Introduction
Gliomas are the most common brain tumors, accounting
for ~30% of central nervous system tumors and 80% of all malignant
brain tumors (1). Due to the
resistance of gliomas to radiotherapy, chemotherapy and adjuvant
therapies, the median survival rate of glioblastomas and high grade
gliomas has not been markedly improved over the past few decades
(2–5). Currently, temozolomide (TMZ) is one of
the most commonly used drugs for the clinical treatment of
glioblastomas (6). TMZ can inhibit
the proliferation of cancer cells via the induction of cell cycle
arrest, and induce tumor cell apoptosis (7,8).
However, there is evidence indicating that the administration of
TMZ also induces tumor chemotherapy resistance via the induction of
autophagy.
Autophagy, an evolutionarily conserved function, is
a cellular self-catabolic degradation process, responsible for the
lysosomal degradation of long-lived proteins as well as aged or
damaged organelles (9). The amino
acids and fatty acids generated during autophagy can be reused and
thus autophagy may be of benefit for sustainable cell survival
(10). A number of studies have
strongly suggested that autophagy is activated in cancer cells
under certain chemotherapy treatments, including TMZ, leading to
tumor chemotherapy resistance (11–13).
Moreover, inhibition of autophagy has been found to enhance the
efficacy of TMZ therapy in glioblastomas (14,15).
MicroRNAs (miRs), a class of non-coding RNAs, 18–25
nucleotides in length, are able to induce mRNA degradation or
suppress protein translation via binding to the 3′-untranslated
regions (3′-UTRs) of mRNA of specific genes (16). Moreover, miRs have been demonstrated
to be involved in a variety of cellular processes, including
proliferation, survival, differentiation, maturation and apoptosis,
as well as autophagy (17,18). Among these miRs, miR-30a has been
found to play a suppressive role in autophagy via the direct
targeting of beclin 1, a key autophagy-promoting gene that is
critical in the regulation of cell survival and death (19). In addition, miR-30a-induced
inhibition of autophagy has been reported to sensitize certain
tumor cells to chemotherapy (20).
For instance, Zou et al found that miR-30a sensitized tumor
cells to cisplatin via the suppression of beclin 1-mediated
autophagy (21). Yu et al
reported that inhibition of autophagy mediated by miR-30a enhanced
imatinib activity against human chronic myeloid leukemia cells
(22). However, to the best of our
knowledge, the detailed role of miR-30a in the regulation of
TMZ-induced autophagy has never been reported in glioblastomas.
In the present study, the aim was to investigate
whether miR-30a has an effect on TMZ-induced autophagy in
glioblastomas. In addition, the involvement of beclin 1 in the
underlying molecular mechanism was explored.
Materials and methods
Cell culture
Human glioblastoma U251 cells were obtained from the
China Cell Culture Center (Shanghai, China). The U251 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin
(all from Thermo Fisher Scientific, Inc.). To mimic chemotherapy,
U251 cells were treated with TMZ (1, 5, 10 or 30 µg/ml) for 6 h,
and then examined by a series of assays.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
TRIzol Reagent (Thermo Fisher Scientific, Inc.) was
used to extract total RNA from U251 cells, in accordance with the
manufacturer's instructions. A RevertAid First Strand cDNA
Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.,
Pittsburgh, PA, USA) was used to reverse transcribe total RNA into
cDNA, according to the manufacturer's protocol. The miRNA
expression was determined using a PrimeScript® miRNA
RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China), in
accordance with the manufacturer's instructions. The PCR conditions
were 95°C for 10 min, and 40 cycles of denaturation at 95°C for 30
sec and annealing/elongation at 60°C for 30 sec. The primer
sequences for miR-30a were: Forward, 5′-GGGGTGTAAACATCCTCGACTG-3′
and reverse, 5′-ATTGCGTGTCGTGGAGTCG-3′. The primer sequences for U6
were: Forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. They were purchased from Shanghai
GenePharma Co., Ltd. (Shanghai, China). All miRNA data are
expressed relative to a U6 small nuclear RNA from the same sample.
Independent experiments were repeated three times. The relative
expression levels of mRNA were analyzed by use of the
2−∆∆Cq method (23).
