Introduction
Diabetes mellitus (DM) is a chronic metabolic
disorder that can affect multiple organs through its duration.
Emerging evidence suggests that DM is linked to an increased risk
of mild cognitive impairment (MCI) and the development of dementia
(1). In 1922, Miles and Root noted
that diabetes has a detrimental effect on the central nervous
system (CNS), which may lead to cognitive dysfunction (2). In 2006, Mijnhout et al proposed
the concept of ‘diabetes-associated cognitive decline’ (DACD) in
order to facilitate further research into this disorder (3).
Our previous studies have indicated that patients
with type 2 diabetes develop cognitive dysfunction and perform
poorly with respect to short term memory,
visuospatial/constructional memory, delayed memory, attention and
language skills (4). Accumulating
evidence suggests that DACD is associated with metabolic
dysfunction in the brain (5),
deficiencies in nerve growth factor (NGF) and oxidative stress
factors, and the inhibition of cell survival signaling pathways
(6). However, the underlying
mechanisms and potential prevention methods have yet to be
elucidated.
The dipeptidyl peptidase-4 (DPP-4) inhibitor
vildagliptin is a novel anti-hyperglycemic drug that may benefit
patients with diabetes in various ways, including by regulating
blood glucose levels with fewer associated adverse events,
increased weight loss and cardiovascular advantages (7). Whether it has neuroprotective effects
and the potential mechanisms underlying these effects, remains to
be studied. The purpose of the present study was to explore whether
vildagliptin was able to prevent the development of cognitive
deficits in a rodent model of diabetes. Using a diabetic rat model,
behavioral tests were performed and the expression levels of
protein kinase B (Akt), phosphorylated (p)-Akt, glycogen synthase
kinase 3β (GSK3β) and p-GSK3β were measured in the brain, in
addition to those of caspase-3, B cell lymphoma-2 (Bcl-2) and Bcl-2
associated X protein (Bax).
Materials and methods
Animals
The animal experiments were conducted with the
approval of the Ethics Committee of Hebei Medical University
(Shijiazhuang, China). A total of 30 male Wistar rats (age, 15
weeks; weight, 220–250 g) were obtained from the Animal Experiment
Center of North China University of Science and Technology
(Tangshan, China) in the present study. Rats were given ad
libitum access to water and were maintained at a temperature of
20–24°C and a humidity of 50±10% with a standard 12-h light/dark
cycle. Following 2 weeks of acclimation, 20 Wistar rats were
randomly divided into two groups (n=10): The DM group and the
vildagliptin-treated diabetic group. A total of 40 mg/kg
streptozotocin (STZ; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was administered via intraperitoneal injection in all rats to
induce diabetes. Establishment of the diabetic models were regarded
as successful if blood glucose levels were >16.7 mmol/l. At 10
weeks following the successful establishment of the models, rats in
the DM group received a dose-matched placebo (saline) and rats in
the vildagliptin-treated group were administered 5 mg/kg
vildagliptin (Galvus; Novartis International AG, Basel,
Switzerland) once a day via oral gavage for 4 consecutive weeks.
The remaining 10 Wistar rats were treated with an equal amount of
normal saline and were designated as the control group. Following
treatment, behavioral tests and biochemical experiments were
conducted in sequence.
Behavioral tests
Spatial learning and memory were performed using the
Morris water maze at 4 weeks following completion of vildagliptin
treatments. At the beginning of the navigation task, the rats were
placed in a black circular water tank (150 cm in diameter and 60 cm
in depth) and allowed to swim for 5 min. They were trained to find
a hidden platform (14 cm in diameter), which was located at the
midpoint of the target quadrant, at a fixed time each day. During
this period, the rats were placed randomly in the water at four
different starting points (corresponding to the different
quadrants). If the rats successfully found the platform during the
Morris water maze test, they would be left on the platform for 15
sec prior to the start of the next training section. On day 5 of
the spatial tests, the platform was removed and the rats were
placed into the water at the same randomly selected starting
points. Maze performance was recorded using a video camera located
above the pool and interfaced with a video tracking system (HVS
Imaging, Hampton, UK). The mean escape latency of a total of 5
trials and the times for which the rats remained in the quadrant,
were then calculated.
Histology
Following completion of the behavioral tests, the
hippocampus was excised and frozen. Sections of the hippocampal
tissue were investigated for neuronal damage using Nissl's staining
method. Rats were anesthetized with sodium pentobarbital (60 mg/kg;
Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and
sacrificed via transcardiac perfusion with cold PBS, and
subsequently fixed with cold 4% paraformaldehyde, containing 0.2%
saturated picric acid, in PBS for 24 h at 4°C. The brains were
removed and the CA1 region of the hippocampus were post-fixed
overnight at 4°C in the aforementioned fixative solution. The
remaining samples were frozen at −80°C. Paraffin-embedded tissue
sections were cut in the coronal plane at a thickness of 5 µm using
a microtome. These sections were de-paraffinized with xylene and
rehydrated using a descending series of alcohol, stained with 0.1%
(w/v) cresyl violet for 10 min at 37°C, and the severity of
neuronal damage was evaluated according to the number of surviving
neurons. Three Nissl-stained sections from each rat was randomly
selected and the number of surviving neurons was counted in three
randomly chosen fields. Samples were observed using an optical
microscope (magnification, ×200; Olympus Corporation, Tokyo,
Japan). The mean number of morphologically intact neurons per 100
µm was calculated using Image J 1.41 software (National Institutes
of Health, Bethesda, MD, USA) in the CA1 hippocampal area in order
to estimate the extent of neuronal damage.
