Introduction
Asthma is airway hyperreactivity caused by
inflammations. Patients with this disease exhibit symptoms such as
wheezing, cough, chest tightness, dyspnea and other major symptoms
(1,2). In the United States, asthma is the most
common cause of hospitalization in the emergency departments
(3). According to the survey data in
2010 from the National Center for Health, 80,000 children were
diagnosed with asthma at the age of 6 years or below (4).
A number of epidemiological studies have revealed
that 25-hydroxyvitamin D [25-(OH)D] deficiency is associated with
many diseases, especially with the occurrence of asthma symptoms
(5,6). Moreover, asthma has a significant
correlation with inflammatory factors. This study assessed the
potential relationship between 25-(OH)D level and inflammatory
factors in children with asthma attack.
Patients and methods
Clinical data
A total of 60 child patients, who were admitted and
treated in the Pediatric Department of the Affiliated
Children'sHospital of Xuzhou Medical University (Xuzhou, China)
from March 2015 to March 2017 during their asthma attack were
selected as the observation group. There were 29 boys and 31 girls,
with an average age of 4.3±1.4 years. The children were diagnosed
according to the Global Initiative for Asthma (GINA) criteria. This
study was approved by the Ethics Committee of the Affiliated
Children's Hospital of Xuzhou Medical University, and informed
consent was obtained from patients and their families. The serum
25-(OH)D levels of the children were detected, with 14.30 ng/ml as
the median level. Children with a 25-(OH)D level >14.30 ng/ml
were included in the high 25-(OH)D group (n=28), and those with a
25-(OH)D level <14.30 ng/ml were included in the low 25-(OH)D
group (n=32). In addition, 30 healthy children were recruited as
the control group. There was no statistically significant
difference for factors including age, sex, body mass index (BMI)
and allergic conditions of the children between the two subgroups
of the observation group, and data were comparable (Table I).
 | Table I.Comparisons of patients general
clinical information. |
Table I.
Comparisons of patients general
clinical information.
|
|
|
| Observation group
(n=60) |
|
|
|---|
|
|
|
|
|
|
|
|---|
| General
information | Control group
(n=30) | All | Low 25-(OH)D group
(n=32) | High 25-(OH)D group
(n=28) | χ2 | P-value |
|---|
| Age (years) | 5.89±0.82 | 4.3±1.4 | 4.2±1.1 | 4.5±1.5 | 1.23 | 0.437 |
| Sex (male/total) | 14/30 | 29/60 | 17/32 | 12/28 | 1.983 | 0.130 |
| BMI | 20.31±1.06 | 20.83±1.28 | 21.08±1.43 | 20.65±1.25 | 1.534 | 0.582 |
| Allergy
(yes/total) | 0/30 | 11 (60)a | 6/32 | 5/28 | 2.943 | 0.075 |
Treatment methods
The children in the observation group received GINA
treatment protocols (7). The
appropriate treatment protocol was selected for each child in
accordance with the control of asthma.
Index detections
Examination of 25-(OH)D and
biochemical indexes
Prior to the study commencing, fasting venous blood
(10 ml) of the observation and control groups was collected in an
anticoagulant tube containing EDTA. Following centrifugation for 10
min at 3,480 × g at 4°C, the serum 25-(OH)D level in the children
was examined using ELISA. An automatic hematology analyzer (LH755;
Beckman Coulter Biomedical GmbH, Munich, Germany) was utilized to
detect the quantities of leukocytes, neutrophils, lymphocytes and
eosinophils. The contents of immunoglobulin G (IgG), immunoglobulin
A (IgA) and immunoglobulin M (IgM) were measured using immune
scatter turbidimetry, Behring Nephelometer (BN) specific protein
analyzer (CSL Behring, Pensylvania, PA, USA).
Blood of the children (10 ml) in the observation
group was drawn at 8 a.m. on day 1, 3 and 7 after treatment,
respectively; ELISA was performed to detect the levels of
interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) (cat. nos.
ab46042 and ab181421; Abcam, Cambridge, MA, USA) in the serum.
