Introduction
According to literature, bilateral lumbar multifidus
(LM) muscles are not symmetrical in acute lower back pain (1,2), chronic
lower back pain (3,4) and lumbar disc disease in patients
(5,6). Research has revealed that the structure
and function of LM muscles are associated with lumbar spinal
stability, and morphological changes of the LM muscles have been
closely related to local pathological changes (7). Research has demonstrated that ~80% of
patients with lumbar disc herniation (LDH) have revealed different
degrees LM muscle atrophy (8). Some
studies have indicated that duration of LDH is positively
correlated with atrophy (9,10). Muscle atrophy has also been suggested
to occur with ageing (11,12). In addition, it is difficult to define
whether LM muscle atrophy is caused by the LM muscle atrophy itself
or whether it is dominated by nerve inhibition (13,14).
Whether these specific and lateral morphological changes represent
a meaningful indicator or consequence of LDH continues to be
debated (10,15). In addition, some scholars have taken
different periods of the same observation object to study LM muscle
changes; however, the existence of interference factors could not
be abandoned (16). Whether there
are morphological changes in the motion segment of LM muscles among
the segments at a certain age and different durations in patients
with LDH is rarely documented in literature (9,10,15–17).
The deep LM present a single-segment distribution of the muscles,
and the majority of clinical assessments of the lumbar spine are
usually based on intervertebral motion segments. However, whether
it is purely from a single section or the entire LM volume, the
evaluation of the morphological changes of the LM muscle may be
restricted for clinical applications.
It remains ambiguous whether LM muscle morphological
and functional changes are caused by spinal balance disorders or
spinal internal and external balance disorders, which result from
LM morphology changes and functional changes of the muscle
(18,19). However, the internal and external
balance of spinal disorders increases intervertebral disc
prominence, and causes the mechanical compression of the nerve root
or a relatively narrow intervertebral foramen (20). Further study is required to clarify
whether level- and side-specific multifidus muscle morphological
changes could be used as a marker for disc pathology and whether
they are associated with the refined duration and age segmentation
in the Chinese population. If LM muscle asymmetry is indeed related
to local pathology and refined by the duration and age
segmentation, this could represent a substantial advance in helping
to adjust and evaluate LM muscle morphological and functional
changes as intervention active factors affecting the functional
unit of the spine, and in evaluation interventions as an objective
evaluation of the curative effect of LDH.
Therefore, the primary aim of the present study was
to analyze multifidus muscle size and bilateral symmetry in the
refined duration and age segmentation of patients with LDH. The
secondary aim was to determine whether different age group patients
with LDH with different pain presentations and duration displayed
different patterns of LM atrophy.
Materials and methods
Patients
A total of 327 subjects (178 male and 149 female)
between the ages of 18 and 65 years were selected through the image
archives of patients imaged between April 2013 and November 2014 at
the Department of Imaging, The Third Affiliated Hospital of
Zhejiang Chinese Medical University (Hangzhou, China), which was
part of a cross-scetional observation study of patients receiving
lumbosacral computed tomography (CT) with symptoms suggestive of
radiculopathy. The referring physicians' requisitions indicated the
presence of radiculopathy or leg pain (21). At the CT facility, all patients that
had a repeat history of the presenting complaint were also
interviewed. The side, type, extent, age, activity level, gender,
dextromanuality, treatment measures and duration of pain were
documented. The extent and activity level were evaluated by VAS
(Visual analogue) and JOA (Japanese Orthopaedic Association Scores)
(22). The inclusion criteria were
as follows: The interval from symptom onset to imaging was between
1 day and several years; the age of the subject was 18–65 years
old; posterolateral disc herniation was only at one disc level;
occurrence was either at L4-L5 or L5-S1, verified on imaging by a
radiologist; symptoms following a nerve root distribution in the
leg on the side of the herniation; recent activity was normal; and
a week of physical activity was <180 points (23). Patients who had received previous
spinal surgery, who had unclear positioning segment or bilateral
lumbocrural pain symptoms, and whose images were of poor quality
were excluded from the present study. The study was approved by the
Health Ethics Research Board of The Third Affiliated Hospital of
Zhejiang Chinese Medical University.
Evaluation of patient groups
According to the time of onset, all patients with
LDH were divided into the following stages: Acute stage (within 1
week); remission stage (within 3 weeks); recovery stage (within 1
month); sub-acute stage (within 3 months); and chronic stage (over
3 months). Additionally, all cases were classified based on age:
Youth (18–45 years) and elderly (>45–65 years).
Instrumentation and procedures
Physical screening
The purpose of this screening was to characterize
each subject's clinical presentation and signs of consistency, in
order to confirm the location of pain in the lower lumbar spine,
for example either L4-L5 or L5-S1, and identify its consistency
with the inclusion criteria.
