Introduction
With the development of gastrointestinal endoscopy,
particularly endoscopic ultrasonography (1), the detection rate of benign submucosal
tumors of the digestive tract has gradually increased (2). Submucosal tumors are often considered
to be relatively benign; however, they have malignant potential,
particularly if they originate from the muscularispropria layer
(3). Gastrointestinal stromal tumor
(GIST), the most common neoplasm that originates from the
muscularispropria layer of the gastrointestinal tract, is diagnosed
as malignant in 10–30% of cases (4).
Additionally, the fear of such tumors often causes psychological
problems for patients and a subsequent medical burden (5).
Endoscopic full-thickness resection (EFR) (6) is a novel nonsurgical method for radical
treatment of submucosal tumors. The application of purse-string
sutures with nylon loops and metal clips using a double-channel
gastroscope is a commonly used treatment method for defects of the
digestive tract resulting from EFR of submucosal tumors (7,8).
However, a double-channel gastroscope is not as commonly used as a
single-channel gastroscope in the majority of endoscopic centers in
China. Thus, the present study aimed to investigate the therapeutic
safety and feasibility of the application of purse-string sutures
with nylon loops and metal clips under single-channel endoscopy in
patients with EFR-induced gastrointestinal wall defects.
A prospective cohort study of 42 patients with
gastrointestinal wall defects after EFR was conducted in the First
People's Hospital of Wujiang District and the Second Affiliated
Hospital of Soochow University (Jiangsu, China). In the present
study, the feasibility and safety of purse-string suture placement
under single- vs. double-channel gastroscopy was assessed.
Materials and methods
Medical ethics
The Medical Ethics Committees of The First People's
Hospital of Wujiang District (Suzhou, China) and The Second
Affiliated Hospital of Soochow University (Suzhou, China) approved
the current study. The inclusion criterion was the presence of a
digestive tract defect following EFR of a submucosal tumor. The
exclusion criteria (6) for patients
were: i) Non-correctable coagulopathy; ii) severe organ failure;
iii) a comorbidity requiring continuous antithrombotic medication;
iv) procedure time >180 min.
Patients
From April 2012 to October 2016 in the endoscopic
centers of the First People's Hospital of Wujiang District and the
Second Affiliated Hospital of Soochow University, a total of 42
patients (age, 49.0±16.6 years; 21 men, 48.3±18.1 years; 21 women,
49.5±16.5 years) with full-thickness defects of the
gastrointestinal wall that had occurred during EFR of submucosal
tumors (27 in the stomach, 5 in the duodenal bulb and 10 in the
rectum) were prospectively investigated. All study participants
provided their written informed consent. Two groups were formed
using opaque sealed envelopes according to a computer-generated
randomized set of numbers. Eighteen patients underwent defect
repair using purse-string suture placement with nylon loops and
metal clips under a single-channel endoscope (research group), and
24 patients underwent purse-string suture placement with nylon
loops and metal clips under a double-channel endoscope (control
group).
Medical instruments
The following medical instruments were used during
treatment: UM-2R (12 MHZ) and UM-3R (20 MHz) miniature ultrasonic
probes (Olympus, Tokyo, Japan), EUM 2000 endoscopic ultrasonography
system (Olympus), GIF-260J with flushing function (Olympus),
GIF-2T260J with double channel (Olympus), ND-201-11802 cap
(Olympus), ITknife2 Electrosurgical Knife (KD-611L; Olympus),
KD-620LR hook knife (Olympus), NM-4i-1 injection needle (Olympus),
FD-410I hot biopsy forceps (Olympus), MAJ-254 and MAJ-340 nylon
loops (Olympus), SD-210U-25 snare (Olympus), HX-600-135 hemostatic
clips (Olympus), VIO 200S electrosurgical unit (Erbe Electromedizin
GmbH, Tübingen, Germany) and CO2 supply system
(Olympus). All procedures were primarily completed by three chief
physicians who had performed more than 100 cases of endoscopic
submucosal dissection (ESD).