Measurement of pulmonary
functions
Before the treatment, and on day 1, 3 and 7 after
treatment, the RSFJ900 pulmonary function detector (RSDQ;
Chongqing, China) was used to measure the forced expiratory volume
in 1.0 sec (FEV1.0), peak expiratory flow (PEF), ratio of time to
reach peak tidal expiratory flow to total expiratory time
(TPTEF/TE) and ratio of volume to PEF and expiratory volume
(VPEF/VE).
Statistical analysis
The GraphPad Prism software Version 5.01 (GraphPad
Software, Inc., La Jolla, CA, USA) was utilized for statistical
analysis. When measurement data were presented as false, the
Chi-square test was performed. Analysis of variance (ANOVA) was
used for comparisons of differences among multiple groups, and
Tukey test was used as post hoc test, and paired t-test was used
for analysis on differences between the two groups. Pearson's
correlation analysis was utilized to investigate the correlations
of the levels of 25-(OH)D, IL-6 and TNF-α with the changes in
pulmonary function indexes. Linear regression analysis was applied
to analyze the correlation of 25-(OH)D with IL-6 and TNF-α.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparisons of patients general
information
As shown in Table I,
there were no statistically significant differences in age, sex,
BMI and allergic conditions of the asthmatic children between the
high and low 25-(OH)D groups (P>0.05). However, the number of
allergic patients in the observation group was significantly
greater than that in the control group (P<0.05).
Correlation of serum 25-(OH)D with
biochemical indexes
The quantities of leukocytes, neutrophils and
eosinophils in patients in the high 25-(OH)D group were lower than
those in the low 25-(OH)D group (P<0.05). The quantities of
leukocytes, neutrophils and eosinophils in patients in the
observation group were significantly increased compared with those
in the control group (P<0.05) (Table
II).
 | Table II.Correlation of serum 25-(OH)D with
biochemical indexes in child patients. |
Table II.
Correlation of serum 25-(OH)D with
biochemical indexes in child patients.
|
|
|
| Observation group
(n=60) |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Biochemical indexes
(cells/mm3) | Control group
(n=30) | All (n=60) | Low 25-(OH)D group
(n=32) | High 25-(OH)D
group(n=28) | χ2 | P-value |
|---|
| No. of
leukocytes | 5.89±0.82 |
7.23±2.18b |
8.21±2.71c |
6.67±1.59c | 2.034 | 0.362 |
| No. of
neutrophils | 3.32±0.56 |
4.01±1.71a |
5.74±2.13c |
3.68±1.27e | 2.851 | 0.048 |
| No. of
lymphocytes | 1.87±0.58 | 2.18±0.94 | 2.28±1.11 | 2.11±0.81 | 1.328 | 0.382 |
| No. of
eosinophils | 0.15±0.09 |
0.47±0.43b |
0.49±0.53d |
0.40±0.30d | 1.284 | 0.321 |
Correlation of serum 25-(OH)D with
humoral immunity
The levels of IgG, IgA and IgM in the low and high
25-(OH)D groups were significantly higher than those in the control
group (P<0.05). Moreover, the levels of immunologic factors in
the low 25-(OH)D group were elevated compared with those in the
high 25-(OH)D group (P<0.05) (Table
III).
 | Table III.Correlation of serum 25-(OH)D level
with immunologic factors (mean ± SD, µg/ml). |
Table III.
Correlation of serum 25-(OH)D level
with immunologic factors (mean ± SD, µg/ml).
