Introduction
Chronic obstructive pulmonary disease (COPD) is a
chronic lung disease characterized with pathological features of
inflammatory changes such as epithelial hyperplasia, hypertrophy of
airway mucosa, and clinical manifestation of partially reversible
airflow obstruction. The prime cause of COPD is smoke either
primary or secondary (1). Moreover,
smoke in the environment is also responsible to a great extent for
the spread of COPD especially in females (2). Clinically, COPD patients often
experience continuous deterioration due to the recurrence of
re-infection symptoms, which has become an important reason for
increased mortality (3,4). Corticosteroid used as anti-inflammatory
agent and vasoconstrictor of small arteries could reduce
inflammatory exudation and reduce airway obstruction, enabling its
wide application in COPD treatment. However, long-term systemic
therapy is likely to pose severe adverse effects on human body.
Changes in physiological and endocrine structure accompanied with
suppression of cellular immune function during pregnancy further
increase the susceptibility to infection. According to the Global
Initiative for Chronic Obstructive Pulmonary Disease (GOLD, version
2009), inhaled steroid medicines are recommended for AECOPD (acute
exacerbations of chronic obstructive pulmonary disease) patients
failed to achieve satisfactory outcomes with bronchodilators or
hospitalized AECOPD patients (5). In
the present study, 120 pregnant women with COPD admitted to the
Department of Intensive Care Union, of The First People's Hospital
of Xuzhou (Xuzhou, China) from April, 2015 to May, 2016 were
recruited to compare the clinical efficiency and adverse events
during treatment with systemic corticosteroids and topical inhaled
corticosteroids. The above tasks were performed by evaluating the
effects of different approaches on inflammatory factors such as
procalcitonin (PCT) and high-sensitivity C-reactive protein
(hs-CRP), so as to provide more appropriate, safe and effective
treatment plan for pregnant COPD patients.
Materials and methods
General materials
One hundred and twenty pregnant women with COPD aged
25–48 years were recruited. Patients were admitted to the
Obstetrics and Gynecology Department of The First People's Hospital
of Xuzhou from April 2015 to May 2016 and signed written informed
consent before initialization of the study. Further, the Ethics
Committee of The First People's Hospital of Xuzhou provided ethical
clearance for the present study. COPD was diagnosed with the
FEV1/FVC ratio (the ratio of the forced expiratory volume in the
first one second to the forced vital capacity of the lungs) after
inhalation of bronchodilator <70% (6). Patients with one or more conditions
listed in the exclusion criteria were excluded (7): Severe heart failure and hepatic and
renal dysfunction; other existing lung diseases; metabolic
disorders and consumptive diseases, such as hyperthyroidism,
cancer, diabetes mellitus, and active tuberculosis; impaired
consciousness.
Experimental methods
Recruited patients conforming to the inclusion
criteria were randomly allocated into three groups: Intravenous
corticosteroid treatment group (n=42), treated with
methylprednisolone (cat. no. MB0668; Pharmacia and Upjohn Co.,
Pfizer, NY, USA) 40 mg, OD, for 7 days; inhaled corticosteroid
treatment group (n=38), treated with budesonide aerosol (cat. no.
H20140511; AstraZeneca AB, Södertälje, Sweden), 2 mg, tid, for 7
days; and control group receiving no corticosteroid treatment.
Patients in all groups received routine symptomatic supportive
care, including continuous low flow oxygen inhalation,
anticholinergic medications and theophylline bronchodilator, and
antibiotics treatment. Gestational weeks and ages were comparable
among patients in the three groups.
Parameter detection
The serum PCT and hs-CRP expression levels were
measured before treatment and after 7 days of treatment. Further,
the clinical parameters such as symptoms, blood gas analysis
parameters, pulmonary function indexes, fasting blood glucose (FBG)
and adverse reactions were recorded.