Suture method for digestive tract
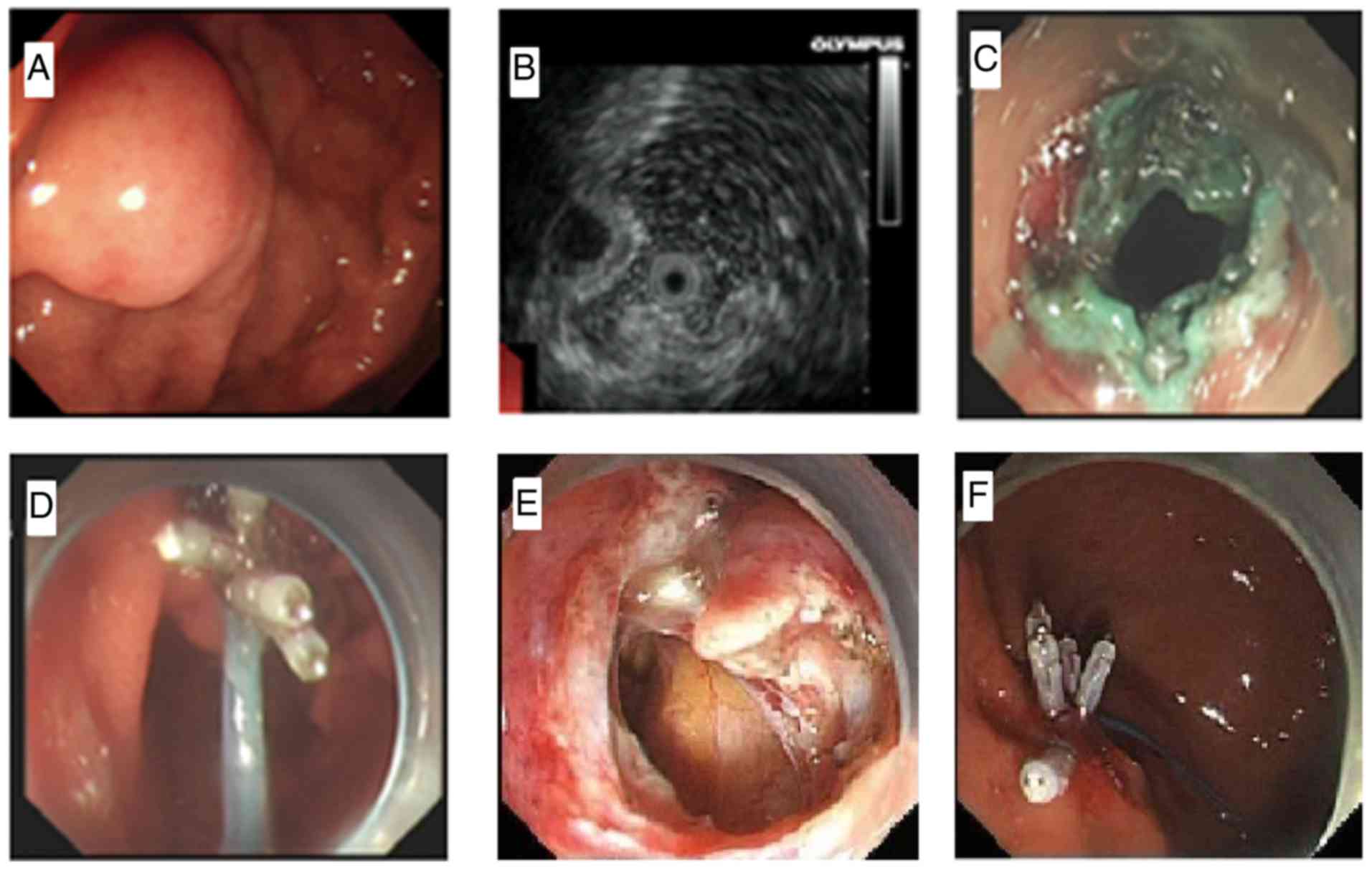
defects
A new suture method was applied in the research
group. First, a nylon loop was fixed on a pusher, and the nylon
loop was then loosened and tied tightly around the front end of the
cap while still holding the handle of the pusher (Fig. 1). Second, the chief endoscopist
inserted the endoscope with the nylon loop until the digestive
tract defect was reached, and a physician assistant loosened the
nylon loop while still holding the handle of the pusher. Third, the
chief endoscopist slowly pulled the endoscope backward while the
assistant pushed the nylon loop forward until the field of vision
was wholly exposed. Fourth, the angle of the metal clips was
adjusted to enable the clips to bring the nylon loop to the distal
end of the wall defect, and the nylon loop was clipped as tightly
as possible into the full layer or muscle layer of the defected
wall. Fifth, clipping of the nylon loop to the other side of the
defected wall with metal clips was continued using a total of four
to six pieces. Sixth, the assistant tightened the handle of the
nylon loop to narrow the nylon loop, closed the defected wall, and
pulled out the nylon loop pusher. Finally, the residual defected
wall was clipped with metal clips if necessary (Fig. 2).
The standard suture method was applied in the
control group. First, the nylon loop and metal clips were inserted
through the two channels of the double-channel endoscope,
respectively. Second, the nylon loop was opened under direct
endoscopic vision, and the loop was then clipped and fixed to the
peripheral edge of the defected wall with metal clips, as in the
research group. Third, the assistant tightened the handle of the
nylon loop to narrow the nylon loop, closed the defected wall, and
pulled out the nylon loop pusher. Finally, the residual defected
wall was clipped with metal clips if necessary.
Postoperative management
Fasting, fluid infusion, nutritional support,
hemostasis and antibiotics were administered as routine treatment.
Nasogastric negative pressure drainage and a semi-reclining
position were adopted following surgery when the resected
submucosal tumor was located in the upper gastrointestinal tract,
and proton pump inhibitors were also administered. Patients were
