Introduction
Pulmonary arterial hypertension (PAH) is a severe
and fatal clinical syndrome that can cause high blood pressure in
the pulmonary artery via right ventricular overload, right heart
failure, and death (1,2). The pathogenesis of PAH is complex and
known to involve endothelial dysfunction, proliferation of smooth
muscle cells, pulmonary arteriolar occlusion, chronic inflammation,
and pulmonary vascular remodeling (3–6). Three
main classes of drugs have been widely used to treat PAH:
Endothelin-1 receptor antagonists, phosphodiesterase type 5
inhibitors, and prostacyclins. However, these drugs can only delay
the course of PAH and cannot cure the disease (7). Recent studies of PAH treatment have
focused on gene and cell therapy in animal models and in some
clinical cases, as these treatments are considered safe and can
attenuate pulmonary vascular remodeling and right ventricular
hypertrophy (8–10). Gene and cell therapy are intended to
promote angiogenesis, increase blood flow, and alleviate
ischemia.
C-X-C chemokine receptor type 4 (CXCR4) is known to
be expressed in cancer cells and stem/progenitor cells, including
endothelial and smooth muscle progenitors (11,12). The
ligand of CXCR4, stromal cell derived factor-1 (SDF-1), recruits
CXCR4-expressing cells to SDF-1-expressing cells at sites of
ischemic injury.
CXCR4 inhibitors have been used to investigate the
function of CXCR4 and inhibit tumor cell migration and
proliferation (13). Furthermore,
inhibition of CXCR4 using small-molecule inhibitors can prevent
pulmonary arterial muscularization in PAH models (14). Mesenchymal stem cells (MSCs) and gene
therapies have emerged as novel tools for the treatment of PAH
(2).
The purpose of this study was to investigate the
involvement of CXCR4 and stem cells such as MSCs in a monocrotaline
(MCT) and chronic hypoxia (CH)-induced model of PAH by measuring
the gene/protein expression levels of CXCR4 and stem cell/MSC
marker genes and proteins. We focused on MSCs from bone marrow,
which can differentiate into endothelial and smooth muscle cells.
Upregulation of CXCR4 and MSC markers in PAH models would suggest
that CXCR4 is involved in the development of PAH. Excessive CXCR4
recruitment of MSCs may cause MSCs not to fully differentiate into
endothelial and smooth muscle cells, or other lung cells, which may
impair lung tissues and vessels and lead to PAH. In this case,
CXCR4 inhibitors may be useful for treating PAH, as inhibition of
CXCR4 would inhibit the recruitment of MSCs.
Materials and methods
Animal models
Male 6-week-old Sprague-Dawley rats (180–230 g;
Tokyo Experimental Animal Company, Tokyo, Japan) were randomly
assigned into two groups with eight rats/group. PAH model rats were
established as previously described (10). Briefly, i) control group, rats were
subcutaneously injected with a single dose of 0.9% saline and
maintained in a chamber with normal air for 5 weeks; ii) PAH group,
rats were subcutaneously injected with a single dose of MCT and
maintained in a hypoxic chamber for 5 weeks. The oxygen
concentration was maintained at 10% by continuously flushing the
chamber with a gas mixture of low %O2 and high
%N2. MCT (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was dissolved in 1 N HCl, neutralized with 1 N NaOH, and diluted
with distilled water to 20 mg/ml. A dose of 60 mg/kg (3 ml/kg) was
administered to the animals (10,15). All
rats had unlimited access to food and water and were weighed
weekly.
All experiments were conducted according to a
protocol approved by the Institutional Animal Experiment Committee
of the Tokyo Women's Medical University (AE16-117). All applicable
international, national, and/or institutional guidelines for the
care and use of animals were followed. All procedures performed in
studies involving animals were in accordance with the ethical
standards of the institution.