Image acquisition
CT images were obtained using a Toshiba 16 row
helical CT scanning imaging system (Toshiba Corp., Tokyo, Japan)
with exposure factors of 120 Kv/375 mAs and 1.5–3.0 mm/s/3.0×4, and
a window level of 35 and window width of 320. The CT procedure in
all subjects included intervertebral segments of standardized
transaxial images and four levels for each section, which were
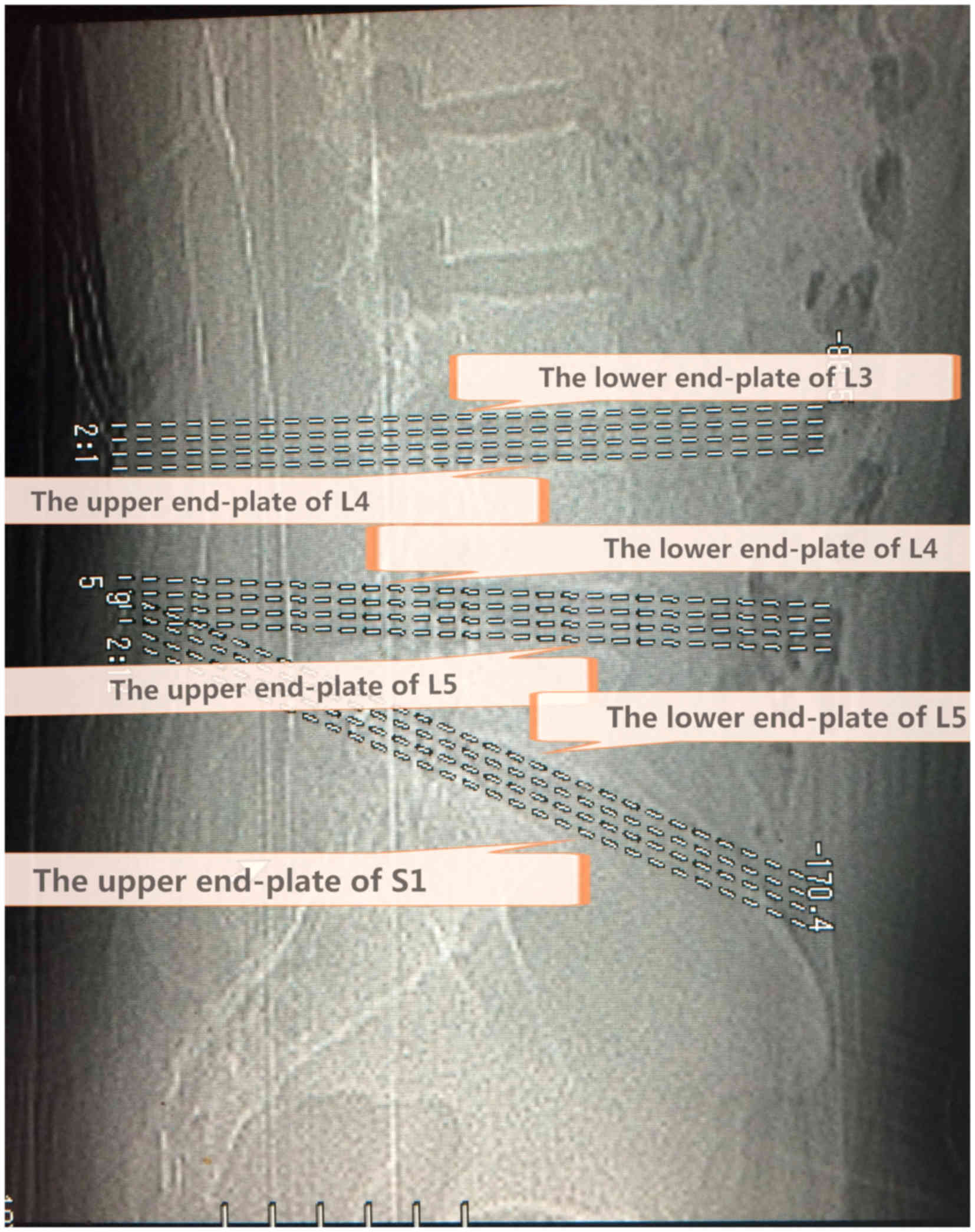
positioned accurately through the end plate of a vertebral body.
The first crossed the lower end plate of L4 and the upper end plate
of L5, while the second crossed the lower end plate of L5 and the
upper end plate of S1 (Fig. 1).
Image analysis
The CT images were obtained by a soft tissue window.
The analysis of isolated multifidus consisted of four steps using a
computer program (Activion v.16, Toshiba Corp., Tokyo, Japan)
written for this purpose. Muscle measurements were obtained at four
levels for each section. Quantitative measurements were performed
by two investigators blinded to the results of previously obtained
information on disc pathology and symptoms, in order to eliminate
potential bias. Image segmentation was performed by means of an
elimination technique based on differences in the grey values of
the pixels. Bone and clear fat deposits were eliminated. Each
section was measured separately for both the right and left
multifidi, and four levels were counted altogether (Fig. 2). The volumes and average for each
side were determined using the following formulae: Volume=Cross
sectional area (CSA) × slice thickness × number of slices; net
relative volume=the affected side of LM muscle volumes-the
unaffected side of the LM muscle volumes; atrophy ratio=the
affected side of LM muscle volumes-the unaffected side of the LM
muscle/the largest side. With reference to the study results
reported by Hides et al (2,24) and
combined with the actual situation of the present research, an
atrophy ratio of 10% or higher was considered atrophy or asymmetry.
The degree of symmetry of the multifidus volume was calculated as a
percentage of the difference between sides, relative to the larger
side [% difference=(largest side-smallest side/largest side)
×100].
Statistical analysis
Data were expressed as the mean ± standard deviation
and were analyzed using SPSS 17.0 software (SPSS Inc., Chicago, IL,
USA). Repeated measures analysis of variance (ANOVA) was conducted
followed by Bonferroni correction. All measurements and images were
recorded by the same observer. The area measurements were repeated
by a second analyst for 30 randomly picked subjects to calculate
the correlation coefficients in interobserver comparisons.