monitored for clinical symptoms and signs, including abdominal
pain, abdominal distension, fever, melena, hematemesis and signs of
peritonitis. Endoscopy was repeated and the mucosal healing
condition was carefully observed 3 months postoperatively. At 6 and
12 months postoperatively, endoscopy was repeated to determine
whether any recurrence of the submucosal tumor had occurred.
End points and subgroup analyses
The primary outcome was the success rate of all
patients that underwent purse-string suture placement with nylon
loops and metal clips, and whether all tumors were resected and
taken out. The secondary outcomes were total procedure time and
treatment outcomes of ESD (decrease in hemoglobin, days of
antibiotic used and average hospital stay). These endpoints were
also compared between the two groups.
Statistical analysis
Statistical evaluations were performed using SPSS
13.0 (SPSS Inc., Chicago, IL, USA). Numerical data are expressed as
the mean ± standard deviation, and categorical variables are
expressed as mean (percentage). Comparisons between the control
group and research group were performed using one-way analysis of
variance for continuous variables. Student's t-test was conducted
for the continuous variable age. The Pearson's chi-square test was
used to test for differences in categorical variables. P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient outcomes
The clinical characteristics of patients are
presented in Table I. No significant
differences were identified between the two groups (P>0.05). All
42 patients underwent purse-string suture placement with nylon
loops and metal clips with a 100% success rate. All tumors (1.0–3.0
cm) were successfully resected and taken out. The procedure time
was 10.5±5.6 min in the research group and 14.6±4.5 min in the
control group, with no significant difference (P>0.05). The mean
hospital stay was 4.5±1.0 days in the research group and 4.5±2.5
days in the control group, with no significant difference
(P>0.05) and no further consultation with a general surgeon or
transfer to the general surgery department was required in any
patient. The detailed results are presented in Table II.
 | Table I.Comparison of clinical characteristics
between the two groups. |
Table I.