Hemodynamic studies and evaluation of
right ventricular hypertrophy
Five weeks after MCT injection, the rats were
anesthetized by isoflurane inhalation. A micro-tip catheter (Millar
Instruments, Houston, TX, USA) was inserted into the right
ventricle (RV) via the right jugular vein to measure the right
ventricular systolic pressure (RVSP). The catheter was connected to
a PowerLab Data Acquisition system and Lab Chart 7 software
(ADInstruments, Dunedin, New Zealand), which were used to record
the data. After hemodynamic evaluation, the rats were sacrificed by
bloodletting. The heart, lungs, and pulmonary arteries (PAs) were
separated and harvested, and then the free wall of the RV was
separated and weighed. The left ventricle and septum (LV+S) were
also separated and weighed. The Fulton index (weight ratio of RV
and LV+S) was measured (10,15).
Immunohistochemical staining
The left and right lower lobes of the rat lungs were
fixed by tracheal infusion of 4% paraformaldehyde (pH 7.4) and
incubated in 4% paraformaldehyde overnight. Lungs were prepared as
paraffin-embedded tissue samples and cut into 4-µm-thick sections.
After deparaffinizing, some sections were stained with hematoxylin
and eosin (H&E). Antigen retrieval was achieved by treating the
sections with Immunosaver (Nisshin EM Co., Ltd., Tokyo, Japan) at
98°C for 45 min in a kitchen electric pot (Zojirushi Corporation,
Osaka, Japan; CD-WU30). After incubation with 0.3%
H2O2 for 30 min to block endogenous
peroxidase activity, the sections were treated with normal goat
serum for 30 min, and then incubated with primary antibodies at 4°C
overnight. Immunohistochemical staining was conducted using
antibodies against α-smooth muscle actin (α-SMA) (1:500;
Sigma-Aldrich; Merck KGaA), proliferating cell nuclear antigen
(PCNA; 1:125; Sigma-Aldrich; Merck KGaA), c-Kit (1:50; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), CD90 (1:50; Santa Cruz
Biotechnology, Inc.), and CXCR4 (1:500; Abcam, Cambridge, UK) as
primary antibodies. The sections were further incubated with a
biotinylated secondary antibody at room temperature for 1 h, and
then with avidin-biotin complex (Vector Laboratories, Peterborough,
UK), followed by diaminobenzidine (Nacalai Tesque, Kyoto, Japan).
Mayer's hematoxylin (Wako Pure Chemical Industries, Ltd., Osaka,
Japan) was used for counterstaining.
α-SMA staining was used to calculate the percent
medial wall thickness (%MT). The external diameter (ED) and MT were
measured in muscularized PAs, whose EDs varied from 50 to 100 µm,
to calculate %MT=(2× MT/ED) ×100 (16). For all evaluations, 20 intra-acinar
PAs per section from each rat were randomly selected. PCNA staining
was used to calculate the vascular occlusion score (VOS), which was
categorized as Grade 0 (no evidence of neointimal formation), Grade
1 (less than 50% luminal occlusion), or Grade 2 (more than 50%
luminal occlusion) (17). For all
evaluations, 20 intra-acinar PAs per section from each rat were
randomly selected.
CXCR4 staining was performed to compare expression
of this protein in PAH rats and control rats, and CD90 and c-Kit
staining were performed to compare the number of stem cells in PAH
rats and control rats. Because CXCR4-positive cells aggregated, it
was impractical to count individual cells; thus, the number of
CXCR4-positive cell aggregates was counted instead. The numbers of
c-Kit and CD90-positive cells around PAs were counted and compared
between the two groups. For each type of staining, we randomly
chose 15–20 microscopic areas from each rat.
RT-qPCR
RNA was isolated from the small PA and surrounding
lung tissue in the left and right lower lobes of the lung using the
RNeasy Mini kit (Qiagen, Hilden, Germany). cDNA was synthesized
using the PrimeScript™ RT Reagent kit (Takara Bio,
Shiga, Japan). qPCR was performed using a Thermo Scientific
PikoReal Real-Time PCR System (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Each sample was analyzed in triplicate. β-actin
mRNA expression was measured for normalization. mRNA expression was
normalized to β-actin expression using the equation
2−∆ΔCq (18). Primer
sequences are listed in Table I.