Inter-rater reliability coefficients were high (0.90–0.99),
indicating that the current measurement technique could be
duplicated by other investigators in a similar fashion. Intraclass
correlation coefficients (ICC) for the intra-rater reliability of
muscle measurements were determined from an independent sample of
30 comparable clinical CTs that were read twice. The intra-rater
ICCs were similar, ranging from 0.90–0.99.. P<0.05 was
considered to indicate a statistically significant difference.
Results
Net relative functional multifidus
muscle volumes
A total of 327 subjects met the inclusion criteria,
including 151 subjects with herniations at L5-S1 levels and 176 at
L4-L5 levels (Table I). ANOVA
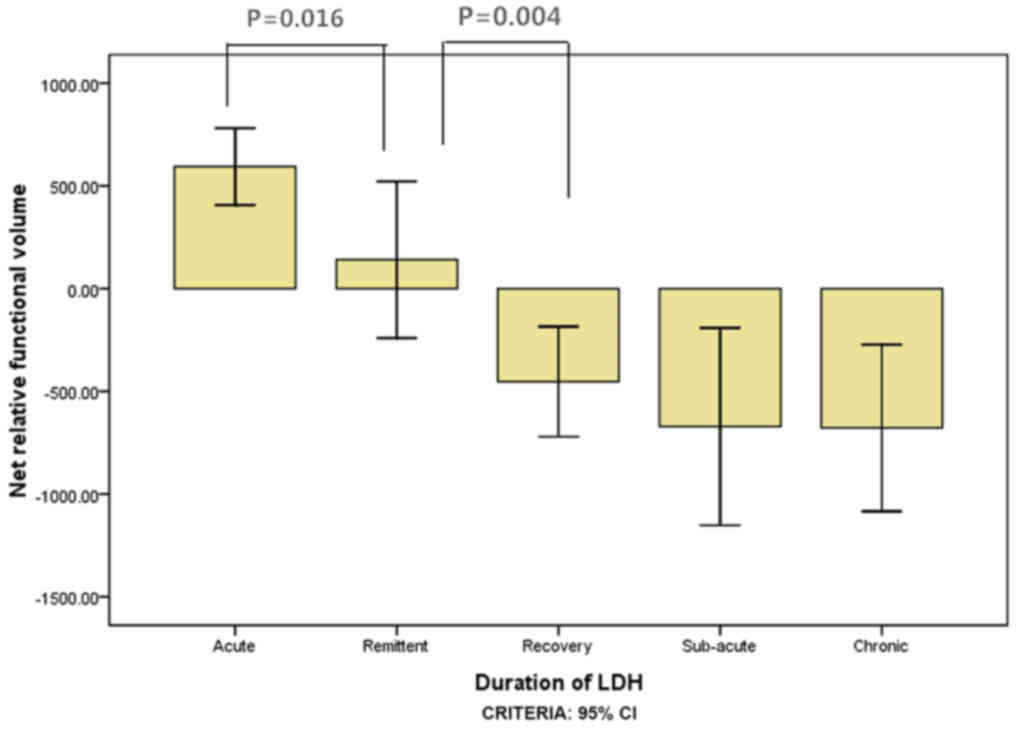
results for the net relative functional multifidus muscle volumes
at L4-L5 were significantly higher within 1 week than within 3
weeks for the elderly group (P=0.016; Fig. 3) and within 3 weeks compared with
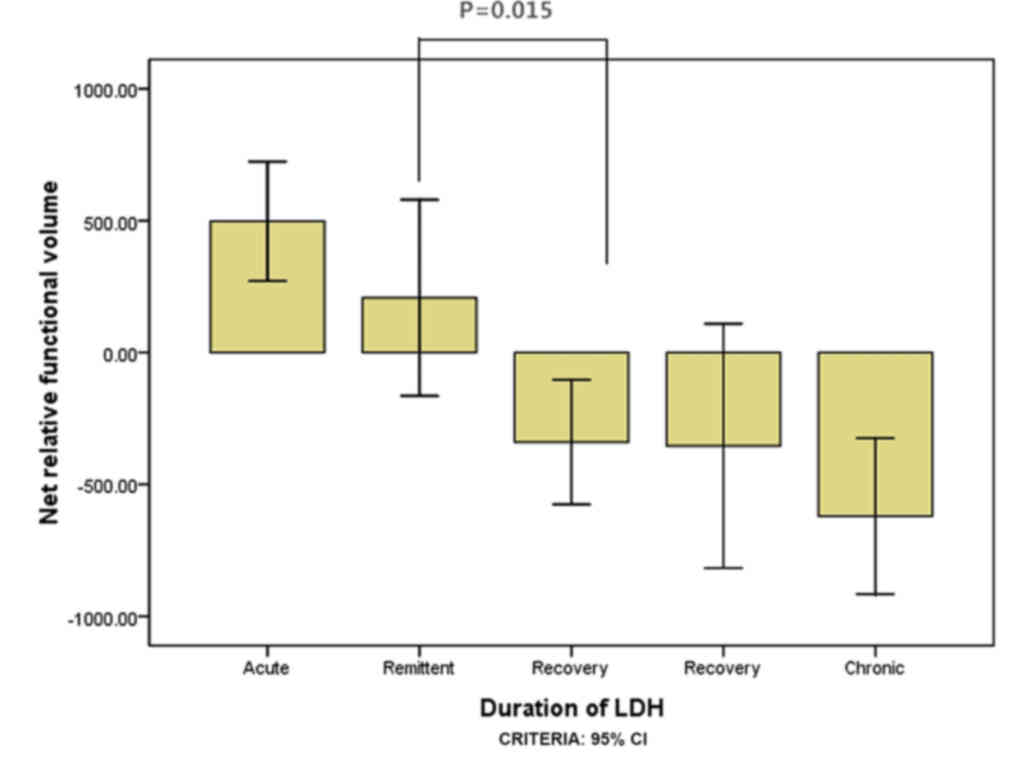
within 1 month for the elderly group (P=0.004; Fig. 3). For the youth group, the multifidus
muscle volumes at L4-L5 were significantly higher within 3 weeks
than within 1 month (P=0.015; Fig.
