Introduction
Traumatic brain injury (TBI) has a high morbidity
and has a significantly negative impact on the lives of patients
and their families (1–4). The U.S. Centers for Disease Control and
Prevention reported that the combined incidence of TBI-associated
hospitalizations, emergency department visits and mortality was
823.7 per 100,000 in 2010 (5).
Intracranial hypertension (ICH) is an important predictor of
mortality in patients with severe TBI and patient outcomes and
mortality can be improved if the ICH is well managed (6–8). In a
meta-analysis, Sadaka and Veremakis (8) reported the effectiveness of
mild-to-moderate therapeutic hypothermia in controlling ICH in
patients with severe TBI. The use of therapeutic hypothermia as a
neuroprotective strategy was first reported in the 1940s (9). A number of studies have demonstrated
that therapeutic hypothermia is useful for improving patient
outcomes and reducing mortality (10–12).
Furthermore, recently published randomized trials reported that
hypothermia had no therapeutic benefit in terms of mortality or
morbidity (13–15). Variation between studies, including
in the duration and degree of hypothermia, may impact their
clinical efficacy.
Multimodal brain monitoring is an important tool
that may provide key information to guide the management of severe
TBI in adults (16,17). A number of studies have reported the
use of non-invasive multimodal brain monitoring of continuous
electroencephalography and the bispectral index (BIS) in the
treatment of patients with severe TBI (sTBI) patients; these
parameters may allow for the development of patient-tailored
treatments and help to determine when the brain is at risk of
injury and the depth of sedation required (18–20).
The present study presents two patients with sTBI
who were admitted to the Center for Craniocerebral Injuries of the
101st Hospital of the People's Liberation Army (Wuxi, China).
Additionally, the present study mainly focused on the value of
multimodal monitoring combined with the effectiveness of
hypothermia management of sTBI.
Case report
Case one
A 17-year-old female was found unconscious on the
stairs at her school in October 2016. The exact mechanism of injury
was unclear. The patient was brought to the emergency department of