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer | Accession no. |
|---|
| MCP1 |
5′-AGCATCCACGTGCTGTCTC-3′ |
5′-GATCATCTTGCCAGTGAATGAG-3′ | AY357296 |
| IL-6 |
5′-CCGGAGAGGAGACTTCACAG-3′ |
5′-ACAGTGCATCATCGCTGTTC-3′ | NG_011640 |
| TNFα |
5′-TGACCCCCATTACTCTGACC-3′ |
5′-GGCCACTACTTCAGCGTCTC-3′ | KY038170 |
| CXCR1 |
5′-GTCGTCATCTATGCCCTGGT-3′ |
5′-GCCAGGTTCAGCAGGTAGAC-3′ | NG_011814 |
| CXCR2 |
5′-CGCTCCGTCACTGATGTCTA-3′ |
5′-GAGTGAGACCACCTTGCACA-3′ | NG_052975 |
| CXCR4 |
5′-GCTGAGGAGCATGACAGACA-3′ |
5′-GATGAAGGCCAGGATGAGAA-3′ | NG_011587 |
| SCF |
5′-TCGTGGCATGTATGGAAGAA-3′ |
5′-TCAGATGCCACCATGAAGTC-3′ | KR815359 |
| c-Kit |
5′-GATCTGCTCTGCGTCCTGTT-3′ |
5′-AGATGGCTGAGAAGTCCCTGT-3′ | NG_007456 |
| CD29 |
5′-AACTGCACCAGCCCATTTAG-3′ |
5′-CCACCTTCTGGAGAATCCAA-3′ | NG_029012 |
| β-actin |
5′-CTAAGGCCAACCGTGAAAAG-3′ |
5′-GCCTGGATGGCTACGTACA-3′ | NM_031144 |
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation (SD). Statistical analyses were performed using
the independent samples t-test in SPSS software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Animal survival rate, RVSP, and right
ventricular hypertrophy measurement
The eight rats in the control group survived and
