Introduction
Peripheral arterial disease (PAD) is an
atherosclerotic process that manifests in the lower extremities,
which is a common complication of end-stage renal disease (ESRD)
(1,2)
and results in a reduced quality of life (3), in addition to an increased risk of
mortality (4–6). While PAD is likely an important marker
of the cardiovascular status, it has not been researched as
thoroughly as coronary artery disease and its pathogenesis in
patients undergoing dialysis is not well understood (7).
Malnutrition is frequently observed in patients with
ESRD (8) and is associated with
inflammation and atherosclerotic complications (9,10).
Malnutrition, assessed by subjective global assessment (SGA)
(11), has also been associated with
indirect cardiovascular risk markers, including carotid
intima-mediated thickness (12) and
patient mortality (13).
There has been an epidemiological rise of obesity in
the general population worldwide; the incidence of obesity is also
increased in those undergoing dialysis, including those with ESRD
(14). Patients with ESRD and obese
sarcopenia, defined as a high visceral fat (VF) content, have
particularly poor outcomes (15).
Furthermore, a previous study has associated visceral fat (VF)
accumulation in patients with ESRD with proatherogenic
hyperlipidemia and inflammation (13). However, it remains unclear how
nutritional status affects the development of PAD in patients
undergoing dialysis.
The present observational and cross-sectional study
analyzed the presence of PAD in patients undergoing hemodialysis
using ankle-brachial index (ABI) and body composition
measurements.
Patients and methods
Patient selection
A total of 210 patients with ESRD undergoing
hemodialysis at the Outpatient Clinic of the Division of Nephrology
of Tianjin Medical University General Hospital (Tianjin, China)
were analyzed during a single outpatient visit between January and
October 2015. The mean age of the patients included in the present
study was 60 years old, with 52% females and 48% males. Patients
undergoing hemodialysis for <3 months or patients unwilling or
unsuitable for measurement by bioelectrical impedance analysis; for
example, due to significant cognitive impairment, inability to
walk, joint replacement or the presence of a cardiac pacemaker,
were excluded. The ethical committee of Tianjin Medical University
General Hospital approved the present study and written informed
consent was obtained from all patients.
ABI measurement
ABI was measured as described by Feigelson et
al (16). An ABI was calculated
for each leg and the lowest value was used to group patients
according to the presence (ABI <0.9) or absence (ABI ≥0.9) of
PAD.
SGA for malnutrition
A dedicated dietician performed a SGA for
malnutrition. The SGA method included six subjective assessments,
of which three were based on the patient's history (weight loss,
presence of anorexia and vomiting) and three were based on the
physician's grading (muscle wasting, presence of edema and loss of
subcutaneous fat) (17). On the
basis of these assessments, the patients' were divided into
non-malnutrition or malnutrition groups, as described previously
(17).
Body composition analysis
The body composition was measured by bioelectrical
impendence analysis (BIA) using an MC-190 Multifrequency Body
Composition Indicator (Tanita Corporation, Tokyo, Japan), with
patients measured whilst standing and without shoes and socks,
according to the manufacturer's protocol. This device uses
multifrequency (5, 50 and 250 kHz) BIA technology and has eight
tactile electrodes, of which four are in contact with the palm and
thumb of each hand, while the other four are in contact with the
feet. The total fat and VF levels are determined by a proprietary
equation developed by the manufacturer. Patients in the
malnutrition and non-malnutrition groups were divided into high VF
and low VF subgroups based upon whether their VF level was above or
below the median, respectively, which was calculated separately for
males and females.
Flow-mediated dilatation (FMD)
Non-invasive endothelium-dependent FMD was measured
for each patient after a 10 min rest period, in a supine position
and with an empty stomach on a non-dialysis day. The brachial
artery, without an artery-venous fistula, was visualized in a
longitudinal section 15 cm above the antecubital fossa using a
Acuson 128XP/10 Ultrasound Machine with a 7 MHz Linear Array
Transducer (Siemens AG, Munich, Germany). The baseline brachial
artery diameter was measured by automated wall tracking using a
Sonoline G50 Digital Ultrasound system (Siemens) as described
previously (18). Furthermore, a
pneumatic cuff was then inflated to 50 mmHg above the systolic
blood pressure (SBP) and kept on the forearm for 5 min to induce
reactive hyperemia. The percentage change in the brachial artery
diameter 1 min after cuff release compared with the baseline
diameter was recorded as the FMD.
