coverslips. Subsequently, colonic epithelial cells were fixed
in 4% paraformaldehyde for 1 h at 37°C and washed with PBS for 5
min at room temperature. Cells were then incubated with DAPI or
TUNEL stain for 30 min at 37°C using the In Situ Cell Death
Detection kit, Fluorescein (Roche Applied Science, Penzberg,
Germany) according to the manufacturer's protocol. Cells were
counted in ≥3 randomly selected fields of view using a fluorescent
microscope (Olympus BX51) and Olympus Stream Image Analysis
software (version 1.0; Olympus Corp.).
PI3K and AKT activity assay
Colonic epithelial cells were homogenized and then
PI3K and AKT activity was analyzed. PI3K and AKT activity was
measured using the PI3K and AKT Fluorimetric Drug Discovery kit
(Enzo Life Sciences, Inc., Farmingdale, NY, USA), according to the
manufacturer's protocol. The fluorescent intensity was analyzed
using the DTX 880 Multimode plate reader (Beckman Coulter, Inc.,
Brea, CA, USA).
Gene knockdown with small interfering
RNA (siRNA)
To silence TGF-β gene expression, colonic epithelial
cells were transfected with 100 pmol of siRNA-TGF-β (cat. no.
1002634), using siRNA-vector (cat. no. 0000110) as the control
(each Applied Biosystems; Thermo Fisher Scientific, Inc.). The
transfection was achieved by using the Cell Line