Western blotting
Following behavioral tests, the hippocampus of each
rat was excised and flash-frozen in liquid nitrogen. Frozen samples
in liquid nitrogen were obtained and lysed in Tissue Protein Lysis
Solution (Thermo Fisher Scientific, Inc., Waltham, MA, USA) which
contained 5% Proteinase Inhibitor Cocktail (Sigma-Aldrich; Merck
KGaA). Protein concentration was determined by using a BCA reagent
(OriGene Technologies, Inc., Beijing, China) method. A total of 25
µg of extracted protein were resolved via SDS-PAGE and transferred
to a polyvinylidene difluoride membrane. The membranes were blocked
in 5% non-fat milk for 2 h at room temperature and washed three
times in PBS with Tween-20. The membranes were probed overnight at
4°C with primary antibodies specific for Bcl-2 (1:500; cat. no.
BS70205; Biogot Technology Co., Ltd., Nanjing, China) and Bax
(1:500; cat. no. BS6420; Biogot Technology Co., Ltd.), and
caspase-3 (1:1,000; cat. no. AB13847; Abcam, Cambridge, UK), p-Akt
(1:1,000; cat. no. AB38449; Abcam), Akt (1:1,000; cat. no. AB8805;
Abcam), GSK3β (1:1,000; cat. no. AB32391; Abcam) and p-GSK3β
(1:1,000; cat. no. AB75745; Abcam), brain-derived neurotrophic
factor (BDNF; 1:1,000, cat. no. AB226843; Abcam), NGF (1:1,000,
cat. no. AB5199; Abcam) and β-actin (1:1,000; cat. no. AB8227;
Abcam), followed by labeling with horseradish peroxidase-conjugated
secondary antibodies (1:1,000; cat. no. AB205718; Abcam) at 4°C for
2 h. Bands were visualized using an enhanced chemiluminescent
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) reagent and were
analyzed using ImageJ 1.41 software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Each experiment was repeated a minimum of 3 times.
Differences among three or more groups were analyzed using one-way
analysis of variance, followed by the Bonferroni post hoc test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
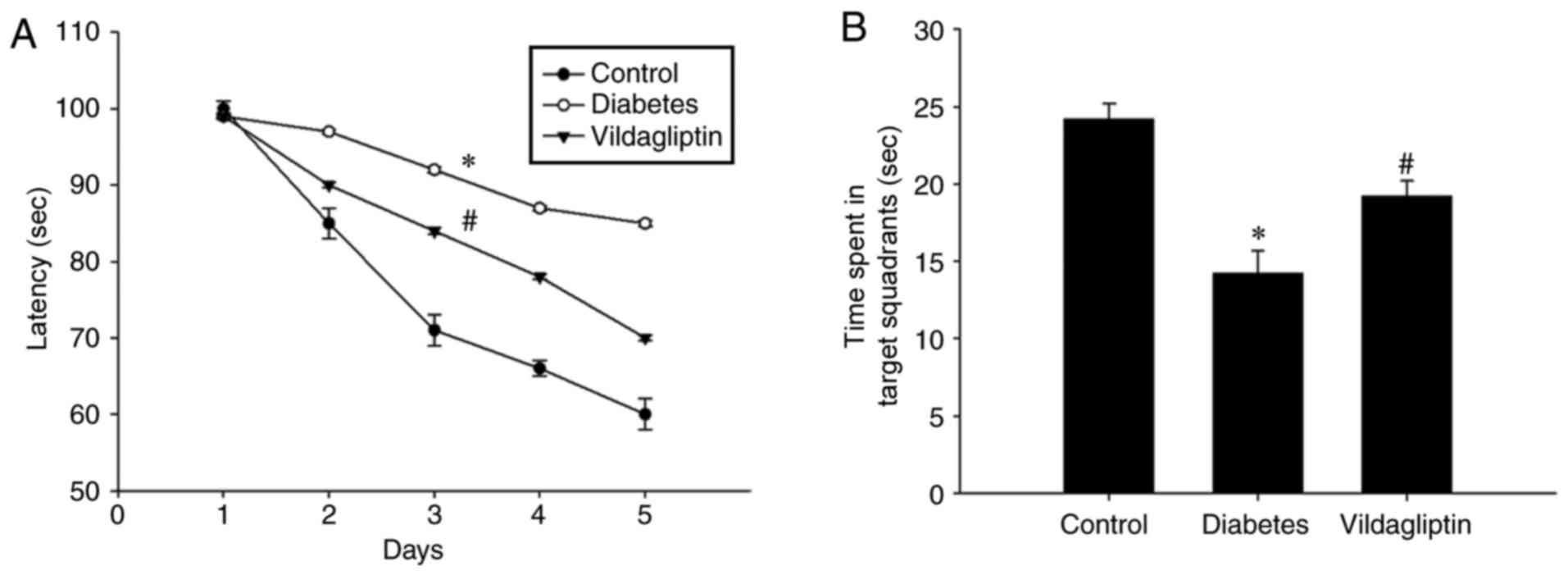
Vildagliptin ameliorates the
impairments to spatial learning and memory observed in the DM
group
It was evaluated whether vildagliptin treatment was
able to improve spatial learning function using the Morris water
maze. As presented in Fig. 1A, STZ
induced a significant spatial learning deficit in the DM group as
compared with the control group, whereas the administration of
vildagliptin significantly reduced the escape latency when compared
with the DM group (P<0.05). Following 4 days of training, the
platform was removed. Under these conditions, the time spent in
target quadrant was significantly lower for the DM group than for
the control group, but was significantly increased in the
vildagliptin treatment group compared with the DM group (Fig. 1B).