the 101st Hospital of the People's Liberation Army by ambulance
within 15 min of being found. Upon arrival of the patient, an
arterial line and central venous catheters were inserted for fluid
and medication administration and her vital signs, ultrasonography
and electroencephalography (EEG) were monitored. Emergency
treatment comprised endotracheal intubation to improve hypoxia and
administration of mannitol (250 ml, 20%) and hypertonic saline (100
ml, 7.5%) to improve cerebral hernia. The patient was examined and
a Glasgow Coma Scale (GCS) score of 4 was determined, as well as
4.0 mm bilaterally fixed pupils and a negative corneal response. A
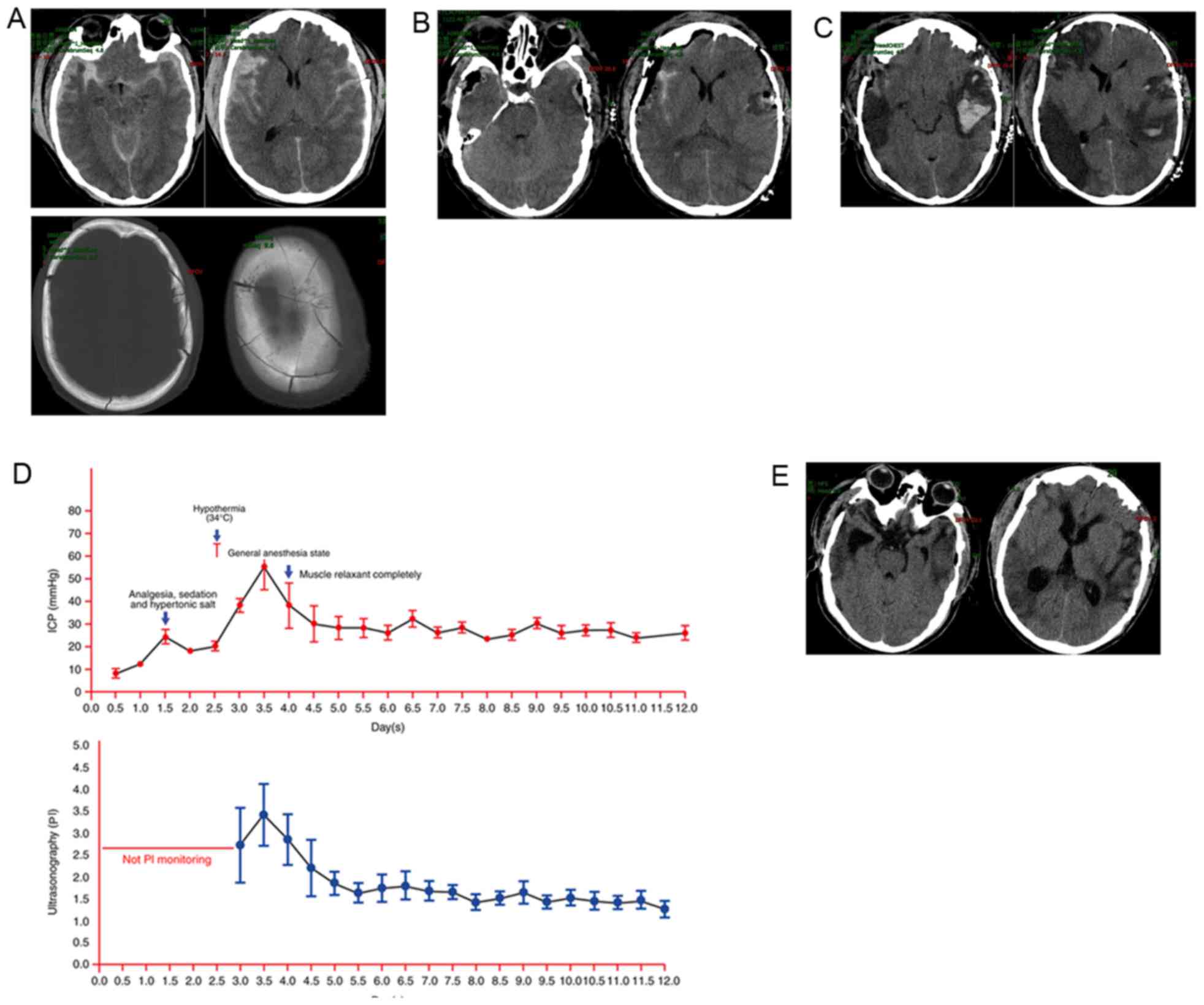
cranial computed tomography (CT) scan revealed subarachnoid
hemorrhage, left occipital bone fracture, right subdural hematoma,
compressed cisterns and a notable midline shift (Fig. 1A). The patient was prepared for
surgery and an intracranial pressure (ICP) probe was implanted.
The ICP prior to surgery was recorded as 47 mmHg.
The patient underwent a craniotomy and right-sided decompressive
craniotomy. The hematoma was removed and the ICP decreased to 5
mmHg; however, intraoperative ICP monitoring revealed that the ICP
progressively increased to 18 mmHg within 1 h. Intraoperative
ultrasound and intraoperative CT scan confirmed an occipital
epidural hematoma (Fig. 1B). The
original incision was extended to remove the epidural hematoma and
the ICP returned to 5 mmHg. The patient was subsequently
transferred to the neurointensive care unit (NICU) where she
underwent a postoperative examination and received continuous BIS,
ICP, CPP and ultrasound monitoring under the care of a neurosurgeon
and an intensivist. The patient's GCS post-surgery was 5 and the
bilateral pupils were 4.0 mm with a negative corneal response. A
subsequent cranial CT scan revealed an improvement of the midline
shift (Fig. 1C).
During the first 24 h following admission, the
primary aim was of treatment was to improve the internal
environment and address any coagulation disorders. Following a 24 h
admission, the CPP and ICP were normal (65–80 and 15–20 mmHg,
respectively). The patient's BIS was 55–65 and ultrasound revealed
a pulsatility index (PI) ~1.35.