Nucleofector® kit L (Lonza Group, Ltd., Basel,
Switzerland) according to the manufacturer's protocol. Cells were
used for further analysis 72 h following transfection.
Statistical analysis
All data are presented as the mean ± standard
deviation of experiments performed in triplicate. Statistical
analysis was completed using SPSS 19.0 statistical software (IBM
Corp., Armonk, NY, USA). Statistical differences between the groups
were assessed using one-way analysis of variance followed by a post
hoc Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Costus root granule treatment
regulates inflammatory cytokine production in colonic epithelial
cells in rats with ulcerative colitis
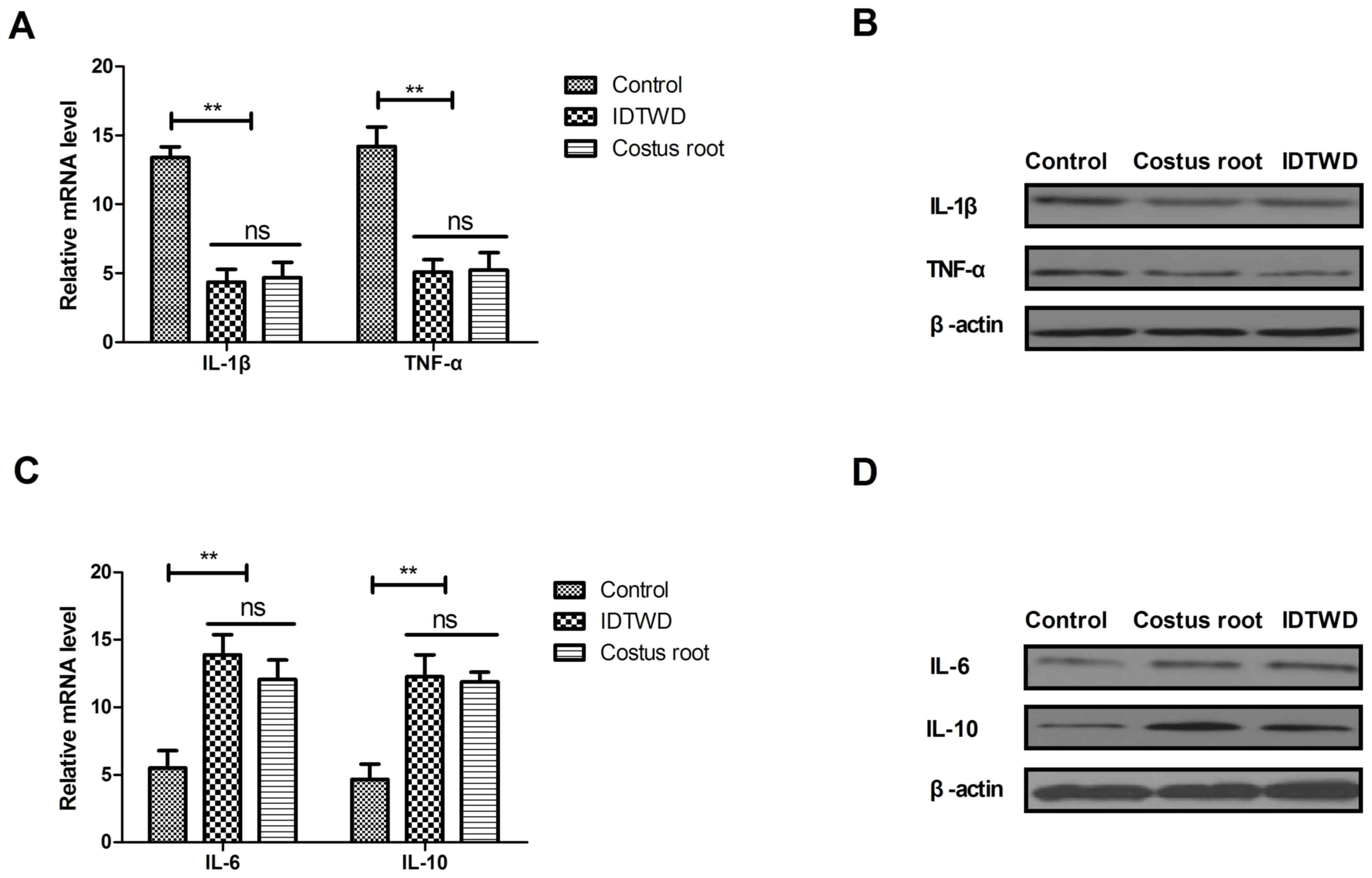
The mRNA expression levels of IL-1β and TNF-α were
significantly downregulated by Costus root granules and IDTWD in
colonic epithelial cells in the experimental groups compared with
the control group (Fig. 1A;
P<0.01). A marked decrease in IL-1β and TNF-α protein expression
was also observed (Fig. 1B). The
mRNA expression levels of IL-6 and IL-10 were significantly
upregulated by Costus root granules and IDTWD in colonic epithelial
cells in the experimental groups compared with the control group
(Fig. 1C; P<0.01). A marked
increase in IL-6 and IL-10 protein expression was also observed
(Fig. 1D). However, no significant