remained active during the experiment. In contrast, two of the
eight rats in the PAH group died during the experiment. The first
died at 3 weeks and 5 days and the second died at 4 weeks and 4
days. Therefore, eight control rats and six PAH rats were used for
the experiments described below.
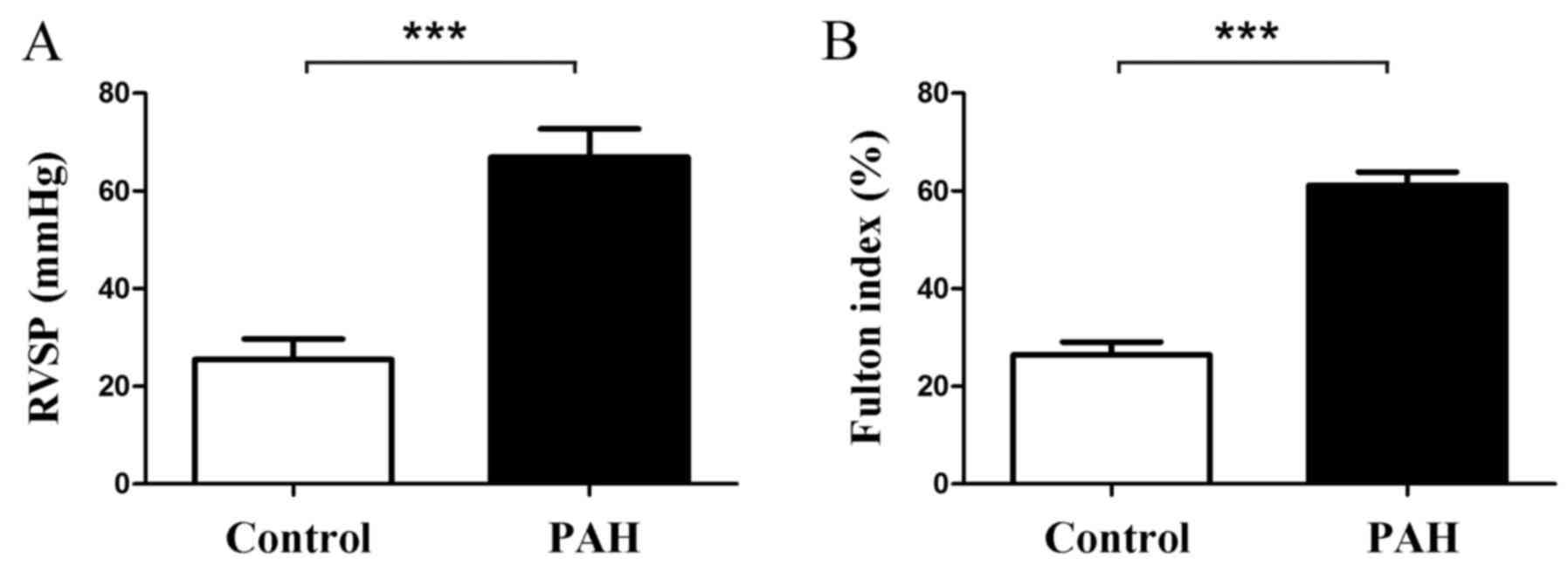
Rats in the PAH group exhibited a significant
increase in RVSP and Fulton index compared with the control group.
RVSP was 66.91±5.76 mmHg in the PAH group and 25.48±4.25 mmHg in
the control group (P<0.001, Fig.
1A); the Fulton index was 61.16±2.72% in the PAH group and
26.43±2.65% in the control group (P<0.001, Fig. 1B).
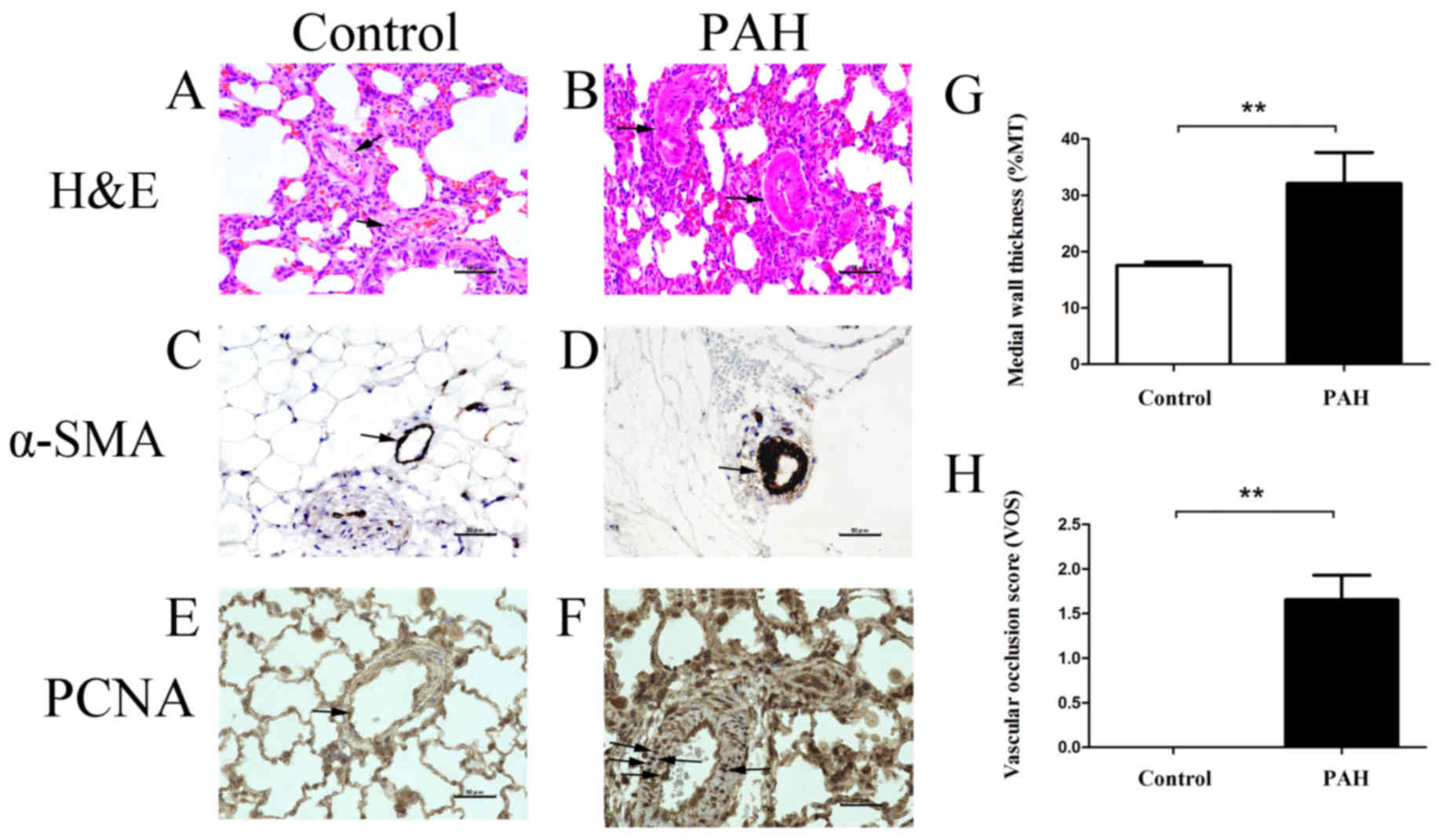
Histopathology
%MT and VOS. H&E staining was performed
to observe differences in the pulmonary artery wall between the
control group (Fig. 2A) and the PAH
group (Fig. 2B). To confirm that the
thicker tissue consisted of smooth muscle cells, lung tissue
sections were immunohistochemically stained using an α-SMA
antibody. Compared with the control group (Fig. 2C), increased proliferation of smooth
muscle cells was observed in the PAH group (Fig. 2D). PCNA-positive proliferating cells
were barely visible in the control group (Fig. 2E), but were distributed throughout