On the second day following admission, the patient's
ICP increased to 25 mmHg, CPP decreased to 55–70 mmHg and PI
increased to 1.6. The patient received analgesia (Fentanyl Citrate,
0.001 mg/kg per 4 h), sedation (Dexmedetomidine, 0.1 µg/kg per
hour) and hypertonic salt (4.5% NaCl) treatment. Following 6 h of
treatment, the ICP decreased to 17 mmHg.
On the third day, the patient's ICP increased to 35
mmHg and the she presented a higher PI (2.3) and lower CPP (45–65
mmHg). CT scans revealed brain swelling (Fig. 1D). At this point, hypothermia was
implemented to control the ICP until coagulation patterns returned
to normal. The specific treatment parameters were as follows:
Temperature, 36°C; target ICP, ~20 mmHg; target CPP, ~70 mmHg.
During the fourth day, ICP, CPP and PI were poorly
controlled; the patient's temperature was decreased to 35°C and she
was deeply sedated with a lower BIS (40–50) and placed on a
ventilator. The ICP, PI and CPP subsequently returned to normal
levels and were within acceptable ranges. The patient was subjected
to hypothermic treatment for a total of 10 days (Fig. 1E).
At 23 days post-injury, the patient had complete
resolution of disease with mild language disorders and a GCS score
of 4. A cranial CT scan revealed that the edema and hematoma were
no longer present (Fig. 1F).
In this case, multimodal brain monitoring was used
to provide important information and insight about the patient's
condition, allowing for precise treatment of TBI. Intraoperative
ultrasound may contribute to a quick diagnosis and better surgical
planning. ICP, CPP, BIS and PI monitoring may provide additional
information and support during the initiation and management of
hypothermia, minimizing complications and maximizing the efficacy
of treatment.
Case two
A 55-year-old male was rendered unconscious
following a fall from a great height (8 m) at his place of work.
The patient was admitted to the emergency department at a Level 2
hospital (Huishan People's hospital, Wuxi, China) via ambulance in
May 2016, within 30 min of injury. Physical examination revealed a
GCS of 6 and 3 mm bilateral pupils with a normal light reflex. A
cranial CT scan revealed subarachnoid hemorrhage, bilateral
subdural hemorrhage and severe cranial comminuted fractures
(Fig. 2A). The patient's initial ICP
was 27 mmHg and he underwent a craniotomy to remove the hematoma
and brain contusion. This patient did not receive decompressive
craniectomy (DC) as ICP levels were 5 mmHg following surgery. The
patient was subsequently sent to the NICU and postoperatively
examined by a neurosurgeon, who reported a GCS of 7 and bilateral
pupils at 3.0 mm with a positive corneal response. A cranial CT
scan revealed improvement of the midline shift and complete removal
of the brain contusion (Fig. 2B);
however, the patient developed a high fever and blood coagulation
disorders on the second day post-surgery and his ICP levels
progressively increased to 28 mmHg. Due to the development of blood
coagulation disorders and the patient's family not consenting to a
second operation or DC.
On the third day post-surgery, the patient's ICP
levels were >37 mmHg and he was referred to the NICU at the
101st Hospital of the People's Liberation Army. When the patient
arrived at the NICU multimodal monitoring was conducted as
described in case 1 and the patient underwent an additional CT
examination. A cranial CT scan revealed brain swelling, cerebral
ischemia and re-bleeding (Fig. 2C).
The ICP levels increased to 56 mmHg with a higher PI (3.4) and
lower CPP (40–55 mmHg). At this point, hypothermia was implemented
to control the ICP. The specific treatment parameters were as
follows: Temperature, 34.5°C; target ICP, ~28 mmHg; target CPP, ~65
mmHg. However, no improvements were observed 1 day following the
initiation of hypothermia and the patient presented with an ICP of
~33 mmHg. The patient was deeply sedated (general anesthesia,
(Fentanyl Citrate, 0.001 mg/kg per 4 h), sedation (Dexmedetomidine,
0.1 µg/kg per hour), BIS ~45, no breathing- and respirator-assisted
respiration). Subsequently, the ICP remained stable, >30 mmHg
and the PI was <1.8. The duration of hypothermia treatment was
15 days (Fig. 2D).