differences in mRNA or protein expression were identified between
the Costus root granule and IDTWD groups. These results suggest
that Costus root granule treatment regulates inflammatory cytokine
production in colonic epithelial cells in rats with ulcerative
colitis.
Costus root granule treatment inhibits
the apoptosis of colonic epithelial cells in rats with ulcerative
colitis
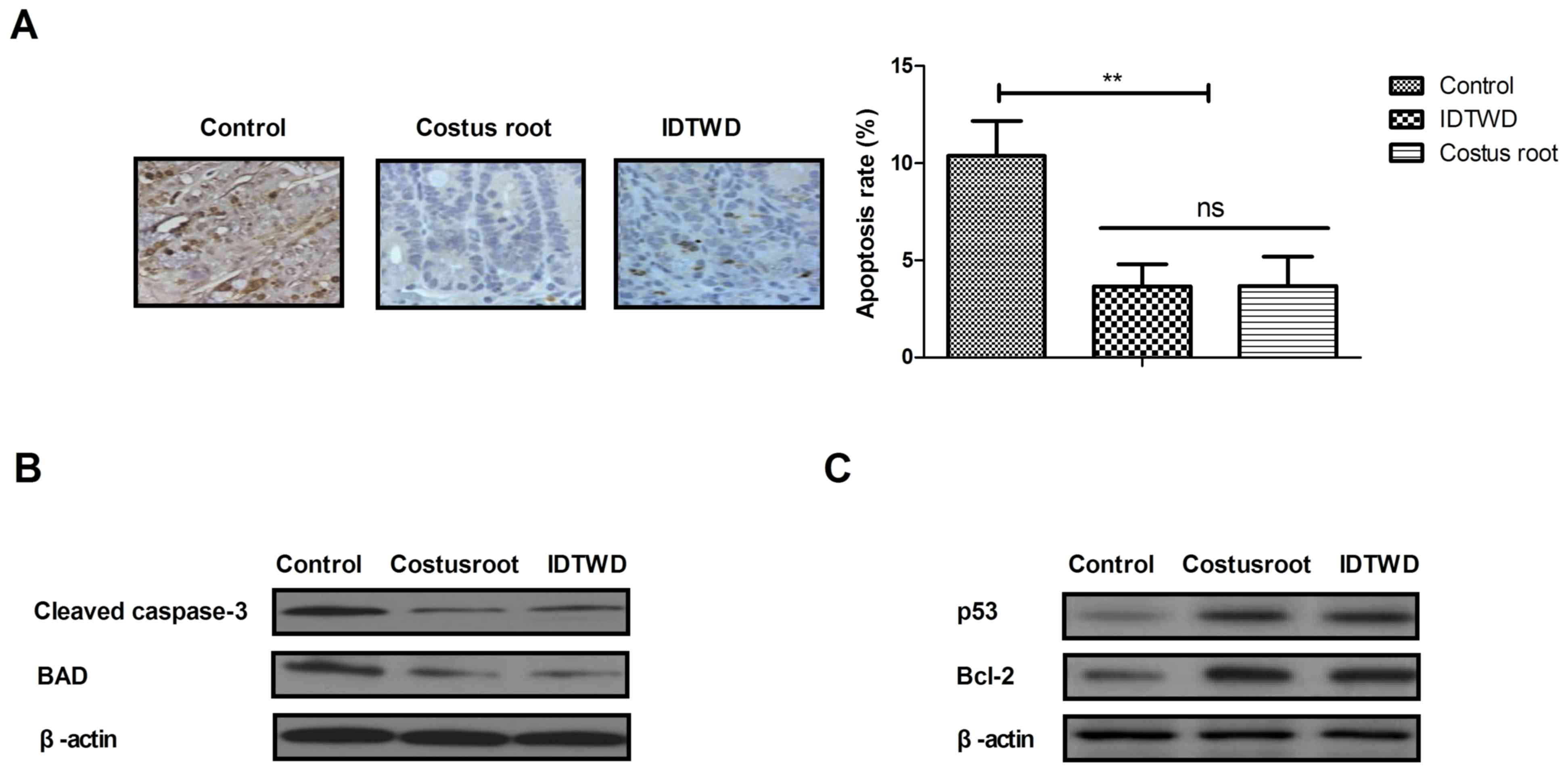
Ulcerative colitis often leads to the apoptosis of
colonic epithelial cells. Immunohistochemical analysis of colonic
epithelial tissues demonstrated that Costus root granules and IDTWD
decreased the level apoptosis (Fig.
2A). In the colonic epithelium of rats with ulcerative colitis,
the level of apoptosis significantly decreased with Costus root
granule and IDTWD treatments compared with the control group
(Fig. 2B; P<0.01). Western blot
analysis demonstrated that Costus root granule and IDTWD
administration downregulated cleaved caspase-3 and BAD in colonic
epithelial cells in the experimental groups (Fig. 2C). Anti-apoptotic protein (p53 and
Bcl-2) expression levels were upregulated by Costus root granule
and IDTWD administration in colonic epithelial cells in the
experimental groups (Fig. 2C). These
results indicate that Costus root granule treatment inhibits the