the lumen and wall of the PA in the PAH group (Fig. 2F). Measurement of the thickness of
the pulmonary artery wall indicated a significant difference in %MT
between the PAH group and the control group: 32.08±5.49% in the PAH
group and 17.52±0.61% in the control group (P=0.002, Fig. 2G). Furthermore, a significant
difference was observed in VOS between the PAH and control groups:
1.66±0.28 for the PAH group and 0 for the control group (P=0.001,
Fig. 2H).
Expression of inflammatory markers in
RT-qPCR
mRNA expression was quantified relative to β-actin
mRNA using the equation 2−∆∆Cq. Rats in the PAH group
exhibited significantly higher gene expression levels of MCP1
(relative expression 0.0439±0.0372 in the PAH group and
0.0029±0.0007 in the control group, P=0.017, Fig. 3A), IL-6 (0.0256±0.0225 in the PAH
group and 0.0032±0.0017 in the control group, P=0.026, Fig. 3B), TNFα (0.1942±0.1940 in the PAH
group and 0.0172±0.0047 in the control group, P=0.036, Fig. 3C), CXCR1 (0.00072±0.00058 in the PAH
group and 0.00013±0.00010 in the control group, P=0.037, Fig. 3D), and CXCR2 (0.0120±0.0080 in the
PAH group and 0.0011±0.0015 in the control group, P=0.036, Fig. 3E).