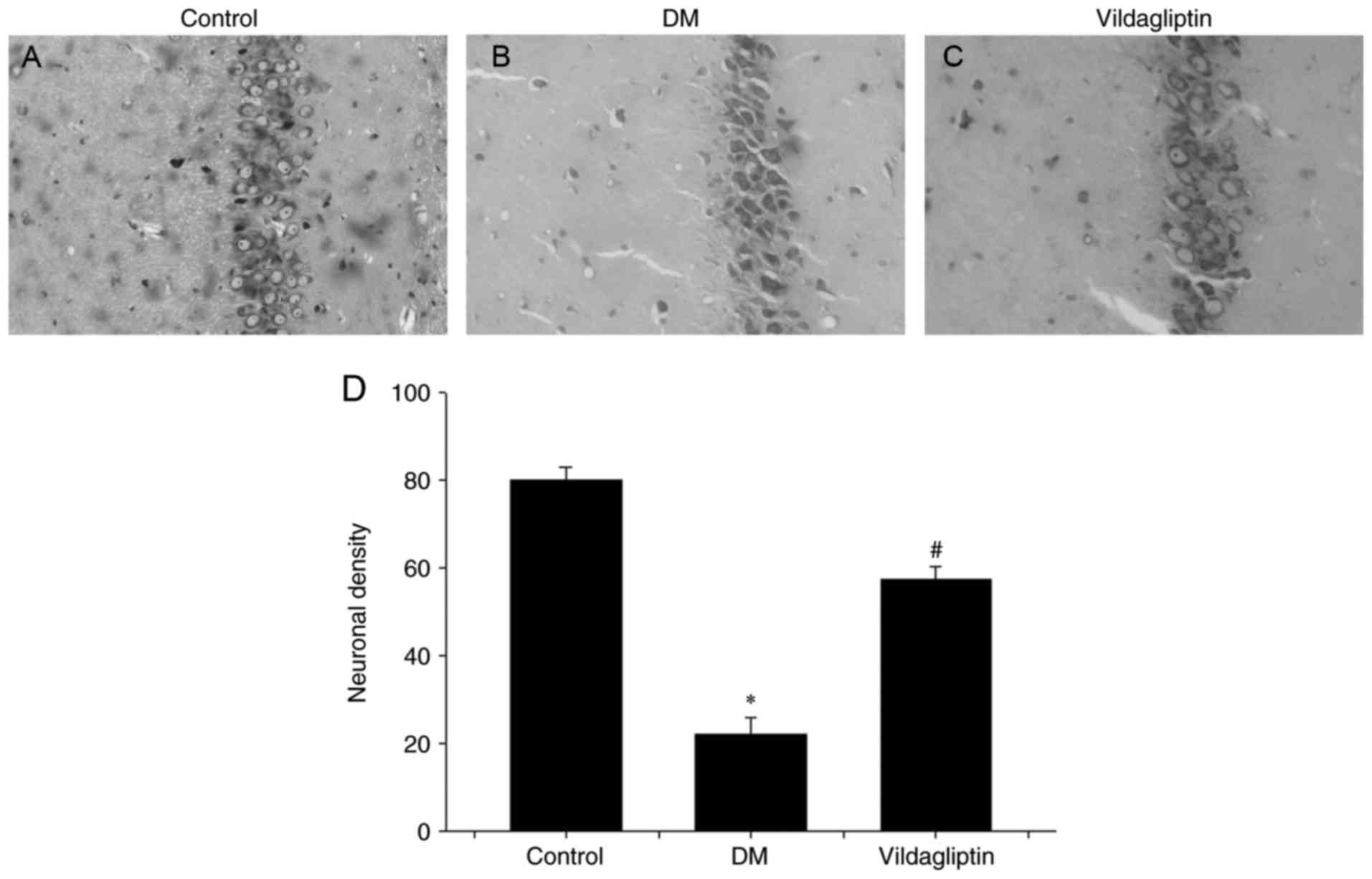
Vildagliptin prevents neuronal cell
loss and attenuates abnormalities in caspase-3, Bax and Bcl-2
expression in the diabetic model
Nissl's staining method was performed to investigate
neuronal alterations in the hippocampal CA1 region of rats in
different groups (Fig. 2). In the DM
group, hippocampal neurons were characterized by pronounced
shrinkage of the neuronal bodies, with the loss of nuclei and
pyknotic pyramidal cells (Fig. 2B).
However, neurons in the control group were large, conical-shaped
cells with well-demarcated amphophilic cytoplasm and round
vesicular nuclei with prominent nucleoli (Fig. 2A). Treatment with vildagliptin
reduced the DM-induced cell loss and pyknotic cells, but
degenerating cells with altered morphology were still present
(Fig. 2C). Vildagliptin exhibited a
significant protective ability against DM-induced neurotoxicity
(Fig. 2D).