At 30 days post-injury, the patient was discharged
to a rehabilitation hospital (Huishan People's hospital, Wuxi,
China) with a GCS of 10. Cranial CT re-examination revealed
decreased cerebral ischemia and complete absorption of brain edema
and hematoma (Fig. 2E).
In this case, ICP, CPP and PI were very important
indices of TBI, which were the target of the hypothermia treatment
conducted in the present study. ICP can be controlled by regulating
the degree of hypothermia and the dose of dehydrant (mannitol,
Beijing Double-Crane Pharmaceutical Co., Ltd., Beijing, China). BIS
monitoring may reflect the depth of coma and sedation;
additionally, the depth of sedation may be regulated to control ICP
and reduce hypothermia-relevant complications.
Discussion
TBI is one of the most common ailments worldwide,
with high morbidity and mortality, (6). Elevated ICP is an important predictor
of mortality in patients with sTBI and controlling the ICP has been
demonstrated to reduce mortality and improve patient outcome
(6,21). However, methods used to control ICP
require further investigation; DC and osmotic therapy are
appropriate options (22); however,
these treatments offer no significant curative effect following the
administration of analgesia, sedation and osmotic therapy (22). Hypothermia is an alternative
treatment that is able to effectively control ICP levels (8,10–12,23,24).
Schreckinger and Marion (23)
indicated that therapeutic moderate hypothermia was as effective or
more effective than other treatments for ICH. Furthermore, there is
no evidence of clinical complications associated with therapeutic
hypothermia. Previous studies have stated that hypothermia does not
confer a therapeutic benefit to mortality or morbidity (13–15,25,26).
However, previous negative findings may be a result of
inappropriate implementation of hypothermia, including the wrong
duration and degree of treatment. Precise monitoring is necessary
to evaluate the degree and duration of therapeutic hypothermia.
Therefore, multimodal monitoring is an important tool that may
provide support for patients who undergo hypothermia treatment.
Both patients described in the present study received long-term
mild to moderate hypothermia treatment and multimodal monitoring
and ICP was successfully controlled.
The majority of multimodal brain monitoring methods
are rapid and non-invasive and no particular contraindications are
usually observed. Implantation of the ICP probe is an invasive
operation that required minor injury; a small proportion of
patients with blood coagulation disorders, old age or unstable
vital signs may be unsuitable for ICP-monitored surgery (22). Therefore, multimodal brain monitoring
may be highly applicable to the general population.
Multimodal brain monitoring is an important tool
used for the rapid diagnosis of TBI. Ultrasound may provide a quick
assessment of intracranial condition. In addition, BIS and PI may
also aid in the determination of disease severity; however, CT
scans are important for the analysis of TBI and cannot be replaced
with other techniques. Additionally, multimodal brain monitoring
has previously been employed to guide the management of sTBI. It is
able to provide clinicians with an early indication of potential
secondary complications in the recovering brain by identifying
diagnostic features, including elevated ICP and decreased CPP
(17) but may also aid the
management of the target temperature and ICP. EEG and BIS serve
important roles in the treatment of critically ill patients,
particularly those suffering from sTBI and can improve their
clinical outcome (19,27–30).
Therefore, monitoring a barbiturate-induced coma using BIS is an
interesting approach as it can provide a continuous suppression
ratio and raw EEG traces, which enable monitoring of cerebral
function (19). Bader et al
(30) reported that the EEG-based
technology in BIS monitors may be an additional tool that can be
used in the ICU. The goal of BIS monitoring in sTBI is to reduce
ICP and secondary injury, thereby improving patient outcome. BIS
scores may reflect the depth of coma and sedation, which may then
be regulated to control ICP. In the cases described in the present
study, both patients underwent BIS monitoring to regulate the depth
of sedation and control ICP; consequently, both patients had good
treatment outcomes.