| Groups | No. | IgG | IgA | IgM |
|---|
| Control | 30 | 8.34±1.88 | 1.58±0.51 | 1.22±0.35 |
| Low 25- | 32 |
29.06±3.65a |
11.62±2.01a |
13.54±1.62a |
| (OH)D |
|
|
|
|
| High 25- | 28 |
17.28±2.34a,b |
6.34±1.25a,b |
5.67±0.84a,b |
| (OH)D |
|
|
|
|
Variations in serum TNF-α and IL-6
contents in the three groups of children
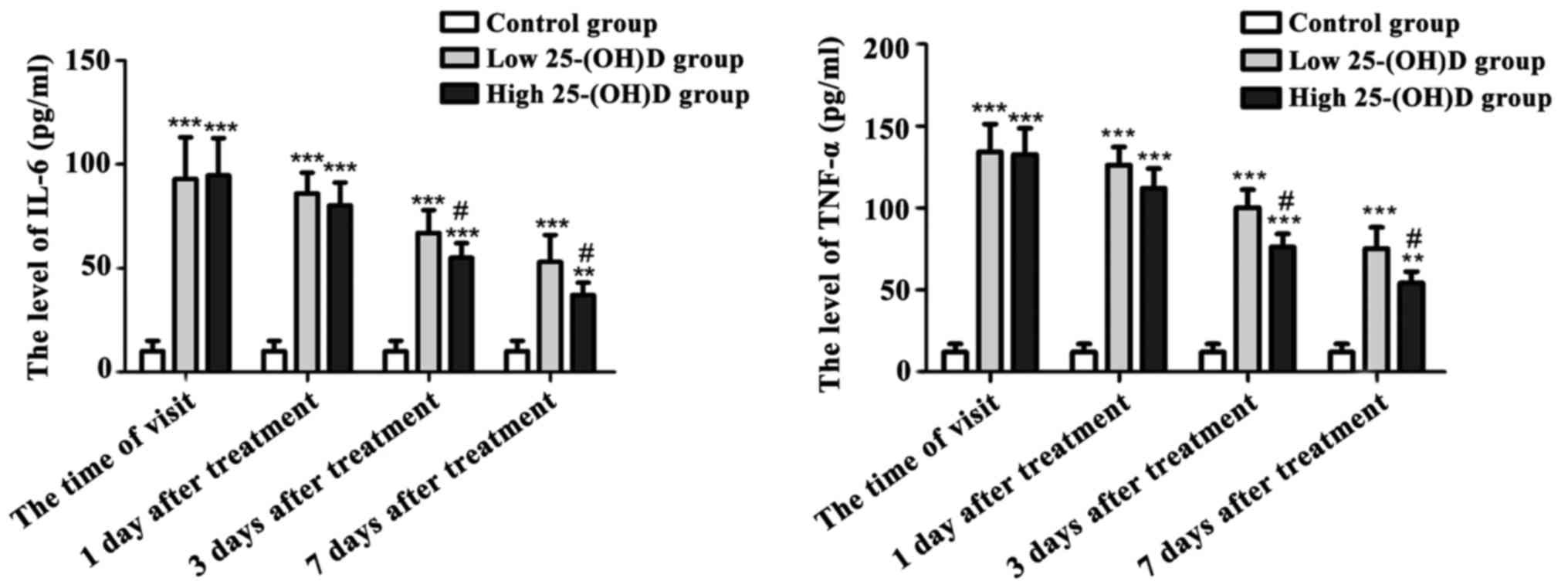
As shown in Fig. 1,
at the time of the first visit and during 7 days of treatment, the
contents of serum IL-6 and TNF-α in asthmatic children of both the
high and low 25-(OH)D groups were significantly higher than those
of the normal control group (P<0.05) with decreasing tendency.
At 3 and 7 days after treatment, the serum IL-6 and TNF-α in the
high 25-(OH)D group was lower compared with that in the low
25-(OH)D group (P<0.05).
Variations in pulmonary function
indexes
The pulmonary functions of the children with acute
attacks in the observation group were monitored, at the time of the
visit, as well as on day 1, 3 and 7 after treatment, respectively.
The results (Table IV) indicated
that the pulmonary functions in the low 25-(OH)D group were
improved on day 3 after treatment, which was manifested as
increased PEF, TPTEF/TE and VPEF/VE compared with those at the time
of the visit. However, those indexes in the high 25-(OH)D group
were improved on day 1 after treatment, which was manifested as
increases in TPTEF/TE and VPEF/VE compared with those at the time
of the visit (P<0.05). Moreover, the treatment effect in the
high 25-(OH)D group was better than that in the low 25-(OH)D group
(P<0.05).
 | Table IV.Variations in pulmonary function
indexes of patients before and after treatment. |
Table IV.