Demographic and clinical data
collection
Previously diagnosed coronary artery disease
(previous myocardial infarction, ischemia detected by cardiogram
abnormality, a history of coronary artery bypass grafting or
coronary stent implantation) or a history of cerebrovascular events
was classified as cardiovascular or cerebrovascular disease (CVD).
A history of smoking (current or former) was defined as a ≥1
pack-year if tobacco use based on patient interviews or chart
documentation.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 15.0; SPSS, Inc., Chicago, IL, USA). Results for
continuous variables are presented as the mean ± standard deviation
and results for categorical variables are presented as percentages.
The statistical significance of differences between the low and
high VF groups (normal nutrition and malnourishment) were
determined using a Student's t-test for comparison between two
variables and one-way multivariate analysis of variance for
continuous variables, while the χ2 test was used for
categorical variables. Bivariate (Pearson) correlation analysis was
conducted to determine the association between PAD and other
variables. Variables that were significantly correlated with PAD
were selected for binary logistic regression analysis to determine
the independent risk factors for PAD. P<0.05 (two-tailed) was
considered to indicate a statistically significant difference.
Results
Clinicopathological characteristics of
patients undergoing hemodialysis based upon their nutritional
status and VF level
A total of 210 patients (mean age, 60 years old; 52%
female and 48% male) were available for analysis in the present
study. The baseline clinicopathological characteristics are
summarized in Table I. Furthermore,
patients with a high VF had a significantly higher body mass index
(BMI) compared with those with a low VF, both in the malnutrition
and non-malnutrition groups (both P<0.05) and the BMI of
malnourished patients with a low VF was significantly lower
compared with that of non-malnourished patients with a low VF (20±2
vs. 22±2.5 kg/m2, respectively; P<0.05). As expected,
the serum albumin level was significantly lower in the malnutrition
group compared with the non-malnutrition groups, in patients with
low VF and with high VF (both P<0.05). Additionally, SBP and
diastolic blood pressure (DBP) was comparable between the groups,
except that the DBP was significantly lower in patients with a high