Transfection
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.)
was used to perform transfection according to the manufacturer's
protocol. Briefly, U251 cells were cultured to 70% confluence, and
resuspended in serum-free DMEM. Serum-free DMEM was used to dilute
Lipofectamine 2000, miR-30a mimic, or scrambled miR mimic,
respectively. The diluted Lipofectamine 2000 was then added to the
diluted miR-30a mimic or diluted scrambled miR mimic. After
incubation for 20 min at room temperature, the mixture was added to
the cell suspension. After incubation at 37°C with 5%
CO2 for 6 h, the medium was replaced by DMEM
supplemented with 10% FBS. Following transfection for 48 h, the
following assays were performed.
MTT assay
An MTT assay was performed to evaluate the cell
proliferation. In brief, 1×104 U251 cells from each
group were plated in a 96-well plate, and incubated for 6, 12, 24
and 48 h at 37°C with 5% CO2. MTT (5 mg/ml; Thermo
Fisher Scientific, Inc.) was then added to each well, and the plate
was incubated for 4 h at 37°C with 5% CO2. The
supernatant was removed, and 100 µl dimethylsulfoxide (Thermo
Fisher Scientific, Inc.) was added to dissolve the precipitate. The
absorbance was detected at 492 nm using the BioTek™ ELX800™
Absorbance Microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA).
Cell apoptosis assay
Cell apoptosis was determined using an Annexin
V-FITC Apoptosis Detection kit (BD Pharmingen, San Diego, CA, USA),
according to the manufacturer's instruction. In brief, U251 cells
were harvested and washed with cold PBS twice. After that, U251
cells (1×106) were resuspended in 200 µl binding buffer
with 10 µl Annexin-V-FITC and 5 µl PI-PE, and incubated in the dark
for 30 min. Following incubation, 300 µl binding buffer was added
and the cells were analyzed by flow cytometry (C6 cytometer;
Beckman Coulter, Inc. (Brea, CA, USA).
Western blotting
U251 cells were solubilized in cold
radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc.) to extract protein, which was separated by 10%
SDS-PAGE (Pierce; Thermo Fisher Scientific, Inc.), and transferred
onto a polyvinylidene difluoride (PVDF) membrane (Pierce). The PVDF
membrane was incubated with rabbit anti-LC3-II polyclonal antibody
(1:50; ab48394; Abcam, Cambridge, MA, USA), rabbit anti-LC3-I
polyclonal primary antibody (1:50; ab128025; Abcam), rabbit
anti-beclin 1 monoclonal antibody (1:100; ab55878; Abcam) and
rabbit anti-GAPDH polyclonal primary antibody (1:100; ab9485;
Abcam), respectively, at 4°C overnight. After washing with PBST
three times, the PVDF membrane was then incubated with mouse
anti-rabbit secondary antibody (1:5,000; ab99697; Abcam) at room
temperature for 40 min. Chemiluminescent detection was conducted
using an ECL kit (Pierce). The protein expression was analyzed
using Image-Pro plus software 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA), and the expression levels were represented as
the density ratio vs. GAPDH.
Bioinformatic analysis and luciferase
reporter gene assay
Targetscan software (version 3.1; targetscan.org/mamm_31/) was used to predict the
putative target of miR-30a. The wild type (WT) or mutant type (MUT)
3′-UTR of the beclin 1 gene (BECN1) was obtained from Yearthbio
(Qingdao, China), amplified from human genomic DNA and then cloned
downstream of the firefly luciferase coding region in the pmirGLO™
Luciferase vector (Promega Corporation, Madison, WI, USA), to
produce pMIR-WT BECN1 and pMIR-MUT BECN1, respectively. After that,
U251 cells were co-transfected with pMIR-WT BECN1 or pMIR-MUT BECN1
vector and miR-30a mimic or scrambled miR mimic, and the pRL-TK
plasmid (Promega Corporation) for internal normalization,
respectively, and cultured for 48 h. The transfected cells were
then lysed using lysis buffer (Promega Corporation). A luciferase
reporter gene assay was then conducted using the Dual-Luciferase
Reporter Assay system (Promega Corporation), in accordance with the
manufacturer's instructions.