Variations in pulmonary function
indexes of patients before and after treatment.
| Groups | Time | No. | FEV1.0 (l) | PEF (l/min) | TPTEF/TE | VPEF/VE |
|---|
| Control |
| 30 | 3.92±0.21 | 10.21±1.85 | 32.43±2.36 | 33.87±2.26 |
| Low 25-(OH)D | At the time of
visit | 32 | 2.03±0.24 | 3.02±0.57 | 13.15±1.32 | 15.25±1.11 |
|
| 1 day after
treatment |
| 2.15±0.31 | 3.86±0.84 | 14.76±1.60 | 16.32±1.52 |
|
| 3 days after
treatment |
| 2.58±0.54 |
4.53±1.05a |
17.05±1.72a |
18.05±1.87a |
|
| 7 days after
treatment |
|
2.74±0.38a |
5.29±1.54a |
22.53±2.04b |
22.35±2.43a |
| High 25-(OH)D | At the time of
visit | 28 | 2.08±0.17 | 3.30±0.66 | 12.53±1.51 |
19.65±1.84a,c |
|
| 1 day after
treatment |
| 2.26±0.30 | 4.01±0.82 |
17.94±2.73a |
19.65±1.84a,c |
|
| 3 days after
treatment |
|
3.01±0.25a,c |
5.84±1.25a,c |
24.62±2.94a,c |
24.46±3.85a,c |
|
| 7 days after
treatment |
|
3.54±0.37a,c |
6.92±1.07b,c |
28.68±3.51a,c |
30.01±3.94a,c |
Correlations of 25-(OH)D, IL-6 and
TNF-α levels with the changes in the pulmonary function indexes in
asthmatic children
Pearson's correlation analysis was conducted for the
levels of 25-(OH)D, IL-6 and TNF-α as well as the pulmonary
function indexes in asthmatic children on day 7 after treatment.
The results (Table V) showed that
25-(OH)D had a positive correlation with the pulmonary function
indexes (P<0.05), while TNF-α and IL-6 were negatively
associated with the pulmonary function indexes (P<0.05).
 | Table V.Correlation analyses of levels of
25-(OH)D, IL-6 and TNF-α with pulmonary function indexes in
asthmatic children. |
Table V.
Correlation analyses of levels of
25-(OH)D, IL-6 and TNF-α with pulmonary function indexes in
asthmatic children.
|
| 25-(OH)D | TNF-α | IL-6 |
|---|
|
|
|
|
|
|---|
| Pulmonary function
index | r-value | P-value | r-value | P-value | r-value | P-value |
|---|
| FEV1.0 | 0.763 | <0.05 | −0.693 | <0.05 | −0.668 | <0.05 |
| PEF | 0.618 | <0.05 | −0.635 | <0.05 | −0.775 | <0.05 |
| TPTEF/TE | 0.821 | <0.05 | −0.743 | <0.05 | −0.612 | <0.05 |
| VPEF/VE | 0.714 | <0.05 | −0.644 | <0.05 | −0.603 | <0.05 |
Correlation analyses of 25-(OH)D with
IL-6 and TNF-α in asthmatic children
Correlation analysis was conducted for the levels of
serum 25-(OH)D, TNF-α and IL-6 in asthmatic children on day 7 after
treatment. As shown in Table VI,
the serum 25-(OH)D level in asthmatic children was negatively
associated with the levels of inflammatory factors TNF-α and IL-6
(P<0.05).
 | Table VI.Regression analyses on 25-(OH)D, IL-6
and TNF-α in asthmatic children. |
Table VI.
Regression analyses on 25-(OH)D, IL-6
and TNF-α in asthmatic children.
| Inflammatory
factor | Regression
coefficient | t-value | P-value |
|---|
| TNF-α | −1.42 | 2.76 | <0.05 |
| IL-6 | −1.88 | 3.04 | <0.05 |
Discussion
Previous findings have shown that 25-(OH)D is a
positive regulatory factor for innate and adaptive immune systems,
which can regulate various immune cells, such as monocytes,
macrophages, lymphocytes and epithelial cells (7,8). In
addition, 25-(OH)D can influence pulmonary functions by mediating
the macrophages (9). Recent clinical
studies have indicated that high-level 25-(OH)D is associated with
good pulmonary functions, which can ameliorate the glucocorticoid
response (10). It is known that
25-(OH)D deficiency is prevalent in child patients with mild to
moderate persistent asthma, accompanied with the possibility of
serious deterioration (11).