apoptosis of colonic epithelial cells in rats with ulcerative
colitis.
Costus root granules improve the
symptoms of ulcerative colitis in a rat model
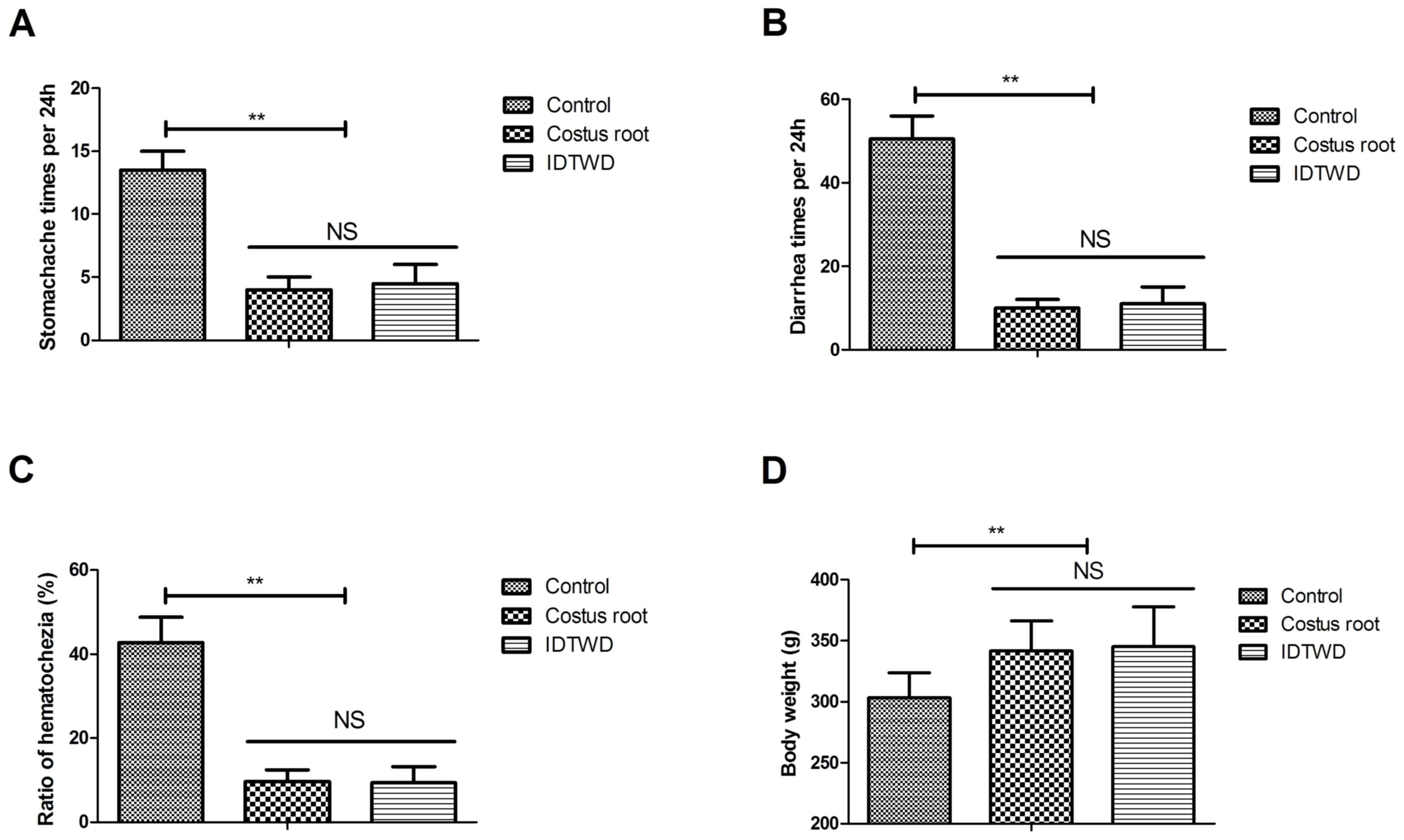
Hematochezia results were collected from all stool
samples in the 24 h period. The efficacy of Costus root granules
was analyzed by measuring preclinical symptoms. Costus root
granules and IDTWD significantly ameliorated stomachache (Fig. 3A; P<0.01), diarrhea (Fig. 3B) and hematochezia (Fig. 3C) in the experimental groups compared
with the control group. Notably, Costus root granule and IDTWD
treatments significantly increased the body weights of rats in the
experimental groups compared with the control group (Fig. 3D; P<0.01). These results suggest
that Costus root granules can be beneficial in the treatment of
ulcerative colitis.
Costus root granules regulate
inflammation through increasing TGF-β-mediated PI3K/AKT
signaling
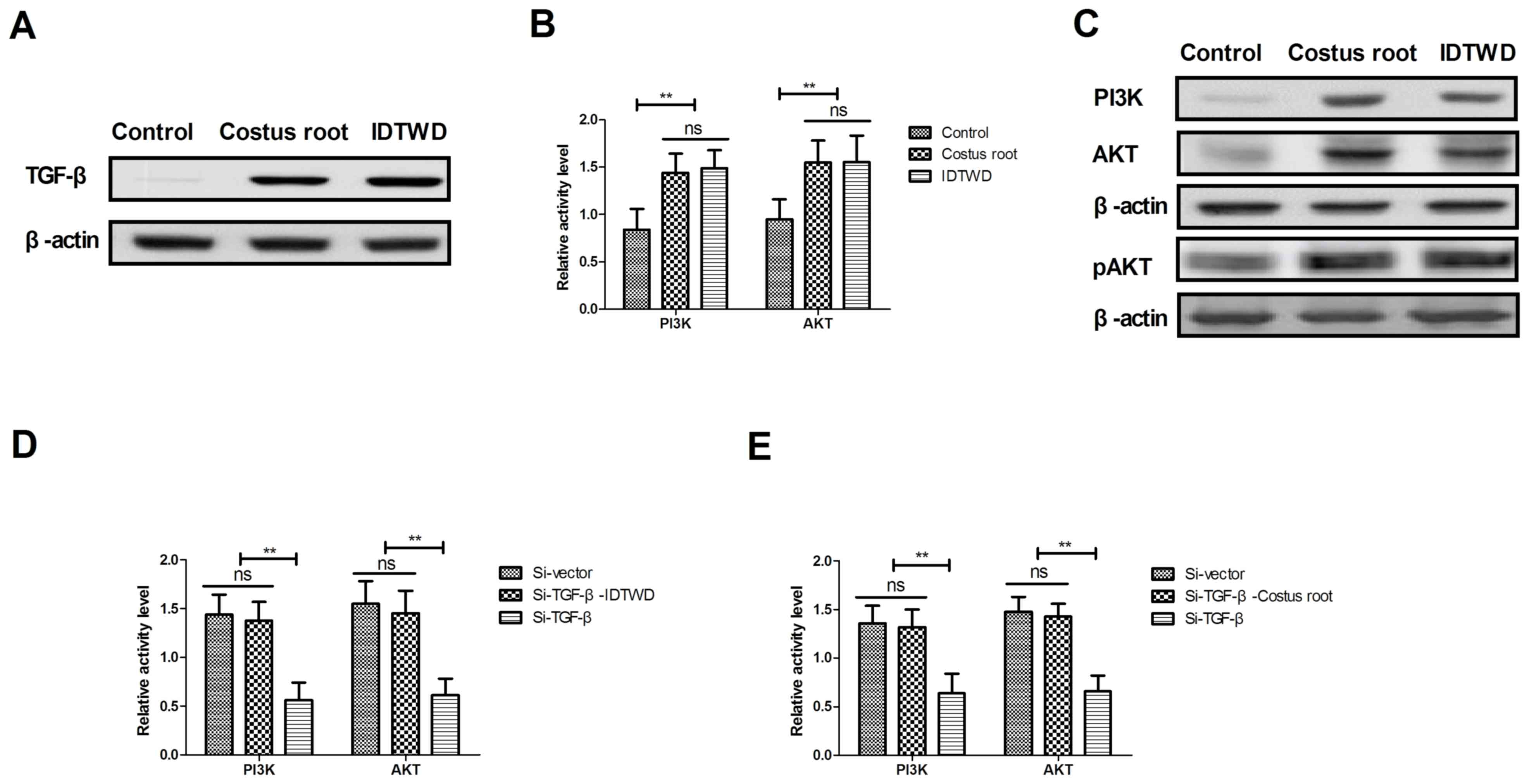
In order to understand the potential molecular
mechanism of Costus root granule-mediated improvement of ulcerative
colitis, TGF-β-mediation of the PI3K/AKT signaling pathway was
studied in colonic epithelial cells isolated from rats. TGF-β
expression levels increased in colonic epithelial cells following
Costus root granule and IDTWD treatments compared with the control