Ultrasonography is easy to use, noninvasive and is
frequently used during early injury assessment, as well during the
assessment of patients with sTBI and elevated ICP. Transcranial
Doppler (TCD) ultrasonography is typically used to determine the PI
of TCD waveforms (31). A
significant correlation between CPP, ICP and PI has been reported
(32,33) and Tan et al (32) suggested that TCD ultrasonography may
be used to predict patient outcome at 6 months post-injury. Mayans
et al (34) reported that
serial TCD monitoring may allow for potentially fatal complications
to be identified in time to allow a life-saving intervention in the
ICU setting. A number of studies have reported that TCD
ultrasonography is of great clinical value for measuring PI in the
management of sTBI (34,35). Furthermore, it has been reported that
PI correlates well with the ICP as measured using invasive methods
(36). Amyot et al (31) summarized the role of TCD for the
evaluation of ICH as follows: i) TCD waveform alterations indicate
an abnormally high ICP, particularly 20 to 30 mmHg; ii) TCD
alterations may indicate that the ICP probe is malfunctioning and
alert NICU personnel; iii) abnormally and globally decreased
patterns of CBFVs with increased PIs indicate the onset of diffuse
intracranial hypertension; and iv) the sudden onset of asymmetrical
CBFVs and PI alterations may indicate a potential midline shift. At
present, ultrasonography is not a viable replacement for ICP as ICP
monitoring is more accurate and may be performed continuously and
in real time. Further randomized controlled trials studies are
required to evaluate the ultrasonography as a potential substitute
for ICP monitoring in sTBI.
The cases presented herein demonstrate that
hypothermia is a highly useful tool for controlling malignant ICH
in sTBI. The present study provides further evidence that
multimodal monitoring is useful in the management and optimization
of therapeutic hypothermia. Additionally, long-term mild
hypothermia may improve sTBI patient outcomes. The ICP, PI and EEG
appear to be associated with patient outcomes and may be used to
guide treatment for TBI in the NICU. A large multicenter,
prospective study is required to validate these results.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and methods
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LKY made substantial contributions to the design of
the study. YHW made substantial contributions to the conception and
design of the study. JHC made substantial contributions to the
conception and design of the study, as well as the acquisition,
analysis and interpretation of data. YNX made substantial
contributions to the conception and design of the study, as well as
the acquisition of data. PPL gave final approval of the version to
be published and made substantial contributions to the conception
and design of the study. JHC, YNX and PPL contributed in drafting
the manuscript and revising it critically for important
intellectual content. MJ contributed in drafting the manuscript and
revising it critically for important intellectual content, as well
as the collection of data.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the 101st Hospital of the People's Liberation Army.
Written informed consent was obtained from all participants.
Consent for publication
All patients provided consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roozenbeek B, Maas AI and Menon DK:
Changing patterns in the epidemiology of traumatic brain injury.
Nat Rev Neurol. 9:231–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corrigan JD, Selassie AW and Orman JA: The
epidemiology of traumatic brain injury. J Head Trauma Rehabil.