Measurement of arterial blood gas parameter oxygen
partial pressure (PaO2) and partial pressure of carbon
dioxide (PaCO2): The arterial blood gas analysis was
performed with blood gas analyzer I-STST1 (300) mode (Abbott
Pharmaceutical Co. Ltd., Lake Bluff, IL, USA).
Pulmonary function test: Spirometry (BTL-08, BTL
Group Ltd., London, UK) was applied to measure the FEV1% pred
(predicted % forced expiratory volume in 1 min) and FEV1/FVC.
PCT and hs-CRP measurement: PCT was measured using
double antibody sandwich immunochromatography Lumat LB 9507
PCT-Analyzer (Berthold Technologies GmbH & Co. KG, Bad wildbad,
Germany); and hs-CRP was measured with turbidimetric immunoassay
(QuikRead, Espoo, Finland).
Determination of clinical
efficiency
The clinical efficiency was measured as
‘ineffective’, ‘effective’, and ‘significantly effective’ based on
the frequency of coughs, the amount of sputum, the degree of
dyspnea, and the auscultation of the lungs (8). The overall efficiency rate was
calculated with the following formula: (significantly effective
cases + effective cases)/total number of cases × 100%.
Statistical analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA)
was used to analyze the data. Quantitative data were described as
mean ± standard deviation, and assessed using F-test. Enumeration
data were expressed as percentages (%) and performed with
Chi-square test to compare the difference. P<0.05 was considered
to indicate a statistically significant difference.
Results
No statistically significant differences were
observed by comparing parameters including age, gestational weeks,
lung function, blood gas analysis, inflammatory factors (PCT,
hs-CRP) and FBG among different groups.
Results of blood gas analysis
The rates of improvement of blood gas analysis (pH,
PaO2, PaCO2) of the inhaled corticosteroid
treatment group and intravenous corticosteroid treatment group were
both significantly higher than those in the control group
(P<0.05; Table I).
 | Table I.Blood gas analysis among the three
groups of patients (mean ± SD). |
Table I.
Blood gas analysis among the three
groups of patients (mean ± SD).
| Parameters | Control group
(n=40) | Intravenous treatment
group (n=42) | Inhalation treatment
group (n=38) | F/χ2
value | P-value |
|---|
| PaCO2
(mmHg) |
|
|
|
|
|
| Before
treatment | 42.98±3.77 | 43.25±4.15 | 41.53±4.56 | 0.333 | 0.836 |
| After
treatment |
40.44±2.86a,c |
37.96±2.96a,b |
38.83±3.55c | 4.997 | 0.023 |
| PaO2
(mmHg) |
|
|
|
|
|
| Before
treatment | 60.68±3.69 | 59.98±4.03 | 61.05±4.92 | 0.579 | 0.566 |
| After
treatment |
63.62±3.48a,c |
66.83±3.14a,b |
66.55±3.36a,b | 2.632 | 0.039 |
| pH |
|
|
|
|
|
| Before
treatment | 7.30±0.04 | 7.32±0.04 | 7.33±0.03 | 0.848 | 0.347 |
| After
treatment |
7.36±0.03a,c |
7.38±0.04a,b |
7.40±0.03c | 4.580 | 0.021 |
Pulmonary function indicators
The pulmonary function indicators (FEV1% pred,
FEV1/FVC) of inhalation treatment group and intravenous treatment
group increased from (42.42±3.29, 47.49±3.55) and (42.22±3.57,
47.35±3.68) to (50.65±3.57, 54.79±3.44) and (49.47±3.37,
53.76±3.15), respectively. Both were significantly higher than that
of the control group (P<0.05; Table
II).
 | Table II.Comparison of pulmonary function
indicators among patients of three groups (mean ± SD). |
Table II.