group (Fig. 4A). The activity levels
of PI3K and AKT significantly increased with Costus root granule
and IDTWD treatments in colonic epithelial cells compared with the
control group (Fig. 4B; P<0.01).
Similar expression levels of the corresponding proteins were
identified (Fig. 4C). In
vitro assays revealed that TGF-β knockdown significantly
inhibited IDTWD (Fig. 4D; P<0.01)
and Costus root granule (Fig. 4E)
promoted activity of PI3K and AKT in colonic epithelial cells
compared with the si-vector group.
TGF-β knockdown also inhibited PI3K and AKT
expression; however, with IDTWD (Fig.
4F) or Costus root granule (Fig.
4G) treatment PI3K and AKT expression increased. TGF-β
knockdown also increased TNF-α and IL-1β expression and decreased
IL-6 and IL-10 expression, which also canceled Costus
root-(Fig. 4H) and IDTWD-regulated
(Fig. 4I) TNF-α, IL-1β IL-6 and
IL-10 expression. No marked differences in protein expression were
identified between colonic epithelial cells treated with Costus
root granules and the colonic epithelial cells in the control
group. Taken together, these results suggest that Costus root
granules regulate inflammation through regulation of TGF-β
mediation of the PI3K/AKT signaling pathway in colonic epithelial
cells isolated from a rat model of ulcerative colitis.