25:72–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Selassie AW, Zaloshnja E, Langlois JA,
Miller T, Jones P and Steiner C: Incidence of long-term disability
following traumatic brain injury hospitalization, United States,
2003. J Head Trauma Rehabil. 23:123–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abbasi HR, Mousavi SM, Akerdi Taheri A,
Niakan MH, Bolandparvaz S and Paydar S: Pattern of traumatic
injuries and injury severity score in a major trauma center in
Shiraz, Southern Iran. Bull Emerg Trauma. 1:81–85. 2013.PubMed/NCBI
|
|
5
|
Centers for Disease Control and
Prevention: Rates of TBI-related emergency department visits,
hospitalizations and deaths-United States, 2001–2010. http://www.cdc.gov/traumaticbraininjury/data/rates.htmlAugust
10–2015
|
|
6
|
Patel HC, Menon DK, Tebbs S, Hawker R,
Hutchinson PJ and Kirkpatrick PJ: Specialist neurocritical care and
outcome from head injury. Intensive Care Med. 28:547–553. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Juul N, Morris GF, Marshall SB and
Marshall LF: Intracranial hypertension and cerebral perfusion
pressure: Influence on neurological deterioration and outcome in
severe head injury. The executive committee of the international
selfotel trial. J Neurosurg. 92:1–6. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sadaka F and Veremakis C: Therapeutic
hypothermia for the management of intracranial hypertension in
severe traumatic brain injury: A systematic review. Brain Inj.
26:899–908. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fay T: Observations on prolonged human
refrigeration. NY State J Med. 40:1351–1354. 1940.
|
|
10
|
Clifton GL, Miller ER, Choi SC, Levin HS,
McCauley S, Smith KR Jr, Muizelaar JP, Wagner FC Jr, Marion DW,
Luerssen TG, et al: Lack of effect of induction of hypothermia
after acute brain injury. N Engl J Med. 344:556–563. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhi D, Zhang S and Lin X: Study on
therapeutic mechanism and clinical effect of mild hypothermia in
patients with severe head injury. Surg Neurol. 59:381–385. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qiu W, Zhang Y, Sheng H, Zhang J, Wang W,
Liu W, Chen K, Zhou J and Xu Z: Effects of therapeutic mild
hypothermia on patients with severe traumatic brain injury after
craniotomy. J Crit Care. 22:229–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nielsen N, Wetterslev J, Cronberg T,
Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J,
Kuiper M, et al: Targeted temperature management at 33°C vs. 36°C
after cardiac arrest. N Engl J Med. 369:2197–2206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hutchison JS, Ward RE, Lacroix J, Hébert
PC, Barnes MA, Bohn DJ, Dirks PB, Doucette S, Fergusson D,
Gottesman R, et al: Hypothermia therapy after traumatic brain
injury in children. N Engl J Med. 358:2447–2456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clifton GL, Valadka A, Zygun D, Coffey CS,
Drever P, Fourwinds S, Janis LS, Wilde E, Taylor P, Harshman K, et
al: Very early hypothermia induction in patients with severe brain
injury (the National Acute Brain Injury Study: Hypothermia II): A
randomised trial. Lancet Neurol. 10:131–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Roux P, Menon DK, Citerio G, Vespa P,
Bader MK, Brophy G, Diringer MN, Stocchetti N, Videtta W, Armonda
R, et al: The international multidisciplinary consensus conference
on multimodality monitoring in neurocritical care: Evidentiary
tables: A statement for healthcare professionals from the
neurocritical care society and the european society of intensive
care medicine. Neurocrit Care. 21 Suppl 2:S2822014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Young AM, Donnelly J, Czosnyka M, Jalloh
I, Liu X, Aries MJ, Fernandes HM, Garnett MR, Smielewski P,
Hutchinson PJ and Agrawal S: Continuous multimodality monitoring in
children after traumatic brain injury-preliminary experience. PLoS
One. 11:e01488172016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Irimia A, Goh SY, Torgerson CM, Stein NR,
Chambers MC, Vespa PM and Van Horn JD: Electroencephalographic
inverse localization of brain activity in acute traumatic brain
injury as a guide to surgery, monitoring and treatment. Clin Neurol
Neurosurg. 115:2159–2165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prins SA, de Hoog M, Blok JH, Tibboel D
and Visser GH: Continuous noninvasive monitoring of barbiturate
coma in critically ill children using the Bispectral index monitor.