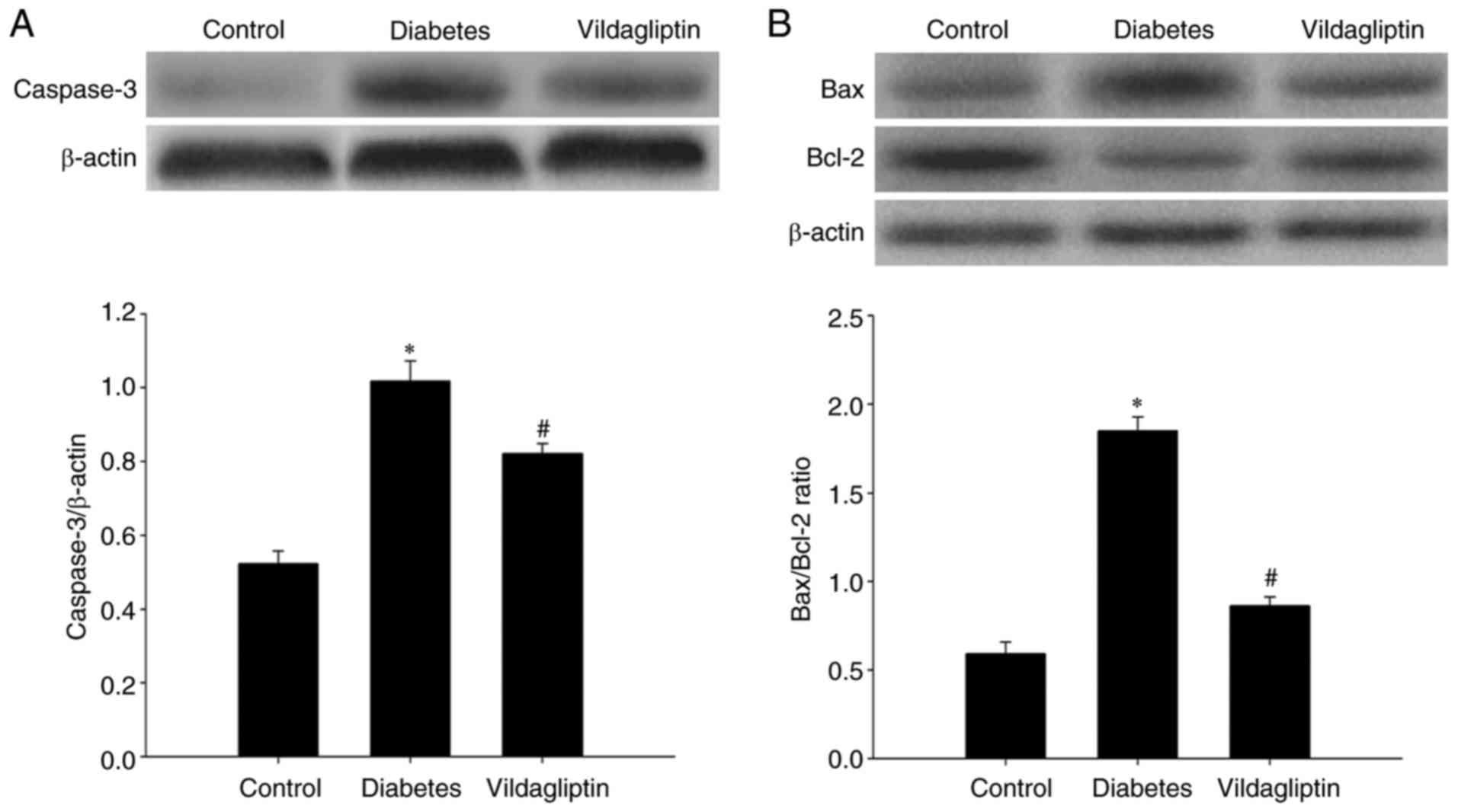
Western blotting was used to detect caspase-3, Bax
and Bcl-2 protein levels in the rat hippocampi. Compared with the
normal control group, hippocampal caspase-3 was significantly
increased in the DM group and significantly downregulated by
vildagliptin treatment compared with the DM group (Fig. 3A). As presented in Fig. 3B, the Bax/Bcl-2 ratio in the rat
hippocampi differed considerably among the three groups. Compared
with the control group (0.591±0.020), STZ-induced DM significantly
increased this ratio (1.856±0.021; P<0.05), whereas the
STZ-induced increases in the Bax/Bcl-2 ratios were attenuated by
treatment with vildagliptin (0.861±0.012; P<0.05).