Discussion
Ulcerative colitis is a condition that may result in
toxic colonic dilatation, intestinal perforation, intestinal
hemorrhage, polyps and colorectal carcinoma (24). The severity of inflammation increases
the risk of ileo-anal anastomotic leak following a pouch procedure
in patients with ulcerative colitis (25,26). In
recent years, the efficacy of traditional Chinese medicines for the
treatment of digestive tract diseases has attracted global interest
(27,28). Costus root is a type of feverfew that
exhibits therapeutic effects on gastrointestinal diseases, liver
metabolic disorders and hypertension (29,30). In
the present study, a complex formula of Costus root granules was
produced and the therapeutic efficacy of the formula was tested on
a rat model of ulcerative colitis. The findings suggest that the
Costus root granule formula exhibits the same anti-inflammatory and
antiapoptotic efficacies as IDTWD in colonic epithelial cells
isolated from rats with ulcerative colitis.
Inflammation serves an important role in the
progression of ulcerative colitis (31,32).
Cuković-Cavka et al (33)
investigated the role of anti-TNF therapy in the treatment of
ulcerative colitis and the outcomes indicated that adequate
long-term maintenance therapy with anti-TNF drugs is beneficial to
patients with ulcerative colitis. IL-1β gene polymorphisms are
associated with genetic susceptibility and steroid dependence in
patients with ulcerative colitis (34). IL-10 has been identified to be
differentially expressed in the small intestine and chronically
inflamed colon of young pigs with dextran sodium sulfate-induced
ulcerative colitis (35). Bernardo
et al (36) demonstrated that
IL-6 promoted immune responses in inflamed areas in patients with
ulcerative colitis, which lead to the induction of a skin-homing
phenotype in dendritic and T cells. In the present study, Costus
root granules downregulated TNF-α and IL-1β expression and
upregulated IL-6 and IL-10 expression, in colonic epithelial cells
isolated from the rat model of ulcerative colitis, indicating an
inhibition of apoptosis.
The apoptosis of colonic epithelial cells has been
observed in patients with ulcerative colitis (37). The present study observed that Costus
root granule treatment suppressed the apoptosis of colonic
epithelial cells by upregulating p53 and Bcl-2 expression. A
previous study also revealed that expression of the TGF-β/mothers
against decapentaplegic homolog (SMAD) signaling pathway was
downregulated in patients with ulcerative colitis; this was
analyzed by pathological and quantitative analyses of TGF-β/SMAD by
immunohistochemistry (38).
Additionally, the PI3K/AKT signaling pathway has been reported to
be involved in the pathogenesis of ulcerative colitis (18). The results of the present study
demonstrated that Costus root granules could inhibit inflammation
through TGF-β-mediation of the PI3K/AKT signaling pathway in
colonic epithelial cells.
In conclusion, the findings of the present study
indicate that Costus root granules inhibit inflammation and the
apoptosis of colonic epithelial cells and improve the symptoms of
ulcerative colitis in rats. Importantly, analyses of potential
mechanisms demonstrated that Costus root granules may inhibit
inflammation through regulated of TGF-β mediation of the PI3K/AKT
signaling pathway in colonic epithelial cells, which may lead to
improvements in stomachache, diarrhea, hematochezia and weight
gain. These results suggest that Costus root granules are an
efficient treatment for patients with ulcerative colitis.
Acknowledgements
The present study was supported by the Tianjin
Science and Technology Correspondent Project (grant no.
16JCTPJC50000).
Competing interests
The authors declare thay they have no competing
interests.
References
|
1
|
Aung PP, Bowker B, Masterpol KS and
Mahalingam M: Disseminated noninterstitial granulomatous dermatitis
as a cutaneous manifestation of the preleukemic state in a patient
with myelodysplasia and ulcerative colitis-apropos a case and
review of the literature. Am J Dermatopathol. 36:e117–e120. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mosli MH, Feagan BG, Sandborn WJ, D'haens
G, Behling C, Kaplan K, Driman DK, Shackelton LM, Baker KA,
Macdonald JK, et al: Histologic evaluation of ulcerative colitis: A
systematic review of disease activity indices. Inflamm Bowel Dis.