Comparison of clinical characteristics
between the two groups.
| Characteristic | Research group | Control group | P-value |
|---|
| No. of patients | 18 | 24 | – |
| Sex
(male:female) | 8:10 | 13:11 | 0.38 |
| Age, years (mean ±
SD) | 48.5±18.2 | 49.5±16.8 | 0.27 |
| Location of the
lesion (S:D:R) | 12:2:5 | 15:3:5 | 0.52 |
| Median maximum tumor
diameter, mm (range) | 23.4 (17.0–30.0) | 23.7 (17.0–30.0) | 0.34 |
| Median maximum
specimen diameter, mm (range) | 29.2 (17.0–30.0) | 30.6 (17.0–30.0) | 0.32 |
 | Table II.Comparison of outcome measures between
the two groups. |
Table II.
Comparison of outcome measures between
the two groups.
| Outcome | Research group
(n=18) | Control group
(n=24) | P-value |
|---|
| Successful repair, n
(%) | 18 (100) | 24 (100) | – |
| Decrease in Hb, g/dl,
mean ± SD | 1.4±0.7 | 1.5±0.8 | 0.177 |
| Procedure time, min,
mean ± SD | 10.5±5.6 | 14.6±4.5 | 0.214 |
| Muscle injury, n
(%) | 4 (22.2) | 6 (25.0) | 0.146 |
| Postoperative
bleeding (hematemesis, melena), n (%) | 0 (0) | 0 (0) | – |
| Postoperative fever,
n (%) | 0 (0) | 0 (0) | – |
| Postoperative
abdominal pain, n (%) | 10 (55.6) | 14 (58.3) | 0.226 |
| Postoperative sepsis,
n (%) | 0 | 0 | – |
| Postoperative GI
tract leakage, n (%) | 0 | 0 | – |
| Antibiotic use, days,
mean ± SD | 1.5±0.5 | 1.5±0.5 | 0.245 |
|
Hydropneumothorax/mediastinal
emphysema/subcutaneous emphysema, n (%) | 0/0/0 (0/0/0) | 0/0/0 (0/0/0) | – |
| Pneumoperitoneum, n
(%) | 8 (44.4) | 10 (41.7) | 0.189 |
| Restart food on POD
3, n (%) | 14 (77.8) | 18 (75.0) | 0.381 |
| Hospital stay, days,
mean ± SD | 4.5±1.0 | 4.5±2.5 | 0.600 |
No cases of hydropneumothorax, mediastinal emphysema
or subcutaneous emphysema occurred during the procedure. Eight
patients in the research group and 10 patients in the control group
underwent abdominal puncture and air drainage due to visible
pneumoperitoneum, with no significant difference between the groups
(P>0.05). No postoperative bleeding (hematemesis, melena) was
observed in either group.
Postoperative follow-up
Postoperative follow-up was performed for all 42
patients (100%). The gastrointestinal wall defects had completely
healed with no residual metal clips or nylon loops 3 months after
EFR treatment. No submucosal tumor recurrences were observed at 6
and 12 months postoperatively.
Discussion
Endoscopic mucosal resection (EMR) and ESD
techniques are now performed worldwide (9). Complications of these procedures
include iatrogenic active perforation or unpredictable iatrogenic
perforation (10). There is an
urgent requirement for endoscopists to repair these
gastrointestinal perforations under EMR/ESD/EFR rather than
transfer these patients to undergo a general operation. Metal clips
are used to close the defects, particularly following ESD and EFR
(11). However, when defects of ≥3
cm or severe mucosal edema are present, closure using metal clips
alone is challenging (12). Recent
developments of the Over-The-Scope Clip system (Ovesco Endoscopy
AG, Tübingen, Germany) (13),
cutting and sewing machines (14)
and artificial repair material (15)
have facilitated suturing of iatrogenic perforations following
EMR/ESD and EFR; however, the costs of these medical devices and
materials are high.