VF in the malnutrition group compared with the non-malnutrition
group (69±10 vs. 78±10, respectively; P<0.05). In the present
study, malnourished patients with a high VF content had worse
endothelial dysfunction (by FMD) compared with patients with a low
VF content. Moreover, in high VF group, malnourished patients
showed decreased FMD than patients with normal nutrition.
 | Table I.Comparison of variables between the
low and high VF mass groups in well-nourished and malnourished
patients undergoing hemodialysis. |
Table I.
Comparison of variables between the
low and high VF mass groups in well-nourished and malnourished
patients undergoing hemodialysis.
|
| Non-malnourished | Malnourished |
|
|---|
|
|
|
|
|
|---|
| Variable | Low VF (n=136) | High VF (n=32) | Low VF (n=20) | High VF (n=22) | Significance |
|---|
| Age (years) | 54±15 | 66±10 | 64±12 | 72±6 | ABCD |
| Gender (male %) | 56 | 50 | 23 | 41 | B |
| DM (%) | 28 | 45 | 30 | 63 | AC |
| CVD (%) | 12 | 35 | 18 | 33 | A |
| Smokers (%) | 27 | 42 | 10 | 27 | NS |
| Dialysis duration
(m) | 13±18 | 17±21 | 15±18 | 11±13 | NS |
| Height (cm) | 160±9 | 160±8 | 155±8 | 159±8 | B |
| Weight (kg) | 57±9 | 67±10 | 47±8 | 61±10 | ABCD |
| BMI
(kg/m2) | 22±2.5 | 26±3 | 20±2 | 25±4 | ABC |
| Alb (g/l) | 39.9±6 | 39.6±4 | 36.8±4 | 35.6±4 | BD |
| SBP (mmHg) | 134±20 | 138±21 | 133±31 | 125±22 | NS |
| DBP (mmHg) | 80±15 | 78±10 | 75±13 | 69±10 | D |
| TG (mmol/l) | 2.4±1.7 | 2.8±1.8 | 2.5±1.6 | 2.9±2 | NS |
| TC (mmol/l) | 5.1±1.1 | 5.0±1 | 6.1±1.5 | 5.2±1.2 | B |
| HDL (mmol/l) | 1.2±0.3 | 1±0.2 | 1.4±0.4 | 1.1±0.3 | ABC |
| LDL (mmol/l) | 3±0.9 | 3±1 | 3.8±1.1 | 3±0.8 | BC |
| Glu (mmol/l) | 5.4±1.7 | 6.3±2.6 | 6.3±2.7 | 6.5±2 | A |
| LnCRP | 0.48±1.5 | 1.2±1.5 | 0.9±1.1 | 2.1±1.4 | ACD |
| Ca (mmol/l) | 2.3±0.4 | 2.4±0.3 | 2.2±0.4 | 2.5±0.3 | NS |
| P (mmol/l) | 1.6±0.4 | 1.5±0.4 | 1.4±0.5 | 1.5±0.4 | NS |
| BUN (mmol/l) | 23.5±6 | 21.5±5 | 19.7±7.5 | 18.6±7.3 | AB |
| Scr (µmol/l) | 918±288 | 880±277 | 749±352 | 792±263 | B |
| KT/V (total) | 1.8±0.5 | 1.8±0.6 | 1.9±0.4 | 1.8±1.8 | NS |
| KT/V (renal) | 0.5±0.6 | 0.4±0.7 | 0.4±0.4 | 0.3±0.3 | NS |
| Fat (kg) | 14±5 | 23±8 | 11±3 | 21±8 | AC |
| Hb (g/l) | 115±17 | 118±15 | 109±21 | 115±20 | NS |
| Total fat (%) | 24±8 | 33±10 | 24±7 | 31±14 | AC |
| FMD (%) | 8.6±5.5 | 7.3±3.6 | 8.6±4.5 | 4.6±2.4 | CD |
| VF | 7.2±3.3 | 12.8±3.5 | 5.6±2.2 | 12.5±3 | ABC |
Risk factors for PAD in patients
undergoing hemodialysis