Statistical analysis
All data are represented as the mean of at least
triplicate samples ± standard deviation. Statistical analysis of
differences was performed by one-way analysis of variance using
SPSS version 17.0 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Treatment with TMZ inhibits
proliferation and induces apoptosis and autophagy in U251
cells
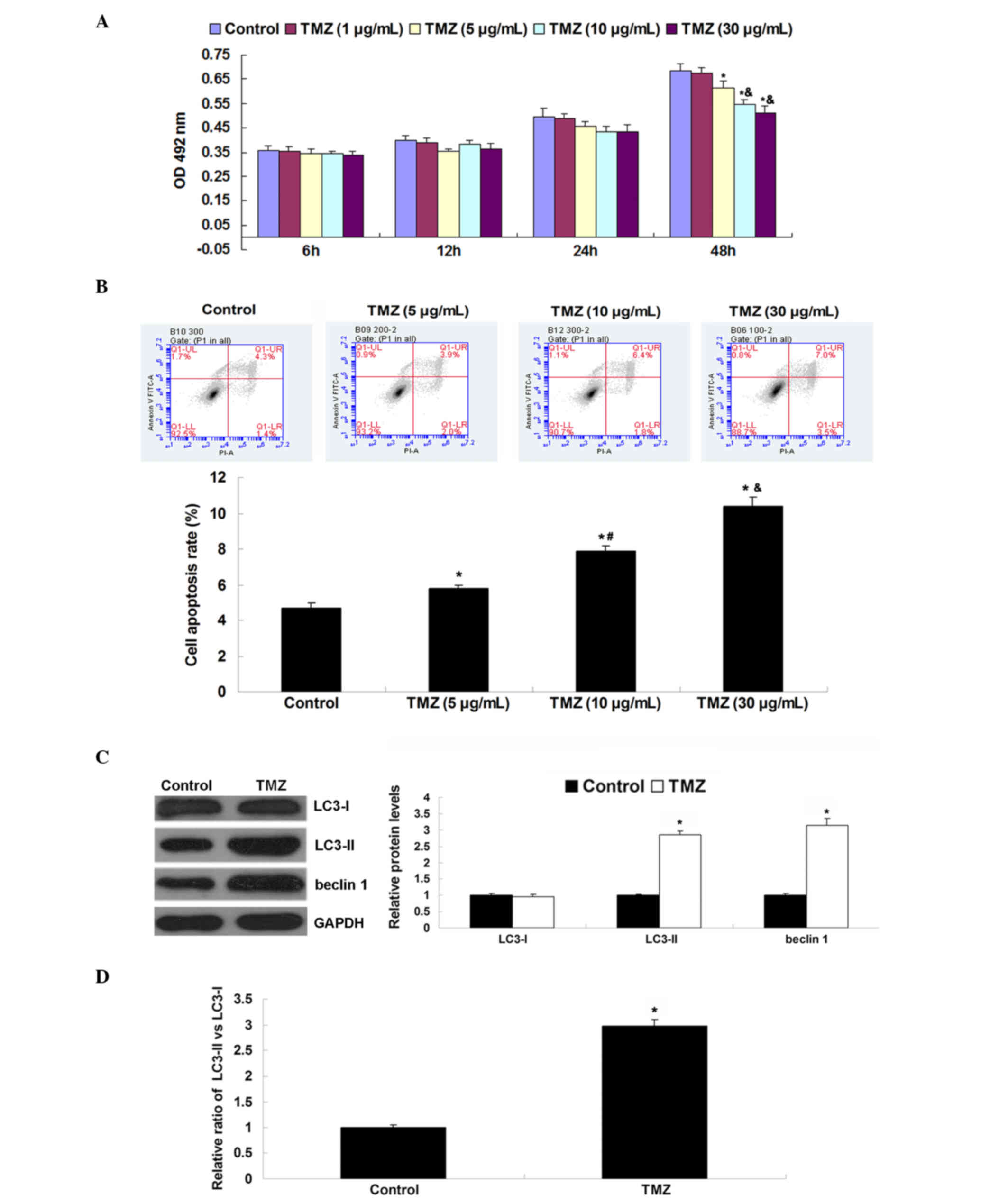
U251 cells were treated with TMZ (1–30 µg/ml).
Following treatment for 6–48 h, an MTT assay was performed to
examine the cell proliferation. As shown in Fig. 1A, the administration of TMZ markedly
inhibited U251 cell proliferation in a concentration-dependent
manner. As 1 µg/ml of TMZ showed no effect on U251 cell
proliferation, this concentration was not used in the following
experiments. Subsequently, the effect of TMZ on U251 cell apoptosis
was examined. The results indicated that treatment with TMZ (5, 10
or 30 µg/ml) significantly induced U251 cell apoptosis in a
concentration-dependent manner (Fig.
1B). Therefore, the highest concentration (30 µg/ml) of TMZ was
used when analyzing the effect of TMZ on protein expression.