In 2012, Zhong et al (7), successfully closed EMR-induced mucosal
defects using a new technique involving purse-string suture
placement with metal clips and nylon loops. However, the
purse-string sutures were usually placed with a double-channel
endoscope in this technique, which limits its widespread clinical
use since many hospitals do not have double-channel endoscopes.
Since April 2012 in the First People's Hospital of
Wujiang District and the Second Affiliated Hospital of Soochow
University the application of purse-string sutures with nylon loops
and metal clips under single-channel endoscopy has been
successfully used to close EFR-induced gastrointestinal defects in
18 patients. Many other techniques have also been applied since
April 2012; for example, the gastric tube insertion method was
initially attempted using a single-channel endoscope. This maneuver
was performed by insertion of a nylon loop into the gastric cavity
first, followed by insertion of the endoscope into the stomach,
opening of the nylon loop under direct endoscopic vision, and
placement of the nylon loop onto the defects with the help of metal
clips. However, because of the hardness of the head of the nylon
loop pusher, rough insertion of the nylon loop pusher through the
patient's throat could easily cause injury. To avoid this
complication, a new technique was developed. This involved
synchronous insertion of the nylon loop pusher along with the
endoscope, temporary fixation of the nylon loop onto the head of
the cap, insertion of the endoscope with the nylon loop to the
gastrointestinal wall defects, loosening of the nylon loop and
removal of the cap, and gradual purse-string suture placement after
metal clip insertion through the channel of the endoscope.
Certain advantages of single-over double-channel
endoscopy were also identified by practicing this maneuver using a
single-channel endoscope in 18 patients. First, when the nylon loop
is inserted into the gastrointestinal tract using a single-channel
endoscope, placement of the loop onto the defect under the guidance
of metal clips is considerably easier than with a double-channel
endoscope, with which the direction of the clips and nylon loops is
relatively fixed. Second, reversal of the endoscope to complete the
suture placement is more difficult with a double- than
single-channel endoscope. Third, better operational space and
flexibility are achieved when the endoscopists and assistants
independently reach the defect with the endoscope and nylon loop
under single-channel endoscopy. Collaboration between the
endoscopist and assistant is essential to complete the purse-string
suture placement.
In the current prospective study, no significant
differences in operation time, occurrence of bleeding, occurrence
of fever, rate of 3-day postoperative resumption of an oral diet or
hospital stay were identified between the research and control
groups. This lack of differences suggests that purse-string suture
placement under single-channel endoscopy is as safe and efficient
as under double-channel endoscopy.
In conclusion, purse-string suture placement with
nylon loops and metal clips under single-channel endoscopy appears
to be as safe, economical, convenient and efficient as that under
double-channel endoscopy. Furthermore, because endoscopists and
assistants manipulate the nylon loop pusher and metal clips
independently under a single-channel endoscope, a single-channel
scope has better operational space and flexibility compared with a
double-channel endoscope, making full-thickness suturing of
digestive tract defects easier to perform. It is worth considering
the widespread application of single-channel endoscopes for repair
of such defects, particularly in hospitals without a double-channel
endoscope.
Acknowledgements
The authors would like to thank Dr Angela Morben for
editing the English text of an earlier version of this
manuscript.
Funding
This study was supported by the Program for the
Talents in Science and Education of Jiangsu Province, China (grant
no. Z2017012) and Program for the Talents in Science and Education
of Wujiang District, Suzhou, China (grant no. WWK201517).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZQ, XL and JZ performed the endoscopy procedure,
designed the study and drafted the manuscript. GY conceiving the
study, designed the study and collected the data. LX collected the
data, identified the picture of the endoscopy, coordinated with the
hospital administration and obtained consent from the patients. HZ
performed statistical analysis, interpreted the data and followed
up with the patients. JT performed the endoscopy procedures and was
responsible for the conception and design of the study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First People's Hospital of Wujiang District and the Second
Affiliated Hospital of Soochow University.