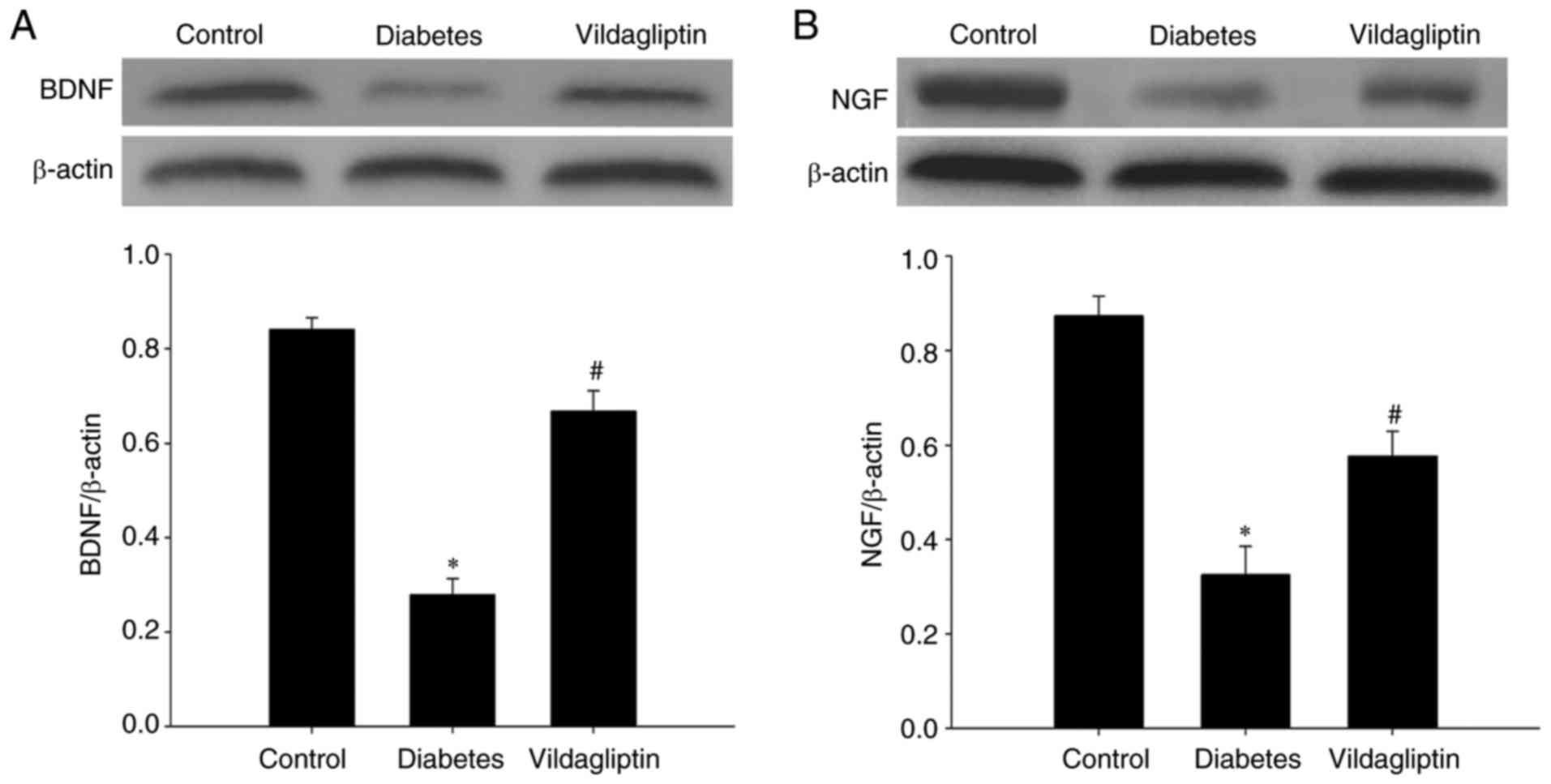
Vildagliptin reverses the decrease in
neurotrophic factor expression
BDNF and NGF, both neurotrophic factors, were also
detected in each group via western blotting. STZ-induced DM
significantly reduced the levels of both BDNF and NGF. By contrast,
vildagliptin treatment significantly attenuated the STZ-induced
decreases (Fig. 4).
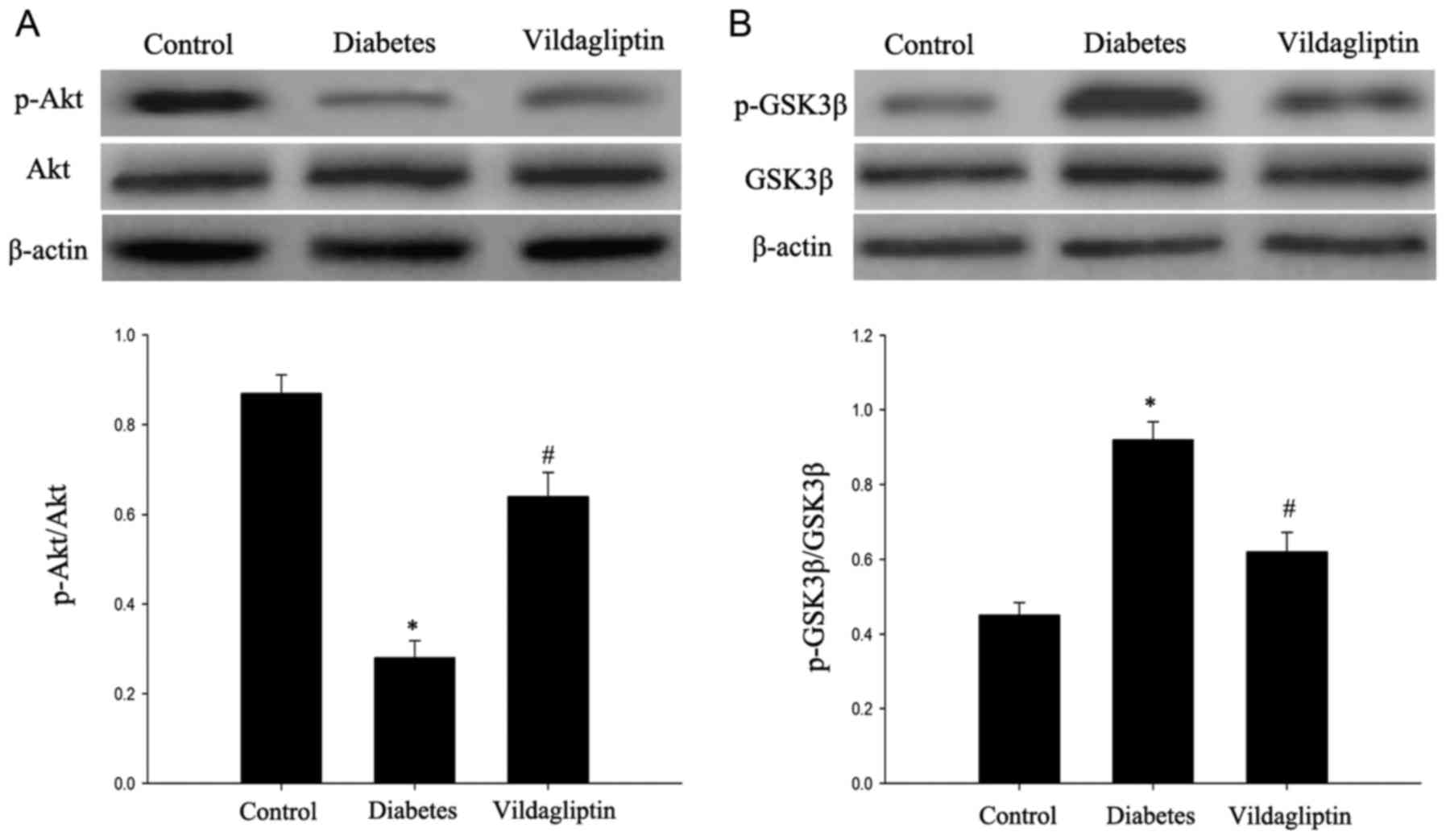
Vildagliptin attenuates the deficit in
the Akt/GSK3β pathway
The Akt/GSK3β pathway, which has been proposed to
serve an important role in cell apoptosis (8), was investigated in the rats. As
presented in Fig. 5, the DM group
exhibited significantly decreased p-Akt expression, in addition to
significantly increased p-GSK3β expression. Vildagliptin treatment
significantly attenuated the decrease in p-Akt expression and
decreased the p-GSK3β expression (P<0.05). By contrast, GSK3β
and Akt total protein levels did not markedly differ among the
three groups.
Discussion
Cognitive impairment is regarded as one of the most
easily underestimated complications of diabetes (9). In the present study, vildagliptin
attenuated STZ-induced spatial learning and memory deficits in
rats, as evaluated using the Morris water maze. Consistent with the
present results, certain prior studies demonstrated that DPP4
inhibitors, including vildagliptin and saxaglipyin may exert
notable neuroprotective effects in animal models of
neurodegeneration, including stroke (10) and Parkinson's disease (11). Furthermore, DPP4 inhibitors were
observed to significantly reduce the plaque load following
long-term treatment in a mouse model of Alzheimer's disease
(12). In the present study, the
pharmacological efficacy of vildagliptin in ameliorating memory
impairment was demonstrated in diabetic rats.
DPP4 inhibitors, a new class of anti-diabetic
agents, mimic many of the actions ascribed to glucagon-like peptide
(GLP)-1 receptor (R) agonists by suppressing DPP4, which act to
increase the level of active GLP-1 in the peripheral blood and then
indirectly diffuse into the brain (13). Mechanisms of action include the
stimulation of insulin and the inhibition of glucagon secretion,
and the preservation of β cell mass via the stimulation of cell
proliferation and the inhibition of apoptosis (14). Unlike GLP-1R agonists, DPP4