Comparison of pulmonary function
indicators among patients of three groups (mean ± SD).
| Parameters | Control group
(n=40) | Intravenous treatment
group (n=42) | Inhalation treatment
group (n=38) | F/χ2
value | P-value |
|---|
| FEV1% (pred) |
|
|
|
|
|
| Before
treatment | 42.41±3.29 | 42.22±3.57 | 42.42±3.29 | 0.287 | 0.919 |
| After
treatment |
44.35±2.65a,c |
49.47±3.37a,b |
50.65±3.57a,b | 3.192 | 0.023 |
| FEV1/FVC (%) |
|
|
|
|
|
| Before
treatment | 47.8±2.68 | 47.35±3.68 | 47.49±3.55 | 0.301 | 0.819 |
| After
treatment |
50.43±3.02a,c |
53.76±3.15a,b |
54.79±3.44a,b | 2.998 | 0.040 |
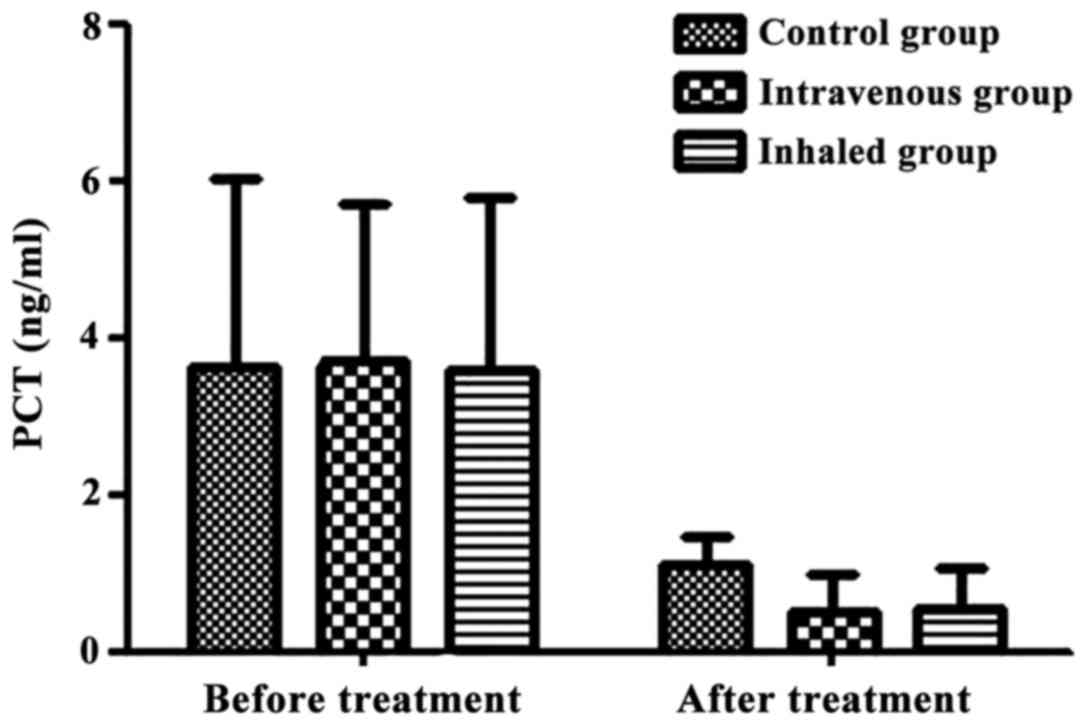
Change in PCT levels
The PCT values before and after treatment of the
control group, intravenous treatment group and inhalation treatment
were 3.64±2.37 vs. 1.10±0.35 ng/ml, 3.71±1.99 vs. 0.50±0.49 ng/ml,
and 3.58±2.20 vs. 0.56±0.49 ng/ml, respectively. It was noted that
the inhibition of inflammatory mediator PCT in
corticosteroid-treated group was remarkably enhanced in comparison
to that of the control group (P<0.05; Fig. 1).