Consent for publication
Patients provided written informed consent for the
publication of their data.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Caletti G, Fusaroli P and Bocus P:
Endoscopic ultrasonography. Digestion. 59:509–529. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rösch T, Lorenz R, Dancygier H, von
Wickert A and Classen M: Endosonographic diagnosis of submucosal
upper gastrointestinal tract tumors. Scand J Gastroenterol. 27:1–8.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chun SY, Kim KO, Park DS, Lee IJ, Park JW,
Moon SH, Baek IH, Kim JH, Park CK and Kwon MJ: Endoscopic
submucosal dissection as a treatment for gastric subepithelial
tumors that originate from the muscularis propria layer: A
preliminary analysis of appropriate indications. Surg Endosc.
27:3271–3279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mandrioli M, Mastrangelo L, Masetti M,
Zanini N, Lega S, Nannini M, Gruppioni E, Altimari A, Dei Tos AP,
Fabbri C and Jovine E: Characterization of malignant
gastrointestinal stromal tumors-a single center experience. J
Gastrointest Oncol. 8:1037–1045. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berzin TM, Blanco PG, Lamont JT and
Sawhney MS: Persistent psychological or physical symptoms following
endoscopic procedures: An unrecognized post-endoscopy adverse
event. Dig Dis Sci. 55:2869–2873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li QL and Zhou PH: Perspective on peroral
endoscopic myotomy for achalasia: Zhongshan experience. Gut Liver.
9:152–158. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong YS, Shi Q, Yao LQ, Zhou PH, Xu MD,
Ma LL and Chen T: Complete closure of gastric wall defect after
endoscopic full-thick resection with metal clips and endoloop
snare. Zhonghua Wei Chang Wai Ke Za Zhi. 15:280–284. 2012.(In
Chinese). PubMed/NCBI
|
|
8
|
Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z,
Xu MD and Yao LQ: Complete closure of large gastric defects after
endoscopic full-thickness resection, using endoloop and metallic
clip interrupted suture. Endoscopy. 45:329–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun W, Wu S, Han X and Yang C:
Effectiveness of endoscopic treatment for gastrointestinal
neuroendocrine tumors: A retrospective study. Medicine (Baltimore).
95:e33082016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim GJ, Park SM, Kim JS, Ji JS, Kim BW and
Choi H: Risk factors for additional surgery after iatrogenic
perforations due to endoscopic submucosal dissection. Gastroenterol
Res Pract. 2017:63534562017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li QL, Yao LQ, Zhou PH, Xu MD, Chen SY,
Zhong YS, Zhang YQ, Chen WF, Ma LL and Qin WZ: Submucosal tumors of
the esophagogastric junction originating from the muscularis
propria layer: A large study of endoscopic submucosal dissection
(with video). Gastrointest Endosc. 75:1153–1158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujishiro M, Yahagi N, Kakushima N,
Kodashima S, Muraki Y, Ono S, Kobayashi K, Hashimoto T, Yamamichi
N, Tateishi A, et al: Successful nonsurgical management of
perforation complicating endoscopic submucosal dissection of
gastrointestinal epithelial neoplasms. Endoscopy. 38:1001–1006.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weiland T, Fehlker M, Gottwald T and
Schurr MO: Performance of the OTSC system in the endoscopic closure
of iatrogenic gastrointestinal perforations: A systematic review.
Surg Endosc. 27:2258–2274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Wu JH, Meng Y, Zhang Q, Gong W and
Liu SD: New devices and techniques for endoscopic closure of
gastrointestinal perforations. World J Gastroenterol. 22:7453–7462.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cios TJ, Reavis KM, Renton DR, Hazey JW,
Mikami DJ, Narula VK, Allemang MT, Davis SS and Melvin WS:
Gastrotomy closure using bioabsorbable plugs in a canine model.
Surg Endosc. 22:961–966. 2008. View Article : Google Scholar : PubMed/NCBI
|