Western blot analysis was conducted to determine the levels of
autophagy-related proteins. As shown in Fig. 1C, higher levels of LC3-II and beclin
1 were observed in TMZ-treated U251 cells compared with the control
group. Furthermore, the ratio of LC3-II to LC3-I in the U251 cells
was also increased following treatment with TMZ (Fig. 1D). These findings indicate that
treatment with TMZ inhibits proliferation, while inducing apoptosis
and autophagy in U251 glioblastoma cells.
Treatment with TMZ decreases the
expression of miR-30a in U251 cells
As miR-30a has been demonstrated to play a
suppressive role in autophagy, the expression level of miR-30a in
U251 cells with or without treatment with TMZ (1–30 µg/ml) was
determined. As shown in Fig. 2, the
expression level of miR-30a was significantly reduced in U251 cells
treated with TMZ in a concentration-dependent manner, when compared
with the control group. Therefore, miR-30a may be involved in
TMZ-induced autophagy in U251 cells.
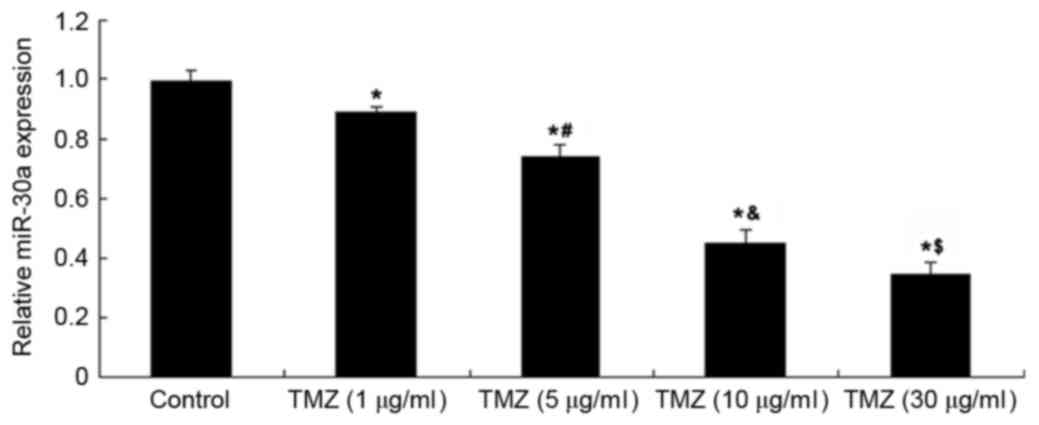
Elevation of miR-30a level suppresses
TMZ-induced autophagy in U251 cells via inhibition of beclin 1
To further determine the role of miR-30a in the
beclin 1-mediated autophagy of TMZ-treated glioblastoma cells, U251
cells were transfected with miR-30a mimic or scrambled miR mimic as
a negative control prior to TMZ treatment. As shown in Fig. 3A, transfection with miR-30a mimic
markedly increased the miR-30a levels, while transfection with
scrambled miR mimic had no effect on the miR-30a level, when
compared with that of the control group. U251 cells were then
treated with TMZ (30 µg/ml) for 6 h, and expression levels of
autophagy markers were examined using western blot assay. As shown
in Fig. 3B, the protein levels
LC3-II and beclin 1 were lower in U251 cells transfected with
miR-30a mimic, compared with the control. Moreover, the ratio of
LC3-II to LC3-I was reduced in miR-30a-overexpressing U251 cells
treated with TMZ, compared with the control cells (Fig. 3C). These findings indicate that
overexpression of miR-30a significantly suppressed TMZ-induced
autophagy in U251 glioblastoma cells.
Overexpression of miR-30a increases
the cytotoxicity of TMZ to U251 cells
Whether miR-30a upregulation could promote the
TMZ-induced inhibition of proliferation and/or TMZ-induced
apoptosis of U251 cells was investigated. As shown in Fig. 4A, MTT assay results showed that the
overexpression of miR-30a significantly suppressed the
proliferation of TMZ (30 µg/ml)-treated U251 cells compared with
the control group. However, transfection with scrambled miR mimic
caused no difference in the proliferation of U251 cells, when
compared with the control group. These data indicate that
overexpression of miR-30a increased the TMZ-induced inhibition of
glioblastoma cell proliferation. It was further observed that an
upregulated level of miR-30a also led to a significant increase in
the apoptosis of TMZ-treated U251 cells, when compared with the
control group (Fig. 4B), indicating
that upregulation of miR-30a also promoted the TMZ-induced
apoptosis of glioblastoma cells. Together, these results indicate
that overexpression of miR-30a increased the cytotoxicity of TMZ to
glioblastoma U251 cells.