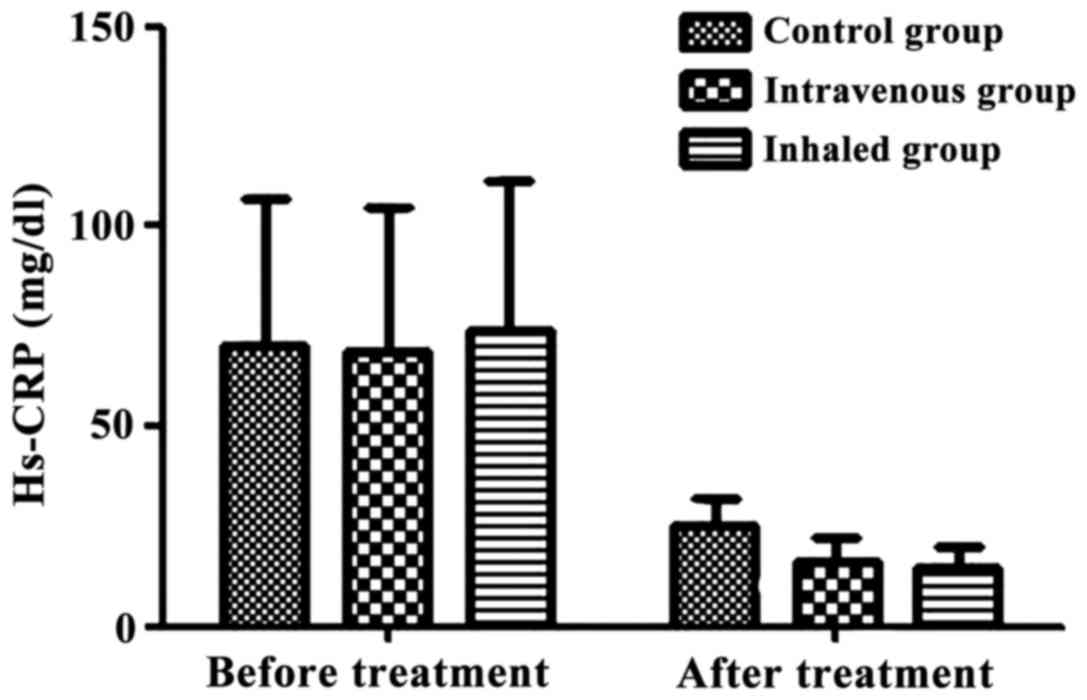
Change in hs-CRP level
The hs-CRP levels before and after treatment of the
control group, intravenous treatment group, and inhalation
treatment were 70.12±36.68 vs. 25.06±6.66 mg/dl, 68.87±36.01 vs.
15.97±5.99 mg/dl, 73.68±37.96 vs. 14.47±5.78 mg/dl. The inhibition
level of inflammatory mediator hs-CRP in corticosteroid-treated
group was remarkably enhanced compared with that of the control
group (P<0.05; Fig. 2).
Change in FBG level
Compared with the control group and inhaled
corticosteroid treatment group, the FBG level of intravenous
corticosteroid treatment group increased significantly (P<0.05)
(Table III).
 | Table III.Comparison of fasting blood glucose
among three groups of patients (mean ± SD). |
Table III.
Comparison of fasting blood glucose
among three groups of patients (mean ± SD).
| Observation
index | Control group
(n=40) | Intravenous
treatment group (n=42) | Inhalation
treatment group (n=38) | F/χ2
value | P-value |
|---|
| FBG (nnmol/l) |
|
|
|
|
|
| Before
treatment | 4.92±0.49 | 4.96±0.55 | 4.85±0.53 | 0.431 | 0.617 |
| After
treatment |
5.01±0.46c |
5.64±0.56a,b |
5.03±0.45c | 7.393 | 0.009 |
Comparisons of rates of clinical
efficiency and adverse events
The overall clinical efficiency rates of inhaled and
intravenous corticosteroid treatment groups were comparable
(P>0.05), and both were higher than that of the control group
(P<0.05). Overall rates of adverse events of inhaled and
intravenous corticosteroid treatment group (such as mouth dry, oral
ulcers, hoarseness) were significantly higher than that of the
control group, and the increase was more pronounced in the
intravenous corticosteroid treatment group (P<0.05; Table IV).
 | Table IV.Comparisons of rates of clinical
efficiency and adverse events among three groups of patients. |
Table IV.