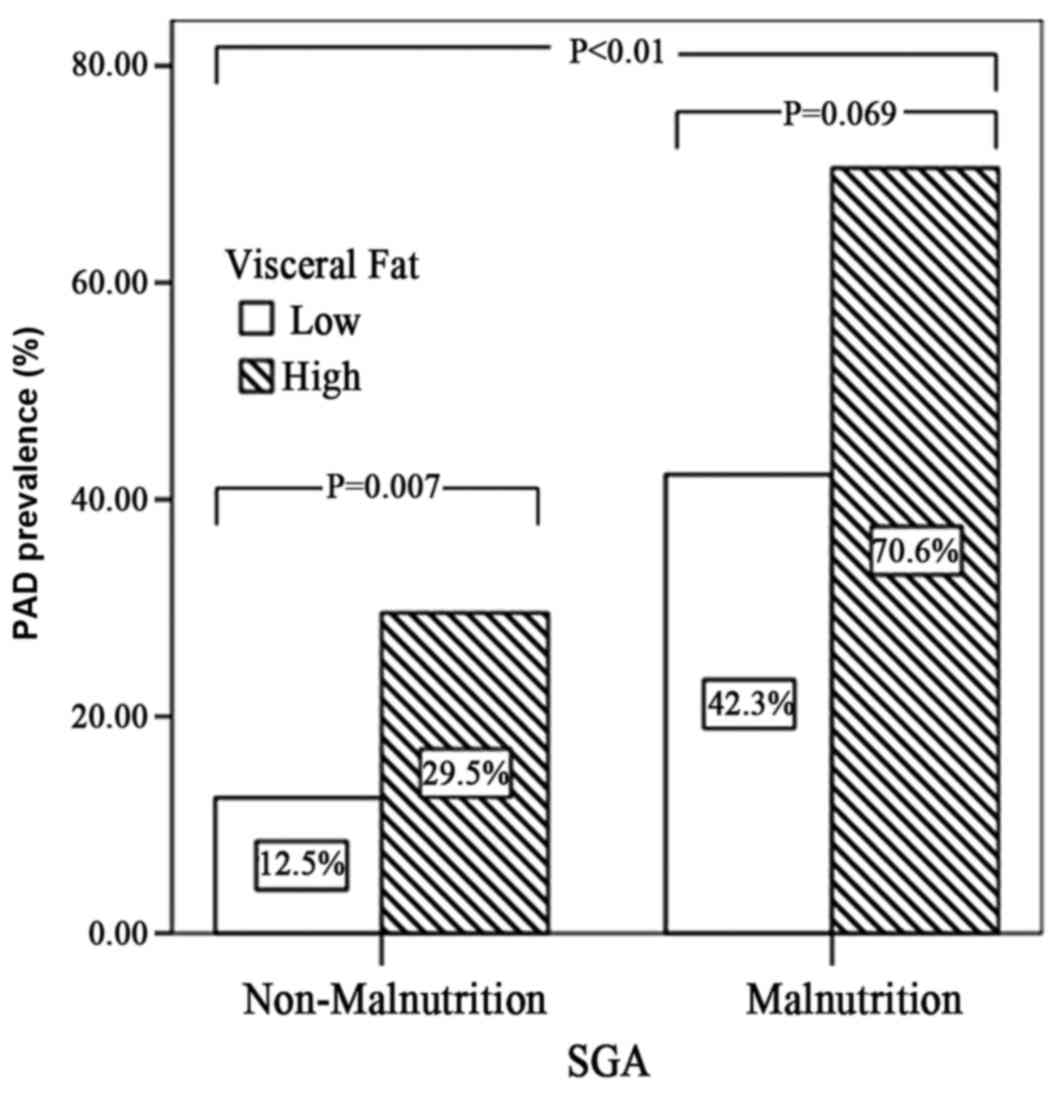
The prevalence of PAD was 28% across all patients
included in the study and it was significantly higher among
malnourished patients compared with non-malnourished patients
(Fig. 1; P<0.01). However,
non-malnourished patients with a high VF had a significantly higher
prevalence of PAD compared with those with a lower VF content (29.5
vs. 12.5%, respectively; P=0.007). Furthermore, bivariate
correlation analysis revealed that the age, diabetic mellitus (DM),
VF, malnutrition, serum albumin level, DBP and log C-reactive
protein (CRP) were significantly correlated with PAD (all
P<0.05) and subsequent logistic regression analysis determined
that age, DM, VF content and malnutrition were independent risk
factors for PAD (all P<0.05) (Table
II).
 | Table II.Univariate and multivariate analysis
of selected variables and peripheral arterial disease patients
undergoing hemodialysis. |
Table II.
Univariate and multivariate analysis
of selected variables and peripheral arterial disease patients
undergoing hemodialysis.
|
| Analysis type |
|---|
|
|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | R-value | P-value | Exp (β) | P-value |
|---|
| Age | 0.304 | <0.010 | 1.036 | 0.047 |
| DM | 0.242 | <0.010 | 2.11 | 0.045 |
| Visceral fat | 0.211 | <0.010 | 1.11 | 0.030 |
| Malnutrition | 0.277 | <0.010 | 4.53 | 0.010 |
| Alb | −0.170 | 0.014 |
|
|
| LnCRP | 0.168 | 0.016 |
|
|
| DBP | −0.226 | <0.010 |
|
|
Discussion
The present study revealed an association between
nutritional status and VF content and the prevalence of PAD in
patients undergoing hemodialysis. Malnourished patients with a high
VF mass (obese sarcopenia) had the highest prevalence of PAD in the
hemodialysis population of the present study. This is similar to
the results of a previous study (15), which identified the detrimental
effects of malnutrition in the presence of obesity by demonstrating
that obese sarcopenia was associated with inflammation and
increased mortality in ESRD patients.
Notably, increased VF was identified to be an
independent risk factor for PAD in the present study. Although no
previous studies, to the best of our knowledge, have explored the
association between VF and PAD in patients with ESRD, it has been
revealed that visceral obesity is associated with uremic
dyslipidemia, inflammation and arterial stiffness (19), in addition to cardiovascular
mortality (20) in patients with
ESRD.