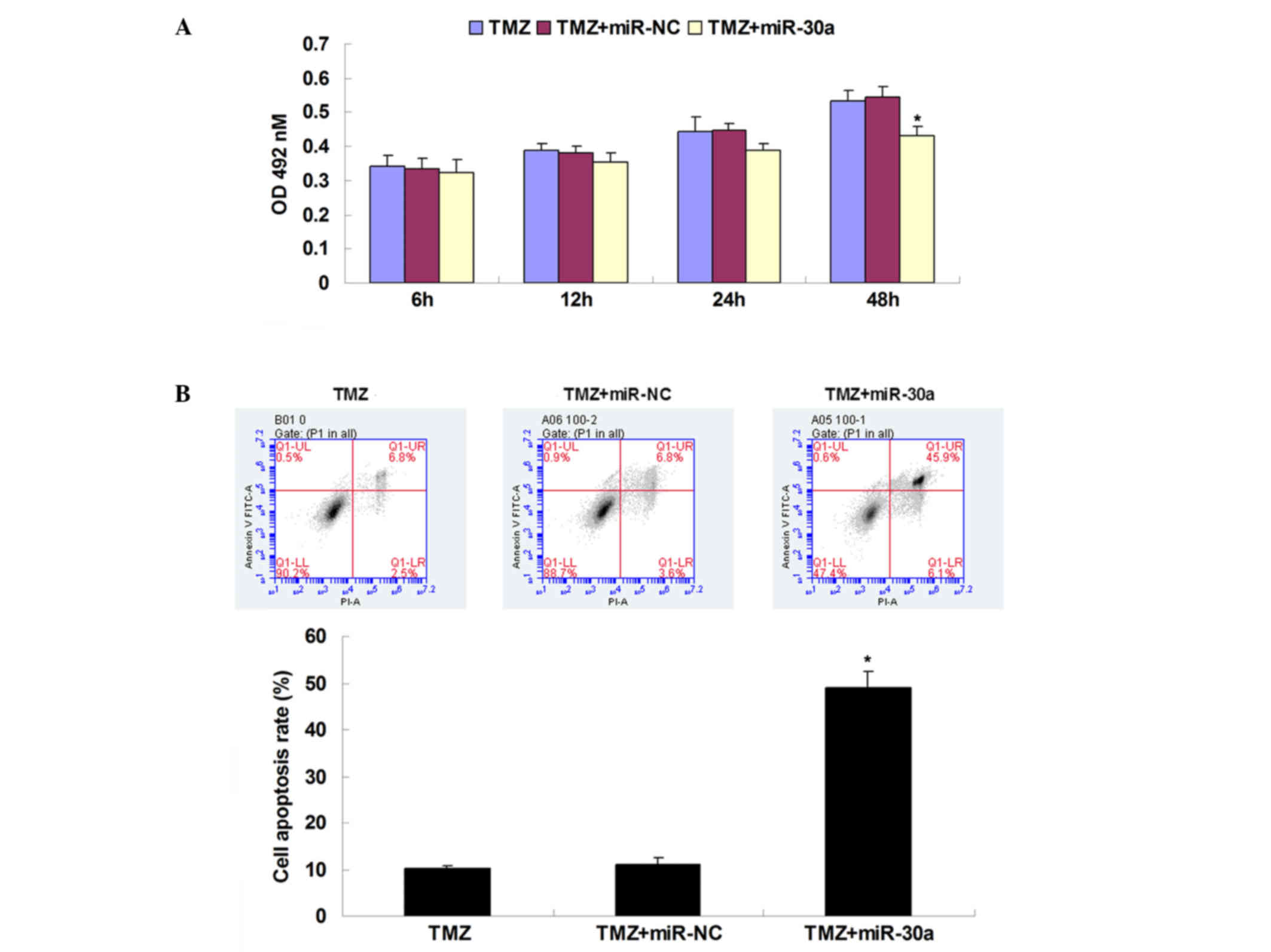
Beclin 1 is a direct target gene of
miR-30a in U251 cells
Finally, the relationship between miR-30a and beclin
1 in U251 cells was examined. Bioinformatic prediction data
indicated that beclin 1 is a direct target gene of miR-30a
(Fig. 5A). To clarify this
relationship, WT or MUT BECN1 3′-UTR was cloned into the pmirGLO
vector, downstream of the firefly luciferase coding region, to
generate pMIR-WT BECN1 and pMIR-MUT BECN1, respectively (Fig. 5B and C). A luciferase reporter assay
was further conducted in the U251 cells. As indicated in Fig. 5D, co-transfection with pMIR-WT BECN1
and miR-30a mimic led to a significant reduction in luciferase
activity; however, co-transfection with pMIR-MUT BECN1 and miR-30a
mimic caused no change in luciferase activity, indicating that
miR-30a could directly bind to the 3′-UTR of beclin 1 mRNA in U251
cells. These results demonstrate that beclin 1 is a direct target
of miR-30a in U251 cells, and suggest that the role of miR-30a in
TMZ-induced autophagy involves the mediation of beclin 1 expression
in U251 cells.