Comparisons of rates of clinical
efficiency and adverse events among three groups of patients.
| Observation
index | Control group
(n=40) | Intravenous
treatment group (n=42) | Inhalation
treatment group (n=38) | F/χ2
value | P-value |
|---|
| Overall efficiency
rate [n (%)] | 26 (65.0) | 38
(90.5)a | 35
(87.5)a | 14.577 | 0.003 |
| Adverse events [n
(%)] | 2
(5.0)b | 16
(38.1)a | 7
(17.5)a,b | 15.203 | 0.004 |
Discussion
Comprehensive treatment approaches for acute
exacerbation of COPD (AECOPD) mainly include bronchial dilation,
anti-inflammatory therapy, and use of corticosteroids to control
airway inflammation (9). Studies
have shown that systemic corticosteroid treatment helps shorten the
rehabilitation time, restored lung function and improved hypoxemia.
Further, it also reduced hospital stay and early recurrence rate.
However, multiple adverse events could be induced by corticosteroid
treatment. This included abnormal lipid metabolism, blood sugar
disturbance, and infection secondary to immune function decline.
Moreover, in a few cases, patients might also experience
respiratory failure (10,11). With local administration of
corticosteroid via inhalation, drugs are inhaled through the airway
into the alveolar, where the highly concentrated local drug
deposition on the lesion surface directly exerts the therapeutic
effect. The hepatic first-pass metabolism of systemic
administration can be avoided, thus significantly reducing the side
effects. However, whether the inhaled corticosteroid could replace
the systemic administration or existence of any potential
difference when compared with systemic corticosteroid therapy
remained a matter of concern.
COPD patients experience persistent airflow
obstruction. Acid-base and electrolyte disorders could be detected
on the basis of blood gas analysis (pH, PaCO2 and
PaO2), This helped to diagnose potential hypoxemia or
hypercapnia and types of respiratory failure, and predict the
outcome. Around half of AECOPDs could be attributed to bacterial
infection-relevant cause (12). As
an acute-phase protein increasing in positive proportion to
infection severity in early onset of infection, CRP level is
insensitive to treatments such as corticosteroid, immunosuppressive
agents, and anti-inflammatory drugs. This enabled CRP as a major
indicator for early diagnosis of COPD and reflector of clinical
treatment outcome (13,14). PCT is the precursor of calcitonin and
significantly increases only during bacterial infection. It is
currently considered that the sensitivity and specificity of serum
PCT in the diagnosis of bacterial inflammation are significantly
better than those of CRP (15,16).
Recent findings showed that treatment course of patients with
respiratory tract infection receiving PCT-based antibiotic therapy
is significantly reduced when compared with patients receiving
treatment guided otherwise. Further, 30-days follow-up did not show
any adverse event, suggesting that PCT could be used as
satisfactory supplementary evidence for diagnosis of AECOPD
(17,18). In the present study, under the same
treatment time, there was no significant difference in the overall
rate of clinical efficiency between the inhaled budesonide
treatment group and the systemic methylprednisolone treatment
group. Both resulted in the decrease of inflammatory mediators
(hs-CRP and PCT). The PCT levels before and after treatment of the
control group, intravenous corticosteroid treatment group and
inhaled corticosteroid treatment group were 3.64±2.37 vs. 1.10±0.35
ng/ml, 3.71±1.99 vs. 0.50±0.49 ng/ml and 58±2.20 vs. 0.56±0.49
ng/ml, and accordingly the hs-CRP levels before and after treatment
were 70.12±36.68 vs. 25.06±6.66 mg/dl, 68.87±36.01 vs. 15.97±5.99
mg/dl and 73.68±37.96 vs. 14.47±5.78 mg/dl, respectively.