20:564–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morelli L, Palmeri M, Tartaglia D,
Guadagni S, Di Candio G and Mosca F: Adenocarcinoma on j-pouch
after proctocolectomy for ulcerative colitis-case report and review
of literature. Int J Colorectal Dis. 29:1171–1173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mennigen R, Sewald W, Senninger N and
Rijcken E: Morbidity of loop ileostomy closure after restorative
proctocolectomy for ulcerative colitis and familial adenomatous
polyposis: A systematic review. J Gastrointest Surg. 18:2192–2200.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park HB, Park HC, Chung CY, Kim JS, Myung
DS, Cho SB, Lee WS and Joo YE: Coexistence of solitary rectal ulcer
syndrome and ulcerative colitis: A case report and literature
review. Intest Res. 12:70–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Papaconstantinou I, Stefanopoulos A,
Papailia A, Zeglinas C, Georgopoulos I and Michopoulos S:
Isotretinoin and ulcerative colitis: A case report and review of
the literature. World J Gastrointest Surg. 6:142–145. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Negoro A, Takano T, Tajiri H, Nezu R,
Kawamura N and Brooks S: A role of colectomy in immune
thrombocytopenic purpura associated with ulcerative colitis: A case
report and a review of the literature. Int J Colorectal Dis.
29:1179–1180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castaño-Milla C, Chaparro M and Gisbert
JP: Systematic review with meta-analysis: The declining risk of
colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther.
39:645–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Önal İK, Beyazit Y, Şener B, Savuk B, Etık
Özer D, Sayilir A, Öztaş E, Torun S, Özın Özderın Y, Demırel Tunç
B, et al: The value of fecal calprotectin as a marker of intestinal
inflammation in patients with ulcerative colitis. Turk J
Gastroenterol. 23:509–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jena G and Trivedi PP: A review of the use
of melatonin in ulcerative colitis: Experimental evidence and new
approaches. Inflamm Bowel Dis. 20:553–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okayasu I: Development of ulcerative
colitis and its associated colorectal neoplasia as a model of the
organ-specific chronic inflammation-carcinoma sequence. Pathol Int.
62:368–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poulsen NA, Andersen V, Møller JC, Møller
HS, Jessen F, Purup S and Larsen LB: Comparative analysis of
inflamed and non-inflamed colon biopsies reveals strong proteomic
inflammation profile in patients with ulcerative colitis. BMC
Gastroenterol. 12:762012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shilpa K, Sangeetha KN, Muthusamy VS,
Sujatha S and Lakshmi BS: Probing key targets in insulin signaling
and adipogenesis using a methanolic extract of Costus pictus and
its bioactive molecule, methyl tetracosanoate. Biotechnol Lett.
31:1837–1841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gireesh G, Thomas SK, Joseph B and Paulose
CS: Antihyperglycemic and insulin secretory activity of Costus
pictus leaf extract in streptozotocin induced diabetic rats and in
in vitro pancreatic islet culture. J Ethnopharmacol. 123:470–474.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quintans Júnior LJ, Santana MT, Melo MS,
de Sousa DP, Santos IS, Siqueira RS, Lima TC, Silveira GO,
Antoniolli AR, Ribeiro LA and Santos MR: Antinociceptive and
anti-inflammatory effects of Costus spicatus in experimental
animals. Pharm Biol. 48:1097–1102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Binny K, Kumar SG and Dennis T:
Anti-inflammatory and antipyretic properties of the rhizome of
costus speciosus (koen.) sm. J Basic Clin Pharm. 1:177–181.
2010.PubMed/NCBI
|
|
17
|
Anyasor GN, Onajobi F, Osilesi O, Adebawo
O and Oboutor EM: Anti-inflammatory and antioxidant activities of
Costus afer Ker Gawl. Hexane leaf fraction in arthritic rat models.
J Ethnopharmacol. 155:543–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang XL, Xu J, Zhang XH, Qiu BY, Peng L,
Zhang M and Gan HT: PI3K/Akt signaling pathway is involved in the
pathogenesis of ulcerative colitis. Inflamm Res. 60:727–734. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao JW, Tang HY, Tan XY and Wang YD:
Effect of Etiasa on the expression of matrix metalloproteinase-2
and tumor necrosis factor-alpha in a rat model of ulcerative
colitis. Mol Med Rep. 6:996–1000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davey G and Wu Z: Attitudes in China
toward the use of animals in laboratory research. Altern Lab Anim.
35:313–316. 2007.PubMed/NCBI
|
|
21
|
Pedersen G, Saermark T, Giese B, Hansen A,
Drag B and Brynskov J: A simple method to establish short-term
cultures of normal human colonic epithelial cells from endoscopic
biopsy specimens. Comparison of isolation methods, assessment of
viability and metabolic activity. Scand J Gastroenterol.