inhibitors cannot pass through the blood-brain barrier (15). To date, DPP4 inhibitors for the
treatment of diabetes have included sitagliptin, vildagliptin,
saxagliptin, alogliptin and linagliptin, among others (16). Vildagliptin is currently available as
an efficient treatment agent for type 2 diabetes (17). It has also exhibited neuroprotective
properties in several animal models of neurodegenerative disorders
(18).
In obese insulin-resistant rats, vildagliptin
displayed marked neuroprotective effects (19,20).
Vildagliptin administration to STZ-treated rats for a period of 30
days has been demonstrated to result in a significant and
dose-dependent reduction of Aβ42, in addition to alleviating
neurotoxicity (21). In rats with
STZ-induced Alzheimer's disease, vildagliptin treatment not only
reduced amyloid β42, but also reduced p-tau, which increases
abnormally and accumulates during disease progression.
Simultaneously, elevated levels of oxidative stress factors,
including tumor necrosis factor-α and interleukin-1β, were
efficiently reduced following vildagliptin treatment (21). Another previous study tested the
neuroprotective effect of vildagliptin on rats with insulin
resistance induced by a 12-week high-fat diet (HFD) consumption.
The drug effectively attenuated the impairment of brain insulin
receptor signaling and improved learning and memory deficits
induced by HFD consumption (22).
Further research demonstrated that vildagliptin is
able to restore the phosphorylation of neuronal insulin receptor,
insulin receptor substance 1 and Akt/PKB, thus preventing neuronal
insulin resistance (23).
Furthermore, vildagliptin is able to decrease brain mitochondrial
ROS production, mitochondrial membrane potential depolarization and
brain mitochondrial swelling in order to ameliorate brain
mitochondrial dysfunction (23).
Consistent with the present study, Zheng et al (24) proposed that mild cognitive impairment
was independently associated with increased DPP4 activity in
elderly patients with type 2 diabetes. This phenomenon may be
partly due to the effect of DPP4 on inflammation and oxidative
stress, which may be regarded as an MCI-related risk biomarker
(24). Nath et al (25) also reported the effect of
vildagliptin on the inhibition of catalase activity.