Combination of increase in arterial blood gas PaO2 and
decrease in PaCO2, in addition to improvement of lung
function indicators FEV1% pred and FEV1/FVC suggested that, local
inhalation and intravenous systemic corticosteroid treatment could
help in the reduction of the inflammatory response, hypoxia, and
effectively improve lung function.
Corticosteroid treatment during COPD acute
exacerbation could improve rate of clinical efficiency and reduce
hospital stay by improving FEV1 and increasing PaO2
(10,19,20).
Budesonide given with oxygen-driven atomization device enters lung
via airway and acts on lung tissue cells and exerts local
anti-inflammatory and antiallergic effect with high selectivity.
However, some patients might experience adverse events including
dry mouth, throat soreness, hoarseness and oral candidiasis.
Corticosteroid provides efficiency by generating active complex by
binding with cytoplasm and hormone receptor on the cell membrane to
promote apoptosis of inflammatory cells. It has been reported to
reduce airway hyperresponsiveness, and relieve bronchospasm and
improve dyspnea (21). In the
present study, we found that the fasting blood glucose (FBG) level
of COPD patients receiving systemic corticosteroid treatment was
significantly increased, while inhaled budesonide did not affect
FBG. In addition, we observed that incidence of adverse events such
as dry mouth, oral ulcers, and hoarseness of systemic
corticosteroid treatment group was higher than local aerosol
inhalation treatment group and control group. The difference might
be attributed to the fact that inhaled corticosteroid generated
high local concentration of drugs, leaving low amount of drugs into
systemic circulation. Therefore no obvious systemic biological
effect was observed without increase of blood sugar levels due to
liver glycogen gluconeogenesis, and the adverse events were
relatively relieved as well.
The approach, dosage and course of clinical
application of corticosteroid should be determined by selecting
individualized protocol based on varying patient population and
disease condition. Considering that the recruited patients in the
study were pregnant women, the side effects secondary to systemic
corticosteroid use might exceed the therapeutic effectiveness.
Therefore, in summary, topical aerosol inhalation is an ideal
administration approach, which might be more suitable for pregnant
COPD patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ contributed significantly to writing the
manuscript and detectin serum PCT and hs-CRP expression levels. FL
and YL analyzed and interpreted clinical parameters.YS measured
arterial blood gas parameter. ZL and GC performed clinical
efficiency. WZ provided statistical analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The First People's Hospital of Xuzhou (Xuzhou, China). Written
informed consents were signed by the patients and/or guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ukawa S, Tamakoshi A, Yatsuya H, Yamagishi
K, Ando M and Iso H: JACC Study Group: Passive smoking and chronic
obstructive pulmonary disease mortality: Findings from the Japan
collaborative cohort study. Int J Public Health. 62:489–494. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeVries R, Kriebel D and Sama S: Outdoor
air pollution and COPD-related emergency department visits,
hospital admissions, and mortality: A meta-analysis. COPD.
14:113–121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohno Y, Koyama H, Yoshikawa T, Matsumoto
K, Aoyama N, Onishi Y, Takenaka D, Matsumoto S, Nishimura Y and
Sugimura K: Comparison of capability of dynamic
O2-enhanced MRI and quantitative thin-section MDCT to
assess COPD in smokers. Eur J Radiol. 81:1068–1075. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diaz-Guzman E and Mannino DM: Epidemiology
and prevalence of chronic obstructive pulmonary disease. Clin Chest
Med. 35:7–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rabe KF, Hurd S, Anzueto A, Barnes PJ,
Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R,
van Weel C and Zielinski J: Global Initiative for Chronic
Obstructive Lung Disease: Global strategy for the diagnosis,
management, and prevention of chronic obstructive pulmonary
disease: GOLD executive summary. Am J Respir Crit Care Med.