 | Figure 5.Beclin 1 is a direct target of miR-30a
in U251 cells. (A) Bioinformatic prediction data indicated that
beclin 1 was a direct target gene of miR-30a. (B and C) WT or MUT
BECN1 3′-UTR was cloned downstream of the firefly luciferase coding
region of the pmirGLO™ vector, to form pMIR-WT BECN1 and pMIR-MUT
BECN1, respectively. (D) U251 cells were co-transfected with
pMIR-WT BECN1 or pMIR-MUT BECN1 vector and miR-30a mimic or miR-NC,
and pRL-TK plasmid for internal normalization, respectively.
Luciferase reporter assay data showed that co-transfection with
pMIR-WT BECN1 and miR-30a mimic significantly decreased the
luciferase activity; however, co-transfection with pMIR-MUT BECN1
and miR-30a mimic caused no change in luciferase activity. Control
cells were transfected with pMIR-WT BECN1 or pMIR-MUT BECN1 vector
and the pRL-TK plasmid. *P<0.05 vs. control. WT, wild type; MUT,
mutant; BECN1, beclin 1; UTR, untranslated region; miR-NC,
scrambled miR mimic. |
Discussion
Autophagy has been demonstrated to be important not
only in the recirculation of degraded components to sustain
metabolic homoeostasis, but also in the prevention of the toxic
accumulation of damaged components (9). Elevated autophagy has been found in a
variety of tumor cells subjected to certain stresses, including
chemotherapy drug treatment, and it has been well established that
autophagy can lead to the chemotherapy resistance of various human
cancers (11–13). Therefore, inhibition of chemotherapy
drug-induced autophagy appears to be a promising strategy for
enhancing the efficiency of chemotherapy in human cancers. In the
present study, it was found that treatment with TMZ not only
inhibited glioblastoma cell proliferation and induced apoptosis in
a concentration-dependent manner, but also induced the activation
of autophagy, which might attenuate the cytotoxicity of TMZ to U251
glioblastoma cells. The study further demonstrated that
overexpression of miR-30a inhibited TMZ-induced autophagy by
targeting beclin 1, and increased the TMZ-induced inhibition of
cell proliferation in addition to TMZ-induced cell apoptosis in
U251 cells.
TMZ has been widely used for the treatment of many
types of human cancers, including glioblastoma. It has been
reported that the antitumor activity of TMZ is achieved through DNA
damage via induction of loop structures as well as DNA condensation
(24). However, treatment with TMZ
often leads to tumor cell resistance to TMZ due to autophagy
(13). Indeed, in the present study,
treatment with TMZ not only caused cytotoxicity but also increased
glioblastoma cell autophagy, as demonstrated by the upregulated
protein levels of beclin 1 and LC3-II, which was probably caused by
the downregulation of miR-30a.
miR-30a has been found to play a key role in
multiple types of human cancers, mainly acting as a tumor
suppressor. For instance, Fu et al reported that miR-30a
suppressed breast cancer cell proliferation and migration by
targeting Eya2 (25). Zhong et
al found that miR-30a suppressed cell migration and invasion
through downregulation of phosphatidylinositol-4,5-bisphosphate
3-kinase catalytic subunit δ in colorectal carcinoma (26). Several studies have indicated that
miR-30a has suppressive effects on autophagy in different cell
types via the direct targeting of beclin 1 (27–29). For
instance, Wang et al found that inhibition of miRNA-30a
alleviated cerebral ischemic injury by increasing beclin 1-mediated
autophagy (30). In the present
study, it was found that treatment with TMZ induced a significant
reduction in miR-30a level in a dose-dependent manner in U251
cells. In addition, beclin 1 has been demonstrated to function as a
key inducer of autophagy (31), and
has been found to participate in the regulation of tumorigenesis
(32). In the present study, the
protein level of beclin 1 was significantly increased in U251 cells
treated with TMZ, while overexpression of miR-30a inhibited the
TMZ-induced upregulation of beclin 1 in U251 cells. As beclin 1 was
found to be a direct target of miR-30a in U251 cells, it is
suggested that the suppressive effect of miR-30a on TMZ-induced
autophagy is achieved through the direct mediation of beclin 1
expression in U251 cells.