In addition to inflammation and oxidative stress,
neuronal apoptosis is an important type of programmed cell death,
which affects cognitive function. It has been documented that GSK3β
is associated with the apoptotic pathway and that GSK3β
overactivity leads to the increased occurrence of plaques and
neuronal loss in neurodegenerative conditions (26). In the present study, downregulation
was observed in the levels of p-Akt and an upregulation of p-GSK3β
was observed in the hippocampus, whereas caspase-3 expression and
the Bax/Bcl-2 ratio were both increased following the induction of
diabetes in the rats. However, vildagliptin attenuated the changes
to p-Akt, p-GSK3β and caspase-3 levels, and to the Bax/Bcl-2 ratio.
The Akt/GSK3β signaling pathway is a cell survival pathway that
inhibits caspase-3 and prohibits apoptosis (27). Akt is a serine/threonine protein
kinase. PI3K can enhance the activity of p-Akt mediated by
phosphoinositide-dependent kinase 1, which affects the
phosphorylation of the downstream protein GSK3β. Activation of the
Akt/GSK3β pathway can induce apoptosis via phosphorylation of the
anti-apoptotic protein myeloid cell leukemia-1, which belongs to
the Bcl-2 family (28). Previous
research has suggested that the inhibition of GSK3β promotes cell
survival; the overexpression of active GSK3β has been demonstrated
to promote neuronal apoptosis (29).
However, whether the anti-apoptotic effect of vildagliptin is
mediated via the Akt/GSK3β pathway requires further
verification.
Neurotrophic factors, such as BDNF and NGF, are
important regulators involved in plasticity and neuronal cell death
(30,31). In the present study, it was
discovered that NGF and BDNF were downregulated in the DM model and
that vildagliptin treatment reversed this downregulation. A
previous study has also indicated that the vildagliptin-induced
amelioration of DACD and the neuroprotective effect observed in the
current study may be due to increased BDNF and superoxide dismutase
activity (32).
In conclusion, the present study demonstrated that
vildagliptin may improve the learning and memory deficits induced
by diabetes and indicated that decreased levels of
apoptosis-related proteins and increased neurotrophic factors may
contribute to these effects. In addition, the activation of Akt and
the inhibition of GSK3β were conducive to the observed effects of
vildagliptin in improving cognitive deficits. Therefore, the
present study provided evidence that may facilitate the development
of vildagliptin as a preventive or therapeutic agent for
diabetes-induced CNS injury. However, the beneficial effects of
vildagliptin and associated mechanisms need to be further
determined in vivo.
Acknowledgements
The authors would like to thank all teachers in the
Animal Experiment Center of North China University of Science and
Technology (Tangshan, China) for their technical support for the
experiments.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
DDZ, NS and HF conceived and designed the study. LM,
WPW and YZZ performed the experiments. JLT, LBT and KK wrote the
manuscript. DDZ, SC and HF reviewed and edited the manuscript. All
authors read and approved the manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted with the
approval of the Ethics Committee of North China University of
Science and Technology.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Koekkoek PS, Kappelle LJ, van den Berg E,
Rutten GE and Biessels GJ: Cognitive function in patients with
diabetes mellitus: Guidance for daily care. Lancet Neurol.
14:329–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miles WR and Root HF: Psychologic tests
applied to diabetic patients. Arch Intern Med (Chic). 30:767–777.
1922. View Article : Google Scholar
|
|
3
|
Mijnhout GS, Scheltens P, Diamant M,
Biessels GJ, Wessels AM, Simsek S, Snoek FJ and Heine RJ: Diabetic
encephalopathy: A concept in need of a definition. Diabetologia.
49:1447–1448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang XY, Liang J, Chen DC, Xiu MH, Yang
FD, Kosten TA and Kosten TR: Low BDNF is associated with cognitive
impairment in chronic patients with schizophrenia.
Psychopharmacology (Berl). 222:277–284. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Svichar N, Shishkin V, Kostyuk E and
Voitenko N: Changes in mitochondrial Ca2+ homeostasis in
primary sensory neurons of diabetic mice. Neuroreport. 9:1121–1125.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
MacGibbon GA, Cooper GJ and Dragunow M:
Acute application of human amylin, unlike beta-amyloid peptides,
kills undifferentiated PC12 cells by apoptosis. Neuroreport.
8:3945–3950. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji LN, Pan CY, Lu JM, Li H, Zhu DL, Li Q,
Li QF, Peng YD, Tian HM, Yao C, Zhao ZG, et al: Efficacy and safety
of combination therapy with vildagliptin and metformin versus
metformin uptitration in Chinese patients with type 2 diabetes
inadequately controlled with metformin monotherapy: A randomized,
open-label, prospective study (VISION). Diabetes Obes Metab.
18:775–782. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao C, Liu Y, Jiang Y, Ding J and Li L:
Geniposide ameliorates learning memory deficits, reduces tau
phosphorylation and decreases apoptosis via GSK3β pathway in
streptozotocin-induced alzheimer rat model. Brain Pathol.
24:261–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Selvarajah D and Tesfaye S: Central
nervous system involvement in diabetes mellitus. Curr Diab Rep.
6:431–438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Darsalia V, Olverling A, Larsson M,
Mansouri S, Nathanson D, Nyström T, Klein T, Sjöholm Å and Patrone
C: Linagliptin enhances neural stem cell proliferation after stroke
in type 2 diabetic mice. Regul Pept. 190–191:25–31. 2014.
View Article : Google Scholar
|
|
11
|
Nassar NN, Al-Shorbagy MY, Arab HH and
Abdallah DM: Saxagliptin: A novel antiparkinsonian approach.