176:532–555. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vestbo J, Hurd SS, Agusti AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta D, Agarwal R, Aggarwal AN, Maturu
VN, Dhooria S, Prasad KT, Sehgal IS, Yenge LB, Jindal A, Singh N,
Jindal SK, et al: for the COPD Guidelines Working Group: Guidelines
for diagnosis and management of chronic obstructive pulmonary
disease: Joint ICS/NCCP (I) recommendations. Lung India.
30:228–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bauer TT, Nilius G, Grüning W and Rasche
K: Diagnosis and therapy of COPD exacerbation. Med Klin Intensivmed
Notfmed. 107:172–178. 2012.(In German). PubMed/NCBI
|
|
9
|
Alía I, de la Cal MA, Esteban A, Abella A,
Ferrer R, Molina FJ, Torres A, Gordo F, Elizalde JJ, de Pablo R, et
al: Efficacy of corticosteroid therapy in patients with an acute
exacerbation of chronic obstructive pulmonary disease receiving
ventilatory support. Arch Intern Med. 171:1939–1946. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaude GS and Nadagouda S: Nebulized
corticosteroids in the management of acute exacerbation of COPD.
Lung India. 27:230–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vestbo J, Hurd SS, Agustí AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rutschmann OT, Cornuz J, Poletti PA,
Bridevaux PO, Hugli OW, Qanadli SD and Perrier A: Should pulmonary
embolism be suspected in exacerbation of chronic obstructive
pulmonary disease? Thorax. 62:121–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Araújo JP, Lourenço P, Azevedo A, Friões
F, Rocha-Gonçalves F, Ferreira A and Bettencourt P: Prognostic
value of high-sensitivity C-reactive protein in heart failure: A
systematic review. J Card Fail. 15:256–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lacoma A1, Prat C, Andreo F, Lores L,
Ruiz-Manzano J, Ausina V and Domínguez J: Value of procalcitonin,
C-reactive protein, and neopterin in exacerbations of chronic
obstructive pulmonary disease. Int J Chron Obstruct Pulmn Dis.
6:157–169. 2011.
|
|
15
|
Hur M, Moon HW, Yun YM, Kim KH, Kim HS and
Lee KM: Comparison of diagnostic utility between procalcitonin and
C-reactive protein for the patients with blood culture-positive
sepsis. Korean J Lab Med. 29:529–535. 2009.(In Korean). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JY, Hwang SJ, Shim JW, Jung HL, Park
MS, Woo HY and Shim JY: Clinical significance of serum
procalcitonin in patients with community-acquired lobar pneumonia.
Korean J Lab Med. 30:406–413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Falsey AR, Becker KL, Swinburne AJ, Nylen
ES, Snider RH, Formica MA, Hennessey PA, Criddle MM, Peterson DR
and Walsh EE: Utility of serum procalcitonin values in patients
with acute exacerbations of chronic obstructive pulmonary disease:
a cautionary note. Int J Chron Obstruct Pulmon Dis. 7:127–135.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Albrich WC, Dusemund F, Bucher B, Meyer S,
Thomann R, Kühn F, Bassetti S, Sprenger M, Bachli E, Sigrist T, et
al: ProREAL Study Team: Effectiveness and safety of
procalcitonin-guided antibiotic therapy in lower respiratory tract
infections in ‘real life’: An international, multicenter poststudy
survey (ProREAL). Arch Intern Med. 172:715–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siddiqui S, Hollins F, Saha S and
Brightling CE: Inflammatory cell microlocalisation and airway
dysfunction: Cause and effect? Eur Respir J. 30:1043–1056. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mikhak Z: Dose-response studies of
fluticasone propionate and budesonide: Classification based on
asthma severity. Allergy Asthma Proc. 27:402–411. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alangari AA: Genomic and non-genomic
actions of glucocorticoids in asthma. Ann Thorac Med. 5:133–139.
2010. View Article : Google Scholar : PubMed/NCBI
|