miR-30a has been found to sensitize tumor cells to
several different chemotherapy drugs. Zou et al (21) found that miR-30a sensitized tumor
cells to cisplatin via the suppression of beclin 1-mediated
autophagy. Furthermore, knockdown of miR-30a has been demonstrated
to increase the expression of beclin 1 and inhibit imatinib-induced
cytotoxicity (23). In the present
study, it was found that elevation of the miR-30a level in
TMZ-treated U251 cells notably increased the cytotoxicity of TMZ to
tumor cells, as demonstrated by the reduced cell proliferation as
well as the increased cell apoptosis.
In conclusion, the present study demonstrated that
treatment with TMZ induced an activation of autophagy as well as a
downregulation of miR-30a, while overexpression of miR-30a
inhibited the expression of beclin 1, and thus suppressed
TMZ-induced autophagy in U251 cells. Inhibition of autophagy by the
elevation of miR-30a expression enhanced the cytotoxicity of TMZ to
U251 cells. Based on these findings, it is suggested that autophagy
may be a promising target for the treatment of TMZ-resistant
tumors.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81201740) and as a project
of the Hunan Province Science and Technology Department (grant no.
2012FJ6075).
References
|
1
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart LA: Chemotherapy in adult
high-grade glioma: A systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu VF, Yang J, Lebrun DG and Li M:
Understanding the role of cytokines in glioblastoma multiforme
pathogenesis. Cancer Lett. 316:139–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sathornsumetee S, Reardon DA, Desjardins
A, Quinn JA, Vredenburgh JJ and Rich JN: Molecularly targeted
therapy for malignant glioma. Cancer. 110:13–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pulkkanen KJ and Yla-Herttuala S: Gene
therapy for malignant glioma: Current clinical status. Mol Ther.
12:585–598. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Messaoudi K, Clavreul A and Lagarce F:
Toward an effective strategy in glioblastoma treatment. Part II:
RNA interference as a promising way to sensitize glioblastomas to
temozolomide. Drug Discov Today. 20:772–779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Çıtışlı V, Dodurga Y, Eroğlu C, Seçme M,
Avcı ÇB and Şatıroğlu-Tufan NL: Temozolomide may induce cell cycle
arrest by interacting with URG4/URGCP in SH-SY5Y neuroblastoma
cells. Tumour Biol. 36:6765–6772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li G, Zhang H, Liu Y, Kong L, Guo Q and
Jin F: Effect of temozolomide on livin and caspase-3 in U251 glioma
stem cells. Exp Ther Med. 9:744–750. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
MicroRNAs in apoptosis, autophagy and necroptosis. Oncotarget.
6:8474–8490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang QW, Wang Y, Wang T, Zhang KB, Jiang
CY, Hu FF, Yuan Y, Bian JC, Liu XZ, Gu JH and Liu ZP:
Cadmium-induced autophagy promotes survival of rat cerebral
cortical neurons by activating class III phosphoinositide
3-kinase/beclin-1/B-cell lymphoma 2 signaling pathways. Mol Med
Rep. 12:2912–2918. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kong Q, Xu LH, Xu W, Fang JP and Xu HG:
HMGB1 translocation is involved in the transformation of autophagy
complexes and promotes chemoresistance in leukaemia. Int J Oncol.
47:161–170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sehgal AR, Konig H, Johnson DE, Tang D,
Amaravadi RK, Boyiadzis M and Lotze MT: You eat what you are:
Autophagy inhibition as a therapeutic strategy in leukemia.
Leukemia. 29:517–525. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang MC, Loh JK, Li YY, Huang WS, Chou CH,
Cheng JT, Wang YT, Lieu AS, Howng SL, Hong YR and Chou AK: Bcl2L12
with a BH3-like domain in regulating apoptosis and TMZ-induced
autophagy: A prospective combination of ABT-737 and TMZ for
treating glioma. Int J Oncol. 46:1304–1316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Wang HD, Zhu L, Cong ZX, Li N, Ji
XJ, Pan H, Wang JW and Li WC: Knockdown of Nrf2 enhances autophagy
induced by temozolomide in U251 human glioma cell line. Oncol Rep.