Neuropharmacology. 89:308–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Amico M, Di Filippo C, Marfella R,
Abbatecola AM, Ferraraccio F, Rossi F and Paolisso G: Long-term
inhibition of dipeptidyl peptidase-4 in Alzheimer's prone mice. Exp
Gerontol. 45:202–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pintana H, Apaijai N, Chattipakorn N and
Chattipakorn SC: DPP-4 inhibitors improve cognition and brain
mitochondrial function of insulin-resistant rats. J Endocrinol.
218:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deacon CF: Therapeutic strategies based on
glucagon-like peptide 1. Diabetes. 53:2181–2189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deacon CF: Dipeptidyl peptidase-4
inhibitors in the treatment of type 2 diabetes: A comparative
review. Diabetes Obes Metab. 13:7–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Messori A, Fadda V, Maratea D, Trippoli S
and Marinai C: Testing the therapeutic equivalence of alogliptin,
linagliptin, saxagliptin, sitagliptin or vildagliptin as
monotherapy or in combination with metformin in patients with type
2 diabetes. Diabetes Ther. 5:341–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drucker DJ and Nauck MA: The incretin
system: Glucagon-like peptide-1 receptor agonists and dipeptidyl
peptidase-4 inhibitors in type 2 diabetes. Lancet. 368:1696–1705.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matteucci E and Giampietro O: Mechanisms
of neurodegeration in type 2 diabetes and the neuroprotective
potential of dipeptidyl peptidase 4 inhibitors. Curr Med Chem.
22:1573–1581. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pintana H, Tanajak P, Pratchayasakul W,
Sa-nguanmoo P, Chunchai T, Satjaritanun P, Leelarphat L,
Chattipakorn N and Chattipakorn SC: Energy restriction combined
with dipeptidyl peptidase-4 inhibitor exerts neuroprotection in
obese male rats. Br J Nutr. 1–9. 2016.PubMed/NCBI
|
|
20
|
Sripetchwandee J, Pipatpiboon N,
Pratchayasakul W, Chattipakorn N and Chattipakorn SC: DPP-4
inhibitor and PPARγ agonist restore the loss of CA1 dendritic
spines in obese insulin-resistant rats. Arch Med Res. 45:547–552.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kosaraju J, Murthy V, Khatwal RB, Dubalab
A, Chinnic S, Nataraj Muthureddy SK and Basavan D: Vildagliptin: An
anti-diabetes agent ameliorates cognitive deficits and pathology
observed in streptozotocin-induced Alzheimer's disease. J Pharm
Pharmacol. 65:1773–1784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pipatpiboon N, Pratchayasakul W,
Chattipakorn N and Chattipakorn SC: PPARγ agonist improves neuronal
insulin receptor function in hippocampus and brain mitochondria
function in rats with insulin resistance induced by long term
high-fat diets. Endocrinology. 153:329–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pipatpiboon N, Pintana H, Pratchayasakul
W, Chattipakorn N and Chattipakorn SC: DPP4-inhibitor improves
neuronal insulin receptor function, brain mitochondrial function
and cognitive function in rats with insulin resistance induced by
high-fat diet consumption. Eur J Neurosci. 37:839–849. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng T, Qin L, Chen B, Hu X, Zhang X, Liu
Y, Liu H, Qin S, Li G and Li Q: Association of plasma DPP4 activity
with mild cognitive impairment in elderly patients with type 2
diabetes: Results from the GDMD study in China. Diabetes Care.
39:1594–1601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nath S, Ghosh SK and Choudhury Y: A murine
model of type 2 diabetes mellitus developed using a combination of
high fat diet and multiple low doses of streptozotocin treatment
mimics the metabolic characteristics of type 2 diabetes mellitus in
humans. J Pharmacol Toxicol Methods. 84:20–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Proctor CJ and Gray DA: GSK3 and p53-is
there a link in Alzheimer's disease? Mol Neurodegener. 5:72010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu S, Begum AN, Jones MR, Oh MS, Beech WK,
Beech B, Yang F, Chen P, Ubeda OJ, Kim PC, et al: GSK3 inhibitors
show benefits in an Alzheimer's disease (AD) model of
neurodegeneration but adverse effects in control animals. Neurobiol
Dis. 33:193–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ragot K, Delmas D, Athias A, Nury T,
Baarine M and Lizard G: α-Tocopherol impairs
7-ketocholesterol-induced caspase-3-dependent apoptosis involving
GSK-3 activation and Mcl-1 degradation on 158N murine
oligodendrocytes. Chem Phys Lipids. 164:469–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ambacher KK, Pitzul KB, Karajgikar M,
Hamilton A, Ferguson SS and Cregan SP: The JNK- and AKT/GSK3β-
signaling pathways converge to regulate puma induction and neuronal
apoptosis induced by trophic factor deprivation. PloS One.
7:e468852012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiao HJ, Li ZZ, Wang LM, Sun W, Yu JC and
Wang B: Association of lower serum Brain-derived neurotrophic
factor levels with larger infarct volumes in acute ischemic stroke.
J Neuroimmunol. 307:69–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li R, Ma J, Wu Y, Nangle M, Zou S, Li Y,
Yin J, Zhao Y, Xu H, Zhang H, et al: Dual delivery of NGF and bFGF
coacervater ameliorates diabetic peripheral neuropathy via
inhibiting schwann cells apoptosis. Int J Biol Sci. 13:640–651.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
El Batsh MM, El Batch MM, Shafik NM and
Younos IH: Favorable effects of vildagliptin on metabolic and
cognitive dysfunctions in streptozotocin-induced diabetic rats. Eur
J Pharmacol. 769:297–305. 2015. View Article : Google Scholar : PubMed/NCBI
|