4); however there was no significant difference between 1 and 3
weeks (P=0.140; Fig. 4). ANOVA
results for the LM muscle net relative functional volumes at L5-S1
were significantly higher at 1 week than 3 weeks in the elderly
group (P=0.004; Fig. 5), and between
3 week and 1 month (P=0.001; Fig.
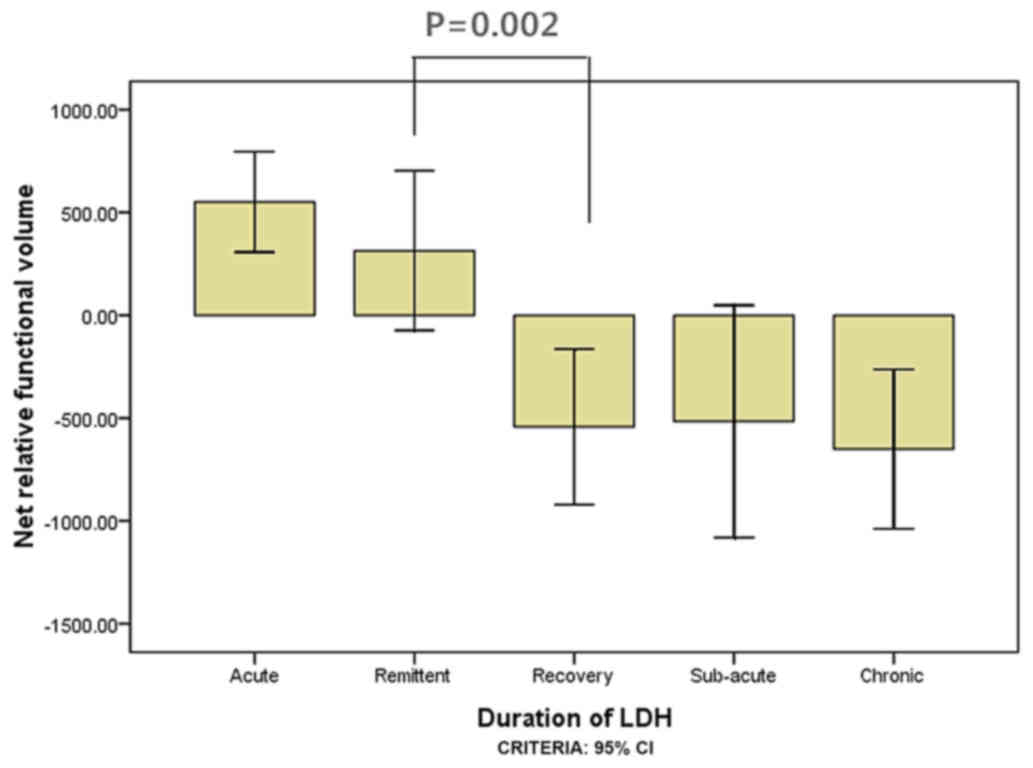
5); however, in the youth group the difference was not
significant between 1 and 3 weeks (P=0.285; Fig. 6) and between 3 weeks and 1 month
(P=0.002; Fig. 6).
 | Table I.Participant characteristics for side
of LDH, gender, age, BMI, VAS and JOA score of LDH in the different
duration groups. |
Table I.
Participant characteristics for side
of LDH, gender, age, BMI, VAS and JOA score of LDH in the different
duration groups.