35:772–780. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Almeida Mde A, Pizzini CV, Damasceno LS,
Muniz Mde M, Almeida-Paes R, Peralta RH, Peralta JM, Oliveira Rde
V, Vizzoni AG, de Andrade CL, et al: Validation of western blot for
Histoplasma capsulatum antibody detection assay. BMC Infect Dis.
16:872016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Christophorou D, Funakoshi N, Duny Y,
Valats JC, Bismuth M, De Chambrun Pineton G, Daures JP and Blanc P:
Systematic review with meta-analysis: Infliximab and
immunosuppressant therapy vs. infliximab alone for active
ulcerative colitis. Aliment Pharmacol Ther. 41:603–612. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Franco AI, Escobar L, Garcia XA, Van
Domselaar M, Achecar LM, Luján DR and García MJ: Mesalazine-induced
eosinophilic pneumonia in a patient with ulcerative colitis
disease: A case report and literature review. Int J Colorectal Dis.
31:927–929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alobaid A, Torlakovic E and Kongkham P:
Primary central nervous system immunomodulatory therapy-induced
lymphoproliferative disorder in a patient with ulcerative colitis:
A case report and review of the literature. World Neurosurg.
84:2074.e15–e19. 2015. View Article : Google Scholar
|
|
27
|
Takayama S and Iwasaki K: Systematic
review of traditional Chinese medicine for geriatrics. Geriatr
Gerontol Int. 17:679–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun GD, Li CY, Cui WP, Guo QY, Dong CQ,
Zou HB, Liu SJ, Dong WP and Miao LN: Review of herbal traditional
chinese medicine for the treatment of diabetic nephropathy. J
Diabetes Res. 2016:57498572016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan H, Liu F, Bligh SW, Shi S and Wang S:
Structure of a homofructosan from Saussurea costus and
anti-complementary activity of its sulfated derivatives. Carbohydr
Polym. 105:152–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hegde PK, Rao HA and Rao PN: A review on
insulin plant (Costus igneus Nak). Pharmacogn Rev. 8:67–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patil DT, Moss AC and Odze RD: Role of
histologic inflammation in the natural history of ulcerative
colitis. Gastrointest Endosc Clin N Am. 26:629–640. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Magnusson MK, Brynjólfsson SF, Dige A,
Uronen-Hansson H, Börjesson LG, Bengtsson JL, Gudjonsson S, Öhman
L, Agnholt J, Sjövall H, et al: Macrophage and dendritic cell
subsets in IBD: ALDH+ cells are reduced in colon tissue of patients
with ulcerative colitis regardless of inflammation. Mucosal
Immunol. 9:171–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cuković-Cavka S, Vucelić B, Urek MC,
Brinar M and Turk N: The role of anti-TNF therapy in ulcerative
colitis. Acta Med Croatica. 67:171–177. 2013.(In Croatian).
PubMed/NCBI
|
|
34
|
Yamamoto-Furusho JK, Santiago-Hernández
JJ, Pérez-Hernández N, Ramirez-Fuentes S, Fragoso JM and
Vargas-Alarcón G: Interleukin 1 β (IL-1B) and IL-1 antagonist
receptor (IL-1RN) gene polymorphisms are associated with the
genetic susceptibility and steroid dependence in patients with
ulcerative colitis. J Clin Gastroenterol. 45:531–535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lackeyram D, Young D, Kim CJ, Yang C,
Archbold TL, Mine Y and Fan MZ: Interleukin-10 is differentially
expressed in the small intestine and the colon experiencing chronic
inflammation and ulcerative colitis induced by dextran sodium
sulfate in young pigs. Physiol Res. 66:147–162. 2017.PubMed/NCBI
|
|
36
|
Bernardo D, Vallejo-Diez S, Mann ER,
Al-Hassi HO, Martínez-Abad B, Montalvillo E, Tee CT, Murugananthan
AU, Núñez H, Peake ST, et al: IL-6 promotes immune responses in
human ulcerative colitis and induces a skin-homing phenotype in the
dendritic cells and Tcells they stimulate. Eur J Immunol.
42:1337–1353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Seidelin JB and Nielsen OH: Attenuated
apoptosis response to Fas-ligand in active ulcerative colitis.
Inflamm Bowel Dis. 14:1623–1629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu X, Xu C, Saud SM, Lu X, Liu L, Fang L,
Zhang X, Hu J and Li W: Effect of kuijie granule on the expression
of TGF-β/Smads signaling pathway in patients with ulcerative
colitis. Evid Based Complement Alternat Med. 2016:26018302016.
View Article : Google Scholar : PubMed/NCBI
|