Crit Care. 11:R1082007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Steinbaugh LA, Lindsell CJ, Shutter LA and
Szaflarski JP: Initial EEG predicts outcomes in a trial of
levetiracetam vs. fosphenytoin for seizure prevention. Epilepsy
Behav. 23:280–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steiner T, Ringleb P and Hacke W:
Treatment options for large hemispheric stroke. Neurology. 57 5
Suppl 2:S61–S68. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carney N, Totten AM, O'Reilly C, Ullman
JS, Hawryluk GW, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon
N, et al: Guidelines for the management of severe traumatic brain
injury, 4th Edition. Neurosurgery. 80:6–15. 2017.PubMed/NCBI
|
|
23
|
Schreckinger M and Marion DW: Marion.
Contemporary management of traumatic intracranial hypertension: Is
there a role for therapeutic hypothermia? Neurocrit Care.
11:427–436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Corry JJ: The use of targeted temperature
management for elevated intracranial pressure. Curr Neurol Neurosci
Rep. 14:4532014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raj R, Bendel S, Reinikainen M, Kivisaari
R, Siironen J, Lång M and Skrifvars M: Hyperoxemia and long-term
outcome after traumatic brain injury. Crit Care. 17:R1772013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kilgannon JH, Jones AE, Shapiro NI,
Angelos MG, Milcarek B, Hunter K, Parrillo JE and Trzeciak S:
Emergency Medicine Shock Research Network (EMShockNet)
Investigators: Association between arterial hyperoxia following
resuscitation from cardiac arrest and in-hospital mortality. JAMA.
303:2165–2171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lafrance WC Jr, Deluca M, Machan JT and
Fava JL: Traumatic brain injury and psychogenic nonepileptic
seizures yield worse outcomes. Epilepsia. 54:718–725. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vespa PM, McArthur DL, Xu Y, Eliseo M,
Etchepare M, Dinov I, Alger J, Glenn TP and Hovda D: Nonconvulsive
seizures after traumatic brain injury are associated with
hippocampal atrophy. Neurology. 75:792–798. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cottenceau V, Petit L, Masson F, Guehl D,
Asselineau J, Cochard JF, Pinaquy C, Leger A and Sztark F: The use
of bispectral index to monitor barbiturate coma in severely
brain-injured patients with refractory intracranial hypertension.
Anesth Analg. 107:1676–1682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bader MK, Arbour R and Palmer S:
Refractory increased intracranial pressure in severe traumatic
brain injury, barbiturate coma and bispectral index monitoring.
AACN Clin Issue. 16:526–541. 2005. View Article : Google Scholar
|
|
31
|
Amyot F, Arciniegas DB, Brazaitis MP,
Curley KC, Diaz-Arrastia R, Gandjbakhche A, Herscovitch P, Hinds SR
II, Manley GT, Pacifico A, et al: A Review of the effectiveness of
neuroimaging modalities for the detection of traumatic brain
injury. J Neurotrauma. 32:1693–1721. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan H, Feng H, Gao L, Huang G and Liao X:
Outcome prediction in severe traumatic brain injury with
transcranial Doppler ultrasonography. Chin J Traumatol. 4:156–160.
2001.PubMed/NCBI
|
|
33
|
Zweifel C, Czosnyka M, Carrera E, de Riva
N, Pickard JD and Smielewski P: Reliability of the blood flow
velocity pulsatility index for assessment of intracranial and
cerebral perfusion pressures in head-injured patients.
Neurosurgery. 71:853–861. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mayans DR, Meads DB and Reynolds PS:
Transcranial doppler identifies a malfunctioning extraventricular
drain. J Neuroimaging. 24:518–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Daboussi A, Minville V, Leclerc-Foucras S,
Geeraerts T, Esquerre JP, Payoux P and Fourcade O: Cerebral
hemodynamic changes in severe head injury patients undergoing
decompressive craniectomy. J Neurosurg Anesthesiol. 21:339–345.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moppett IK and Mahajan RP: Transcranial
Doppler ultrasonography in anaesthesia and intensive care. Br J
Anaesth. 93:710–724. 2004. View Article : Google Scholar : PubMed/NCBI
|