|
| Stage of duration of
LDH |
|---|
|
|
|
|---|
| Characteristic | Acute (L/R) | Remittent (L/R) | Recovery (L/R) | Sub-acute (L/R) | Chronic (L/R) |
|---|
| Side, n
(left/right) |
|
|
|
|
|
| L4-L5
segment | 21 (8/13) | 15 (9/6) | 13 (10/3) | 12 (8/4) | 10 (5/5) |
|
| 22 (10/12) | 18 (9/9) | 16 (7/9) | 11 (9/2) | 13 (6/7) |
| L5-S1
segment | 31 (11/20) | 17 (8/9) | 13 (7/6) | 13 (5/8) | 15 (5/10) |
|
| 30 (11/19) | 21 (10/11) | 11 (3/8) | 13 (6/7) | 12 (4/8) |
| Gender, n
(male/female) |
|
|
|
|
|
| L4-L5
segment | 17/4 | 10/5 | 9/4 | 7/5 | 7/3 |
|
| 11/11 | 11/7 | 5/11 | 4/7 | 6/7 |
| L5-S1
segment | 18/13 | 9/8 | 6/7 | 11/2 | 9/6 |
|
| 13/17 | 8/13 | 5/6 | 6/7 | 6/6 |
| Age, years |
|
|
|
|
|
| L4-L5
segment | 34.71±8.31 | 37.33±7.37 | 35.46±6.50 | 36.25±7.98 | 36.70±7.99 |
|
| 53.18±6.28 | 53.33±5.60 | 51.13±4.50 | 53.36±5.52 | 52.08±3.55 |
| L5-S1
segment | 36.10±6.56 | 34.88±9.87 | 34.15±6.43 | 35.85±4.95 | 36.67±5.42 |
|
| 53.20±5.96 | 52.00±4.50 | 54.64±6.55 | 54.08±4.52 | 52.42±5.76 |
| BMI,
kg/m2 |
|
|
|
|
|
| L4-L5
segment | 21.17±1.84 | 21.17±1.58 | 21.05±1.65 | 21.29±1.54 | 21.14±1.96 |
|
| 21.43±1.85 | 21.35±1.63 | 21.23±1.61 | 21.25±1.89 | 20.55±1.59 |
| L5-S1
segment | 21.14±1.76 | 21.07±1.68 | 21.34±1.63 | 21.34±1.80 | 20.29±1.36 |
|
| 21.30±1.88 | 21.35±1.51 | 21.31±1.62 | 20.64±1.84 | 20.86±1.93 |
| VAS |
|
|
|
|
|
| L4-L5
segment | 4.71±1.15 | 4.07±0.70 | 3.23±1.24 | 2.83±1.40 | 2.50±1.08 |
|
| 4.68±1.23 | 3.89±0.68 | 3.25±1.24 | 2.81±1.17 | 2.62±1.26 |
| L5-S1
segment | 4.74±1.06 | 3.88±0.70 | 3.08±1.26 | 2.69±1.32 | 2.47±1.36 |
|
| 4.63±1.19 | 3.80±0.98 | 3.09±1.30 | 2.77±1.24 | 2.50±1.68 |
| JOA score |
|
|
|
|
| L4-L5
segment | 11.10±3.69 | 12.13±2.88 | 12.85±3.46 | 13.00±3.52 | 13.7±3.78 |
|
| 11.00±4.20 | 12.17±2.81 | 12.75±3.49 | 12.91±3.05 | 14.15±4.12 |
| L5-S1
segment | 11.10±3.69 | 11.59±2.85 | 12.77±4.25 | 12.77±3.96 | 14.8±4.31 |
|
| 10.87±4.34 | 12.00±3.18 | 12.73±3.95 | 12.92±2.90 | 14.92±4.19 |
Relative functional atrophy ratio of
lumbar multifidus muscles
Results demonstrated that age influenced the degree
of LM muscle atrophy in the L5-S1 segment at the chronic stage,
with the elderly group having a significantly lower net relative
functional volume than the youth group (P=0.048; Table II). At the L4-L5 and L5-S1 segments,
all patients with unilateral LDH in the youth group had <10% (vs
0.10) asymmetry for atrophy ratio of LM in the size of their
multifidus muscles. At the L4-L5 segment, patients with unilateral
LDH in the elderly group had >10% (vs. 0.10) asymmetry for
atrophy ratio of LM in the size of their multifidus muscles within
the course of 3 months, while in the L5-S1 segment, asymmetry
degree reached the standard of asymmetry within the course of 1
month, and reached 16 (vs 0.16) over 3 months (Table II). The reduced rate of the net LM
muscle volume was positively correlated with VAS scores, age,
duration and JOA scores (P<0.05; Table III).
 | Table II.Net relative functional volume and
relative functional atrophy ratio of lumbar multifidus muscles for
the various stages of LDH duration and ages at the L4-L5 and L5-S1
segmental levels. |
Table II.
Net relative functional volume and
relative functional atrophy ratio of lumbar multifidus muscles for
the various stages of LDH duration and ages at the L4-L5 and L5-S1
segmental levels.
|
| Segmental
level |
|---|
|
|
|
|---|
|
| L4-L5 | L5-S1 |
|---|
|
|
|
|
|---|
| Duration of
LDH | Youth group | Elderly group | P-value | Youth group | Elderly group | P-value |
|---|
| Acute |
|
|
|
|
|
|
| Net
volume | 497.70±510.47 | 593.84±421.64 |
| 551.23±665.25 | 726.99±759.27 |
|
| Atrophy
ratio | 0.08±0.07 | 0.11±0.09 |
| 0.10±0.13 | 0.12±0.14 |
|
| Remittent |
|
|
|
|
|
|
| Net
volume | 207.42±671.38 | 140.52±767.18 |
| 314.78±755.11 | 107.52±654.84 |
|
| Atrophy
ratio | 0.09±0.06 | 0.11±0.09 |
| 0.09±0.09 | 0.09±0.08 |
|
| Recovery |
|
|
|
|
|
|
| Net
volume | −340.02±391.26 | −452.62±503.18 |
| −542.42±626.21 | −857.58±604.20 |
|
| Atrophy
ratio | 0.05±0.06 | 0.10±0.06 |
| 0.09±0.06 | 0.15±0.10 |
|
| Sub-acute |
|
|
|
|
|
|
| Net
volume | −354.10±729.14 | −671.97±672.10 |
| −516.48±934.97 | −674.87±570.32 |
|
| Atrophy
ratio | 0.10±0.11 | 0.13±0.11 |
| 0.11±0.07 | 0.14±0.09 |
|
| Chronic |
|
|
|
|
|
|
| Net
volume | −620.56±353.97 | −678.97±671.02 |
| −650.67±699.39 |
−1214.99±1019.73 | 0.048 |
| Atrophy
ratio | 0.09±0.05 | 0.13±0.12 |
| 0.15±0.11 | 0.16±0.13 |
|
 | Table III.Correlation analysis for the duration
of LDH, VAS and JOA scores. |
Table III.