Epidemiological studies have revealed that intake of VitD during
pregnancy can lower the incidence rate of asthma in children
(12). According to the suggestions
of the National Center for Health, the ideal level of serum
25-(OH)D in healthy children is ≥30–40 ng/ml (75–100 nmol/l)
(13). In the present study, it was
found that the overall level of serum 25-(OH)D in asthmatic
children was lower than that in the healthy controls. Detection of
the pulmonary function indexes suggested that the pulmonary
functions in the low 25-(OH)D group were improved at 3 days after
treatment, while those in the high 25-(OH)D group were improved at
1 day after the treatment. In addition, the serum 25-(OH)D level
had a positive correlation with pulmonary functions.
Asthma and airway inflammation are triggered by
cytokines associated with T helper cell type 2, such as IL-4, IL-6
and IL-13 (14). In particular, IL-6
and IL-13 play important roles in the development of airway
hyperreactivity (15). Moreover, the
synergistic effects between the synthesis of specific
immunoglobulin E and airway remodeling can lead to the formation of
IL-6 receptor α-subunit, further inducing the occurrence of
inflammations (16,17). The research by Hinks et al
(18) revealed that the increased
TNF-α, IL-6 and IL-13 levels can obviously induce the occurrence of
asthma and skin inflammations in the animal models. In addition,
25-(OH)D has the function of immunoregulation and host defense in
addition to its influence on calcium and skeletal balance. Simpson
et al found that 25-(OH)D can overcome the glucocorticoid
response in patients with severe asthma by virtue of IL-10, which
is an immunologic factor for cluster of differentiation 4 + T cell
expressions (19). In addition,
25-(OH)D has a negative correlation with the severity of allergy
and asthma, including a number of eosinophils, markers of serum IgE
level and TNF-α level (20,21).
In the present study, the quantities of leukocytes,
neutrophils and eosinophils in patients were increased compared
with those in the control group, and the indexes in the high
25-(OH)D group were lower than those in low 25-(OH)D group.
However, there was no difference in lymphocytes between the two
groups, which may owing to a relatively small number of
participants in the two groups. The Pearson's correlation analysis
revealed that the 25-(OH)D level had a positive correlation with
the pulmonary function indexes. By contrast, the inflammatory
factors TNF-α and IL-6 were negatively asociated with the pulmonary
function indexes.
In conclusion, findings of the present study have
shown that the level of 25-(OH)D was decreased in children
suffering from asthma attack, which was associated with the
inflammatory mediators, IL-6 and TNF-α, as well as pulmonary
functions. Therefore, the level of 25-(OH)D can be employed as an
indicator for the prevention and control of childhood asthma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW wrote the manuscript and helped with measurement
of pulmonary functions. YP and ZZ contributed to index detections
and statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Affiliated Children's Hospital of Xuzhou Medical University
(Xuzhou, China) and informed consent was obtained from patients and
their families.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
National Asthma Education and Prevention
Program: Expert Panel Report 3 (EPR-3): Guidelines for the
diagnosis and management of asthma-summary report 2007. J Allergy
Clin Immunol. 120:94–138. 2007. View Article : Google Scholar
|
|
2
|
Fitzpatrick AM, Teague WG, Holguin F, Yeh
M and Brown LA: Severe asthma research program: Airway glutathione
homeostasis is altered in children with severe asthma: Evidence for
oxidant stress. J Allergy Clin Immunol. 123:146–152. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fitzpatrick AM, Gaston BM, Erzurum SC and
Teague WG: National Institutes of Health/National heart, lung, and
blood institute severe asthma research program: Features of severe
asthma in school-age children: Atopy and increased exhaled nitric
oxide. J Allergy Clin Immunol. 118:1218–1225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong GW, Ko FW, Hui DS, Fok TF, Carr D,
von Mutius E, Zhong NS, Chen YZ and Lai CK: Factors associated with
difference in prevalence of asthma in children from three cities in
China: Multicentre epidemiological survey. BMJ. 329:4862004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wjst M, Altmüller J, Braig C, Bahnweg M
and André E: A genome-wide linkage scan for 25-OH-D(3) and
1,25-(OH)2-D3 serum levels in asthma families. J Steroid Biochem
Mol Biol. 103:799–802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chawes BL, Bønnelykke K, Jensen PF, Schoos
AM, Heickendorff L and Bisgaard H: Cord blood 25(OH)-vitamin D
deficiency and childhood asthma, allergy and eczema: The COPSAC2000
birth cohort study. PLoS One. 9:e998562014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dizier MH, Besse-Schmittler C,
Guilloud-Bataille M, Annesi-Maesano I, Boussaha M, Bousquet J,
Charpin D, Degioanni A, Gormand F, Grimfeld A, et al: Genome screen
for asthma and related phenotypes in the French EGEA study. Am J
Respir Crit Care Med. 162:1812–1818. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cranney A, Horsley T, O'Donnell S, Weiler
H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, et al:
Effectiveness and safety of vitamin D in relation to bone health.