29:394–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu J, Liu ZG, Liu XM, Chen FR, Shi HL,
Pangjesse CS, Ng HK and Chen ZP: Glioblastoma stem cells resistant
to temozolomide-induced autophagy. Chin Med J (Engl).
122:1255–1259. 2009.PubMed/NCBI
|
|
16
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartoszewska S, Kochan K, Piotrowski A,
Kamysz W, Ochocka RJ, Collawn JF and Bartoszewski R: The
hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1α
expression in human endothelial cells through a negative feedback
loop. FASEB J. 29:1467–1479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou S, Zhang P, Liang P and Huang X: The
expression of miR-125b regulates angiogenesis during the recovery
of heat-denatured HUVECs. Burns. 41:803–811. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He R, Peng J, Yuan P, Xu F and Wei W:
Divergent roles of BECN1 in LC3 lipidation and autophagosomal
function. Autophagy. 11:740–747. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng B, Zhu H, Gu D, Pan X, Qian L, Xue
B, Yang D, Zhou J and Shan Y: MiRNA-30a-mediated autophagy
inhibition sensitizes renal cell carcinoma cells to sorafenib.
Biochem Biophys Res Commun. 459:234–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zou Z, Wu L, Ding H, Wang Y, Zhang Y, Chen
X, Chen X, Zhang CY, Zhang Q and Zen K: MicroRNA-30a sensitizes
tumor cells to cis-platinum via suppressing beclin 1-mediated
autophagy. J Biol Chem. 287:4148–4156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu Y, Yang L, Zhao M, Zhu S, Kang R,
Vernon P, Tang D and Cao L: Targeting microRNA-30a-mediated
autophagy enhances imatinib activity against human chronic myeloid
leukemia cells. Leukemia. 26:1752–1760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kondo N, Takahashi A, Mori E, Noda T,
Zdzienicka MZ, Thompson LH, Helleday T, Suzuki M, Kinashi Y,
Masunaga S, et al: FANCD1/BRCA2 plays predominant role in the
repair of DNA damage induced by ACNU or TMZ. PLoS One.
6:e196592011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu J, Xu X, Kang L, Zhou L, Wang S, Lu J,
Cheng L, Fan Z, Yuan B, Tian P, et al: Mir-30a suppresses breast
cancer cell proliferation and migration by targeting Eya2. Biochem
Biophys Res Commun. 445:314–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhong M, Bian Z and Wu Z: Mir-30a
suppresses cell migration and invasion through downregulation of
PIK3CD in colorectal carcinoma. Cell Physiol Biochem. 31:209–218.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X,
Liu CG and Yang JM: Regulation of autophagy by a beclin 1-targeted
microRNA, miR-30a, in cancer cells. Autophagy. 5:816–823. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang J, Huang C, Luo Y, Liu S and Chen X:
Role of MiR-30a in cardiomyocyte autophagy induced by Angiotensin
II. J Renin Angiotensin Aldosterone Syst. 16:1–5. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Z, Wang T, Liu Z, Zhang G, Wang J,
Feng S and Liang J: Inhibition of autophagy by MiR-30A induced by
mycobacteria tuberculosis as a possible mechanism of immune escape
in human macrophages. Jpn J Infect Dis. 68:420–424. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang P, Liang J, Li Y and Li J, Yang X,
Zhang X, Han S, Li S and Li J: Down-regulation of miRNA-30a
alleviates cerebral ischemic injury through enhancing beclin
1-mediated autophagy. Neurochem Res. 39:1279–1291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He Y, Zhao X, Subahan NR, Fan L, Gao J and
Chen H: The prognostic value of autophagy-related markers beclin-1
and microtubule-associated protein light chain 3B in cancers: A
systematic review and meta-analysis. Tumour Biol. 35:7317–7326.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Elgendy M, Ciro M, Abdel-Aziz AK, Belmonte
G3, Dal Zuffo R, Mercurio C, Miracco C, Lanfrancone L, Foiani M and
Minucci S: Beclin 1 restrains tumorigenesis through Mcl-1
destabilization in an autophagy-independent reciprocal manner. Nat
Commun. 5:56372014. View Article : Google Scholar : PubMed/NCBI
|