Correlation analysis for the duration
of LDH, VAS and JOA scores.
|
| Age group |
|---|
|
|
|
|---|
|
| Youth | Elderly |
|---|
|
|
|
|
|---|
| Factor | L4-L5 segment | L5-S1 segment | L4-L5 segment | L5-S1 segment |
|---|
| Duration of
LDH |
|
|
|
|
|
Rho | 0.962 | 0.929 | 0.949 | 0.936 |
|
P-value | 0.009 | 0.023 | 0.014 | 0.019 |
| JOA score |
|
|
|
|
|
Rho | 0.973 | 0.920 | 0.970 | 0.961 |
|
P-value | 0.005 | 0.027 | 0.006 | 0.009 |
| VAS |
|
|
|
|
|
Rho | 0.984 | 0.969 | 0.975 | 0.971 |
|
P-value | 0.002 | 0.006 | 0.005 | 0.006 |
Discussion
The findings of the present study on muscle relative
functional net volume and asymmetry suggest that LM muscles could
be used as selective objective indicators of localized nerve root
pathologies in LDH patients. The morphological change in LM muscles
on the symptomatic side in accord with the protrusion segment was
obvious. Furthermore, LM muscle atrophy was obvious with a symptom
duration of <1 month, which was earlier than in previous reports
(9,15,17). The
age considered in future study could influence LM muscle morphology
(11). The net LM volume reduction
rate was positively correlated with the duration of LDH (Table III). This could be considered as an
asymmetry at no less than 10% of the volume of functional asymmetry
on the average volume for the atrophy index. The net relative
volume of LM changed with age, segment and lumbocrural pain
duration. This finding revealed that VAS, JOA score and duration
were significantly and positively correlated with age and spinal
segment.
However, there were factors that affected CSA
measurements, such as the measurement itself, the state of the body
and the chosen level of measurement. Compared with CSA, using the
volume in the present research had many advantages. For example,
the segmental level of the study had a constant volume, the local
situation had a smaller influence than it did on CSA and the volume
did not easily change. Furthermore, it ensured that the sample was
selected for consistency. Against the pathological changes of LM
muscles, CSA assumptions may be expected on the basis of the
innervation of the intervertebral disc herniation segment below.
Furthermore, intervertebral disc volume assumptions may be closer
to the actual innervations, including a wider range, at least
(10,25). According to the level of the
intervertebral disc, the relative volume of LM muscle changes were
mostly accompanied by functional changes, which appeared to be
closer to clinical practice. Hides et al (24) suggested that the use of a 10% or
greater LM muscle asymmetric CSA is a potential indicator of
abnormal function of the spine. However, a multifidus muscle
asymmetry of no less than 10% of the relative functional volume
could also be considered to be atrophy in the present study.
Research has suggested that the duration of LDH is
positively associated with the degree of muscle atrophy (9,15,17).
Although some researchers have reported that more LM asymmetry was
found in acute symptoms, unilateral lumbar back and magnetic
resonance determine the segment degeneration of suspicious
segmental pathological changes (1).
It remains controversial whether morphological changes in specific
segments and sides of the LM muscle may be considered as a symbol
of the pathological changes of intervertebral disc disease
(15).
Research has demonstrated that during the onset of
early symptoms of LDH with lower back pain, which affects the
opposite side to the muscle, the diameter of the LM muscle was
larger than the unaffected side and was positively correlated with
duration (17). Durations of LDH of
<6 weeks demonstrated a more obvious asymmetrical difference in
LM muscle, which could be a local disk or selective indication of
sensitive nerve root lesions among patients with LDH (15). With <4 months of pain, LM muscle
atrophy was on a specific side and on the local pain area (26). When the duration of LDH was <3
months, LM muscle atrophy was not obvious (26). However, when the duration was >3
months, the CSA of bilateral LM muscles decreased, and the affected
side atrophy was more obvious (9).
In addition, the studies of some scholars on LM changes in patients
with <12 days of acute lower back pain (1) revealed that no lower back pain
persisted for >1 day (24). A
study by Barker et al (26)
used the MR to measure the LM CSA in patients with an average
16-week history of unilateral lower back pain. A study by Hyun
et al (27) reported the
average duration of lower back pain as >17 months, and a study
by Kulig et al (28) reported
an average of ~6 months. These subjects were not a research history
of the system analysis in these studies, the analysis of age was
also not a certain limit, and other merger confounding factors such
as body mass index, gender and physical activity index were not
comprehensively considered; thus, these conclusions could have
differences.
In the present study, the onset of symptoms of
subjects ranged from 1 day to >3 months, including the acute
stage. These were divided into the acute, remission, recovery,
sub-acute and chronic phases, which fully reflected the course of
morphological change over the duration. In the duration of <1
week in the acute stage, the affected side of the LM muscle was not
atrophic, and this significantly increased with time. It may be
interpreted as pseudohypertrophy or spasticity. Some researchers
have considered that it may be caused by muscle spasm (17). However, another possible mechanism
was hypotrophy (13). In MR images,
edema signal changes may occur with the loss of nerve control
within a few days or weeks (13).