Evid Rep Technol Assess. 158:1–235. 2007.
|
|
9
|
Panda DK, Miao D, Tremblay ML, Sirois J,
Farookhi R, Hendy GN and Goltzman D: Targeted ablation of the
25-hydroxyvitamin D 1α -hydroxylase enzyme: Evidence for skeletal,
reproductive, and immune dysfunction. Proc Natl Acad Sci USA.
98:7498–7503. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu PT, Stenger S, Li H, Wenzel L, Tan BH,
Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al: Toll-like
receptor triggering of a vitamin D-mediated human antimicrobial
response. Science. 311:1770–1773. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sutherland ER, Goleva E, Jackson LP,
Stevens AD and Leung DY: Vitamin D levels, lung function, and
steroid response in adult asthma. Am J Respir Crit Care Med.
181:699–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Litonjua AA and Weiss ST: Is vitamin D
deficiency to blame for the asthma epidemic? J Allergy Clin
Immunol. 120:1031–1035. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van der Meer IM, Karamali NS, Boeke AJ,
Lips P, Middelkoop BJ, Verhoeven I and Wuister JD: High prevalence
of vitamin D deficiency in pregnant non-Western women in The Hague,
Netherlands. Am J Clin Nutr. 84:350–353; quiz 468–469. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martins D, Wolf M, Pan D, Zadshir A,
Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R and Norris
K: Prevalence of cardiovascular risk factors and the serum levels
of 25-hydroxyvitamin D in the United States: Data from the Third
National Health and Nutrition Examination Survey. Arch Intern Med.
167:1159–1165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Björnsdottir US and Cypcar DM: Asthma: An
inflammatory mediator soup. Allergy. 54:55–61. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jayaram L, Pizzichini E, Lemière C, Man
SF, Cartier A, Hargreave FE and Pizzichini MM: Steroid naive
eosinophilic asthma: Anti-inflammatory effects of fluticasone and
montelukast. Thorax. 60:100–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beasley R, Roche W and Holgate ST:
Inflammatory processes in bronchial asthma. Drugs. 37:117–122;
discussion 127–136. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hinks TSC, Brown T, Lau LC, Rupani H,
Barber C, Elliott S, Ward JA, Ono J, Ohta S, Izuhara K, et al:
Multidimensional endotyping in patients with severe asthma reveals
inflammatory heterogeneity in matrix metalloproteinases and
chitinase 3-like protein 1. J Allergy Clin Immunol. 138:61–75.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simpson JL, Scott R, Boyle MJ and Gibson
PG: Inflammatory subtypes in asthma: Assessment and identification
using induced sputum. Respirology. 11:54–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vilarrasa N, Vendrell J, Maravall J, Elío
I, Solano E, San José P, García I, Virgili N, Soler J and Gómez JM:
Is plasma 25(OH) D related to adipokines, inflammatory cytokines
and insulin resistance in both a healthy and morbidly obese
population? Endocrine. 38:235–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peterson CA and Heffernan ME: Serum tumor
necrosis factor-alpha concentrations are negatively correlated with
serum 25(OH)D concentrations in healthy women. J Inflamm (Lond).
5:102008. View Article : Google Scholar : PubMed/NCBI
|