The underlying molecular mechanisms of the
association between adiposity and CVD are not fully understood
(21); however, it is well known
that adipose tissue, in addition to storing energy in the form of
triglycerides, is a highly active endocrine tissue that secretes a
large variety of cytokines, several of which are thought to be
associated with cardiovascular disease (13). Furthermore, systemic inflammation is
considered to be an important mediator of CVD in patients with
ESRD, while VF has been identified to be positively correlated with
low-grade inflammation in this patient group (22). In the present study, patients with a
higher VF mass had significantly higher CRP levels, particularly if
they were also malnourished. Similarly, levels of antiatherogenic
high-density lipoprotein q43 known to be low in obese patients with
ESRD (22) and the present study
identified that patients with increased abdominal fat had a more
atherogenic lipid profile.
A risk factor for PAD is endothelial dysfunction,
which has previously been determined to be associated with obesity
in non-uremic individuals (13). In
the present study, malnourished patients with a high VF content had
worse endothelial dysfunction. This confirms earlier reports of an
association between fat content and FMD (13), in addition to between FMD and the
adipokines, adiponectin, leptin and visfatin, in patients
undergoing dialysis (13). The
present study did not show an association between FMD and PAD
prevalence, which is contrary to what previous studies have
identified. However, the patients undergoing hemodialysis in the
present study had several risk factors for PAD, including
hypertension, dyslipidemia, malnutrition, obesity, diabetes,
smoking, CRP level and CVD complications, which could contribute to
a lower FMD independently of PAD (23).
The present study had a number of limitations,
including that a causal relationship between PAD and obese
sarcopenia could not be determined by a cross-sectional observation
study and the estimation of the body composition of patients
undergoing hemodialysis by BIA may not be as accurate as that of
general subjects due to volume overload. In addition, the levels of
atherogenetic adipokines, including leptin and adiponectin, were
not tested in the present study.
In conclusion, obesity and malnutrition were
demonstrated to have a synergistic effect on increasing the risk of
PAD in patients undergoing hemodialysis. The association between a
high VF content and increased risk of PAD has attracted the
attention of nephrologists for cardiovascular disease detection and
prevention, particularly due to the increasing prevalence of
obesity in patients with ESRD (14).
The present study presents an association between PAD and obese
sarcopenia, which implies that some intervention is required to
decrease the incidence of cardiovascular complications in patients
with ESRD. Further studies are required to clarify whether
improving nutritional status and decreasing visceral fat-mass may
help to reduce cardiovascular complications in patients with
ESRD.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Tianjin Natural
Science Foundation Youth Project (grant no. 15JCQNJC12500).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST contributed to the study design and data
analysis. KZ collected and analyzed data. PX contributed to the
design and drafting of the manuscript, agrees to be accountable for
all aspects of the work and gave final approval of the version to
be published.
Ethics approval and consent to
participate
The ethics committee of Tianjin Medical University
General Hospital approved the present study and written informed
consent was obtained from all patients.
Consent for publication
All patients provided written informed consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu JH, Lin HH, Yang YF, Liu YL, Kuo HL,
Wang IK, Chou CY and Huang CC: Subclinical peripheral artery
disease in patients undergoing peritoneal dialysis: Risk factors
and outcome. Perit Dial Int. 29:64–71. 2009.PubMed/NCBI
|
|
2
|
Chen SC, Su HM, Chang JM, Liu WC, Tsai JC,
Tsai YC, Lin MY, Hwang SJ and Chen HC: Increasing prevalence of
peripheral artery occlusive disease in hemodialysis patients: A
2-year follow-up. Am J Med Sci. 343:440–445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tian SL, Tian XK, Han QF, Axelsson J and
Wang T: Presence of peripheral arterial disease predicts loss of
residual renal function in incident CAPD patients. Perit Dial Int.