The smaller side was ipsilateral to the reported side of the
symptoms within a course of 1 month. The relative volumes and CAS
of LM muscles in the bilateral side decreased, and the affected
side of muscle atrophy was most obvious with durations of >3
months. However, in actuality, LM muscle atrophy may not be
detected through in a larger sample size, and a wider range of the
course of the span of cases of clinical symptoms of initial samples
should be covered to clarify this problem. Based on this
consideration, the present research attempted to eliminate the
influence of confounding factors by a more detailed clinical
symptoms onset span, respectively, and to study the effect of the
duration of LDH on LM morphology.
The correlation analysis on net relative volume
changes with age, segment and lumbocrural pain duration, VAS and
JOA revealed that VAS, JOA and lumbocrural pain duration was
significantly positively correlated with age and spinal segment.
Previous research has demonstrated that asymmetric LM was quite
common in adults without a history of lower back pain, since
asymmetry prior to onset may mask these pathological changes
(14). Among various kinds of
research on the history of lower back pain, participants were
selected without consistent standards, such as no history of lower
back pain (29), no more than a day
of lower back pain (24) and no
limit to the reporting activities of lower back pain (30).
In the present study, the LM of intervertebral disc
volume was due to asymmetric lumbar pathological changes, which was
supported through the observed asymmetry of muscles in all studies.
LM asymmetry was associated with local pathological changes. The
present study did not consider the LM change above or below the
protrusion level; however, asymmetrical findings suggested that LM
may be particularly sensitive, or lumbar pathological changes, such
as those described in this article, were >3 weeks after the
symptoms of pathological changes. In addition, it may exist by
segmental LM muscular atrophy caused by segmental instability, and
may aggravate these pathological changes. Considering the
composition of muscles, LM had an obvious trend with a fattier
infiltration in the affected side than in the unaffected side. In
the same course, the elderly group had more fatty infiltration than
younger groups. Patients with chronic lower back pain have been
demonstrated to be positively associated with different degrees of
muscle atrophy at different times (16), this may reflect the effect of age on
the muscle function and structure of adaptation. The present study
indicated that the occurrence of muscle atrophy occurred more
frequently in the elderly group. Studies have demonstrated that age
should be given attention and considered, and the age group is
influenced by the degree of muscle atrophy (31–33). In
other words, the age factor must be taken into consideration in
later studies but should be considered in conjuction with other
factors, which was similar to previous studies (11).
Future research should include additional samples,
further subdivisions of participants into age groups, and provide a
more detailed forecast of lumbar muscle size, shape and structure
definition of age-related changes. Relative volume changes of
muscles of adjacent segments (above and below) should also be
considered. In addition, participants should be further
investigated through different activity types and intensities. The
limitations of the present study were determined by the
cross-sectional study, and the sample size was limited by the time
span. The subjects were not matched to the right or left side or by
gender. The relative volume changes of muscles of the adjacent
segments (above and below) were also not considered.
In conclusion, the results of the present study
suggest that the multifidus muscle atrophy in LDH was considerable
and bilateral. The reduced rate of relative functional net LM
volume were positively correlated with the age, duration, VAS
scores and JOA scores. The results suggest that the multifidus
muscle may be selectively responsive to, or indicative of,
localized lumbar disc or nerve root pathology in LDH patients with
a symptom duration of less than 1 month. Although the findings
observed were not reliable markers of lumbar pathology on an
individual level, the importance of early intervention of LM
atrophy was obvious and concerns of confounding factors, including
age, activity level and gender, were taken into account in clinical
or research settings.
Acknowledgements
We would like to thank all of the consultants in the
Departments of Orthopedics and Imaging, The Third Affiliated
Hospital of Zhejiang Chinese Medical University (Hangzhou, China)
for agreeing to allow their patients to be involved in the present
study. The present work was supported by grants from the Key
Project of Zhejiang Province Science and Technology Plan of
Traditional Chinese Medicine (grant no. 2011ZZ007, received by
Xin-miao Yao) and the National Project On Inheritance Workshop Of
Famous TCM Experts (grant no. 201420).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hides JA, Stokes MJ, Saide M, Jull GA and
Cooper DH: Evidence of lumbar multifidus muscle wasting ipsilateral
to symptoms in patients with acute/subacute low back pain. Spine
(Phila Pa 1976). 19:165–172. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hides JA, Richardson CA and Jull GA:
Multifidus muscle recovery is not automatic after resolution of
acute, first-episode low back pain. Spine (Phila Pa 1976).
21:2763–2769. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gibbons LE, Latikka P, Videman T, Manninen
H and Battié MC: The association of trunk muscle cross-sectional
area and magnetic resonance image parameters with isokinetic and
psychophysical lifting strength and static back muscle endurance in
men. J Spinal Disord. 10:398–403. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkkola R, Rytökoski U and Kormano M:
Magnetic resonance imaging of the discs and trunk muscles in
patients with chronic low back pain and healthy control subjects.
Spine (Phila Pa 1976). 18:830–836. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshihara K, Shirai Y, Nakayama Y and
Uesaka S: Histochemical changes in the multifidus muscle in
patients with lumbar intervertebral disc herniation. Spine (Phila
Pa 1976). 26:622–626. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao WP, Kawaguchi Y, Matsui H, Kanamori M
and Kimura T: Histochemistry and morphology of the multifidus
muscle in lum-bar disc herniation: Comparative study between
diseased and normal sides. Spine (Phila Pa 1976). 25:2191–2199.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Danneels LA, Vanderstraeten GG, Cambier
DC, Witvrouw EE and De Cuyper HJ: CT imaging of trunk muscles in
chronic low back pain patients and healthy control subjects. Eur
Spine J. 9:266–272. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kader DF, Wardlaw D and Smith FW:
Correlation between the MRI changes in the lumbar multifidus
muscles and leg pain. Clin Radiol. 55:145–149. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong YB, Xu HS and Li JH: Cross-sectional
area of LMmuscle in different stages of LDH. Clin Edu Gen Pra.