32:67–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jimenez ZN, Pereira BJ, Romão JE Jr,
Makida SC, Abensur H, Moyses RM and Elias RM: Ankle-brachial index:
A simple way to predict mortality among patients on hemodialysis-a
prospective study. PLoS One. 7:e422902012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Otsubo S, Kitamura M, Wakaume T, Yajima A,
Ishihara M, Takasaki M, Ueda S, Sugimoto H, Otsubo K, Kimata N, et
al: Association of peripheral artery disease and long-term
mortality in hemodialysis patients. Int Urol Nephrol. 44:569–573.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian SL, Tian XK, Han QF and Wang T:
Peripheral arterial disease predicts overall and cardiovascular
mortality in peritoneal dialysis patients. Ren Fail. 34:1010–1014.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuzawa R, Aoyama N and Yoshida A:
Clinical characteristics of patients on hemodialysis with
peripheral arterial disease. Angiology. 66:911–917. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Locatelli F, Fouque D, Heimburger O,
Drüeke TB, Cannata-Andía JB, Hörl WH and Ritz E: Nutritional status
in dialysis patients: A European consensus. Nephrol Dial
Transplant. 17:563–572. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Papagianni A, Kokolina E, Kalovoulos M,
Vainas A, Dimitriadis C and Memmos D: Carotid atherosclerosis is
associated with inflammation, malnutrition and intercellular
adhesion molecule-1 in patients on continuous ambulatory peritoneal
dialysis. Nephrol Dial Transplant. 19:1258–1263. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oka RK and Alley HF: Differences in
nutrition status by body mass index in patients with peripheral
artery disease. J Vasc Nurs. 30:77–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pecoits-Filho R, Lindholm B and Stenvinkel
P: The malnutrition, inflammation, and atherosclerosis (MIA)
syndrome-the heart of the matter. Nephrol Dial Transplant. 17 Suppl
11:S28–S31. 2002. View Article : Google Scholar
|
|
12
|
Choi HY, Lee JE, Han SH, Yoo TH, Kim BS,
Park HC, Kang SW, Choi KH, Ha SK, Lee HY and Han DS: Association of
inflammation and protein-energy wasting with endothelial
dysfunction in peritoneal dialysis patients. Nephrol Dial
Tranplant. 25:1266–1271. 2010. View Article : Google Scholar
|
|
13
|
Zoccali C: The obesity epidemics in ESRD:
From wasting to waist? Nephrol Dial Transplant. 24:376–380. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Honda H, Qureshi AR, Axelsson J,
Heimburger O, Suliman ME, Barany P, Stenvinkel P and Lindholm B:
Obese sarcopenia in patients with end-stage renal disease is
associated with inflammation and increased mortality. Am J Clin
Nutr. 86:633–638. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feigelson HS, Criqui MH, Fronek A, Langer
RD and Molgaard CA: Screening for peripheral arterial disease: The
sensitivity, specificity, and predictive value of noninvasive tests
in a defined population. Am J Epidemiol. 140:526–534. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Young GA, Kopple JD, Lindholm B, Vonesh
EF, De Vecchi A, Scalamogna A, Castelnova C, Oreopoulos DG,
Anderson GH, Bergstrom J, et al: Nutritional assessment of
continuous ambulatory peritoneal dialysis patients: An
international study. Am J Kidney Dis. 17:462–471. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee JW, Lee HR, Shim JY, Im JA, Kim SH,
Choi H and Lee DC: Viscerally obese women with normal body weight
have greater brachial-ankle pulse wave velocity than nonviscerally
obese women with excessive body weight. Clin Endocrinol (Oxf).
66:572–578. 2007.PubMed/NCBI
|
|
18
|
Postorino M, Marino C, Tripepi G and
Zoccali C: CREDIT (Calabria Registry of Dialysis and
Transplantation) Working Group: Abdominal obesity and all-cause and
cardiovascular mortality in end-stage renal disease. J Am Coll
Cardiol. 53:1265–1272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fantuzzi G and Mazzone T: Adipose tissue
and atherosclerosis: Exploring the connection. Arterioscler Thromb
Vasc Biol. 27:996–1003. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mafra D, Guebre-Egziabher F and Fouque D:
Body mass index, muscle and fat in chronic kidney disease:
Questions about survival. Nephrol Dial Transplant. 23:2461–2466.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berg AH and Scherer PE: Adipose tissue,
inflammation, and cardiovascular disease. Circ Res. 96:939–949.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Axelsson J, Qureshi Rashid A, Suliman ME,
Honda H, Pecoits-Filho R, Heimbürger O, Lindholm B, Cederholm T and
Stenvinkel P: Truncal fat mass as a contributor to inflammation in
end-stage renal disease. Am J Clin Nutr. 80:1222–1229. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuang L and Wong ND: Abstract 38: HDL
cholesterol, inflammation and peripheral arterial disease in U.S.
adults. Circulation. 129 Suppl 1:A382014.
|