12:256–259. 2014.
|
|
10
|
Beneck GJ and Kulig K: Multifidus atrophy
is localized and bilateral in active persons with chronic
unilateral low back pain. Arch Phys Med Rehabil. 93:300–306. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valentin S, Licka T and Elliott J: Age and
side-related morphometric MRI evaluation of trunk muscles in people
without back pain. Man Ther. 20:90–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fortin M, Yuan Y and Battié MC: Factors
associated with paraspinal muscle asymmetry in size and composition
in a general population sample of men. Phys Ther. 93:1540–1550.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kamath S, Venkatanarasimha N, Walsh MA and
Hughes PM: MRI appearance of muscle denervation. Skeletal Radiol.
37:397–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niemeläinen R, Briand MM and Battié MC:
Substantial asymmetry in paraspinal muscle cross-sectional area in
healthy adults questions its value as a marker of low back pain and
pathology. Spine (Phila Pa 1976). 36:2152–2157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Battié MC, Niemelainen R, Gibbons LE and
Dhillon S: Is level- and side-specific multifidus asymmetry a
marker for lumbar disc pathology? Spine J. 12:932–939. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu WW, Hu ZJ, Fan SW, Xu WB, Fang XQ and
Zhao FD: Influencing of chronic low back pain on multifidus muscle
atrophy. Zhongguo Gu Shang. 27:207–212. 2014.(In Chinese).
PubMed/NCBI
|
|
17
|
Du ZF, Chi HJ and Li AH: Study on changes
of multifidus in patients with lumber disc herniation. J Clin Exper
Med. 11:608–609. 2012.
|
|
18
|
Panjabi MM: A hypothesis of chronic back
pain: Ligament subfailure injuries lead to muscle control
dysfunction. Eur Spine J. 15:668–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mörl F and Bradl I: Lumbar posture and
muscular activity while sitting during office work. J Electromyogr
Kinesiol. 23:362–368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bergmark A: Stability of the lumbar spine:
A study in mechanical engineering. Acta Orthop Scand suppl.
230:1–54. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schoenfeld AJ and Weiner BK: Treatment of
lumbar disc herniation: Evidence-based practice. Int J Gen Med.
3:209–214. 2010.PubMed/NCBI
|
|
22
|
Hiyama A, Watanabe M, Katoh H, Sato M,
Sakai D and Mochida J: Evaluation of quality of life and
neuropathic pain in patients with low back pain using the Japanese
Orthopedic Association Back Pain Evaluation Questionnaire. Eur
Spine J. 24:503–512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aadahl M and Jorgensen T: Validation of a
new self-report instrument for measuring physical activity. Med Sci
Sports Exerc. 35:1196–1202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hides J, Gilmore C, Stanton W and
Bohlscheid E: Multifidus size and symmetry among chronic LBP and
healthy asymptomatic subjects. Man Ther. 13:43–49. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boom HP, van Spronsen PH, van Ginkel FC,
van Schijidel RA, Castelijns JA and Tuinzing DB: A comparison of
human jaw muscle cross-sectional area and volume in long-anf
short-face subjects, using MRI. Arch Oral Bio. 153:273–281. 2008.
View Article : Google Scholar
|
|
26
|
Barker KL, Shamley DR and Jackson D:
Changes in the crosssectional area of multifidus and psoas in
patients with unilateral back pain: The relationship to pain and
disability. Spine (Phila Pa 1976). 29:E515–E519. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hyun JK, Lee JY, Lee SJ and Jeon JY:
Asymmetric atrophy of multifidus muscle in patients with unilateral
lumbosacral radiculopathy. Spine (Phila Pa 1976). 32:E598–E602.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kulig K, Scheid AR, Beauregard R, Popovich
JM Jr, Beneck GJ and Colletti PM: Multifidus morphology in persons
scheduled for single-level lumbar microdiscectomy: Qualitative and
quantitative assessment with anatomical correlates. Am J Phys Med
Rehabil. 88:355–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yanik B, Keyik B and Conkbayir I: Fatty
degeneration of multifidus muscle in patients with chronic low back
pain and in asymptomatic volunteers: Quantification with chemical
shift magnetic resonance imaging. Skelet Radiol. 42:771–778. 2012.
View Article : Google Scholar
|
|
30
|
Marras WS, Jorgensen MJ, Granata KP and
Wiand B: Female and male trunk geometry: Size and prediction of the
spine loading trunk muscles derived from MRI. Clin Biomech
(Bristol, Avon). 16:38–46. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anderson DE, D'Agostino JM, Bruno AG,
Manoharan RK and Bouxsein ML: Regressions for estimating muscle
parameters in the thoracic and lumbar trunk for use in
musculoskeletal modelling. J Biomech. 45:66–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fortin M, Videman T, Gibbons LE and Battié
MC: Paraspinal muscle morphology and composition: A 15-yr
longitudinal magnetic resonance imaging study. Med Sci Sports
Exerc. 46:893–901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meakin JR, Fulford J, Seymour R, Welsman
JR and Knapp KM: The relationship between sagittal curvature and
extensor muscle volume in the lumbar spine. J Anat. 222:608–614.
2013. View Article : Google Scholar : PubMed/NCBI
|