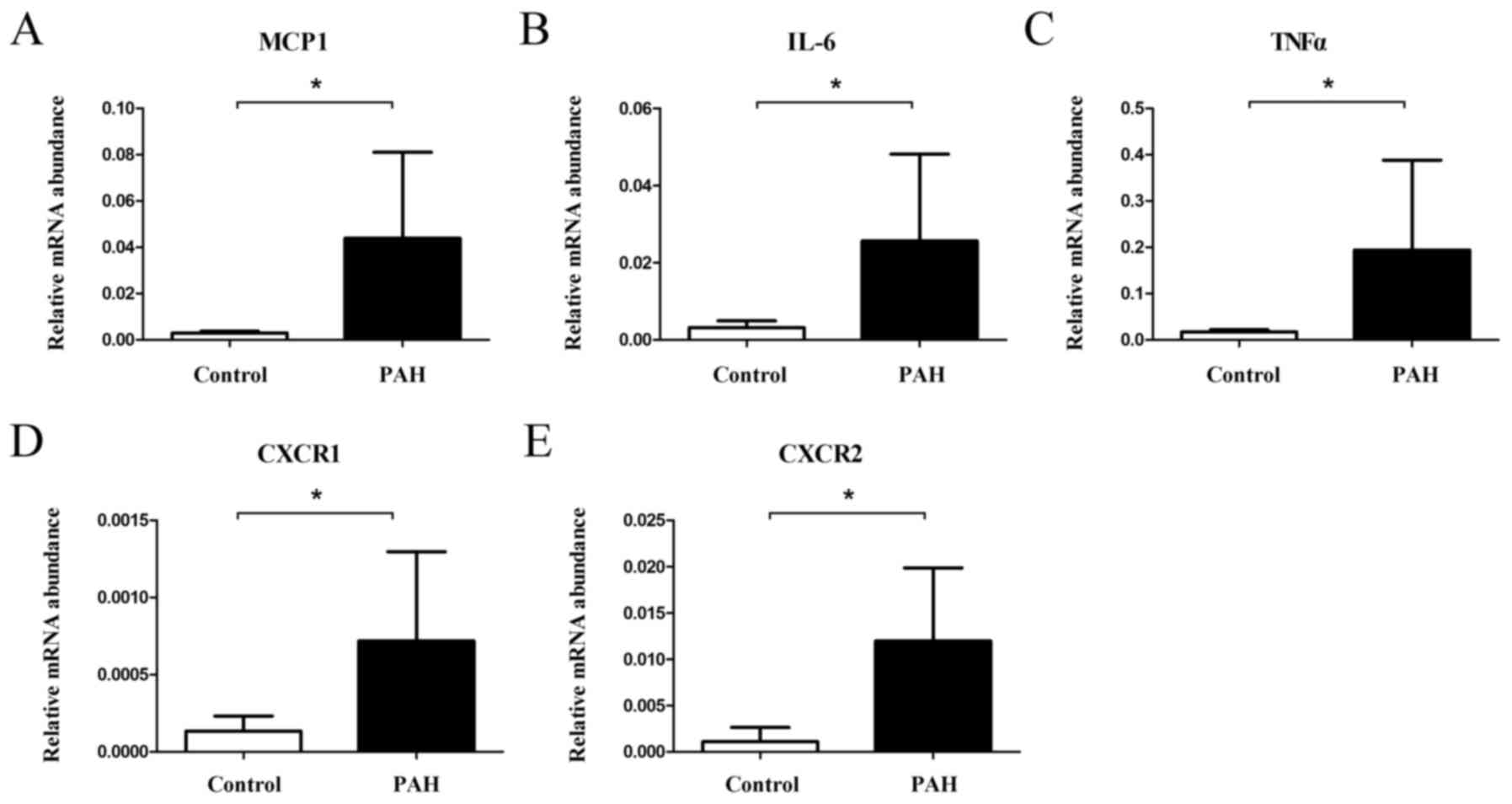 | Figure 3.mRNA expression of inflammation genes
increased in PAH. (A) MCP1, (B) IL-6, (C) TNFα, (D) CXCR1, and (E)
CXCR2 mRNA levels increased in PAH animals compared to controls
(*P<0.05). PAH, pulmonary arterial hypertension; MCP1, monocyte
chemoattractant protein 1; IL-6, interleukin-6; TNFα, tumor
necrosis factor α; CXCR1, C-X-C chemokine receptor type 1; CXCR2,
C-X-C chemokine receptor type 2. |
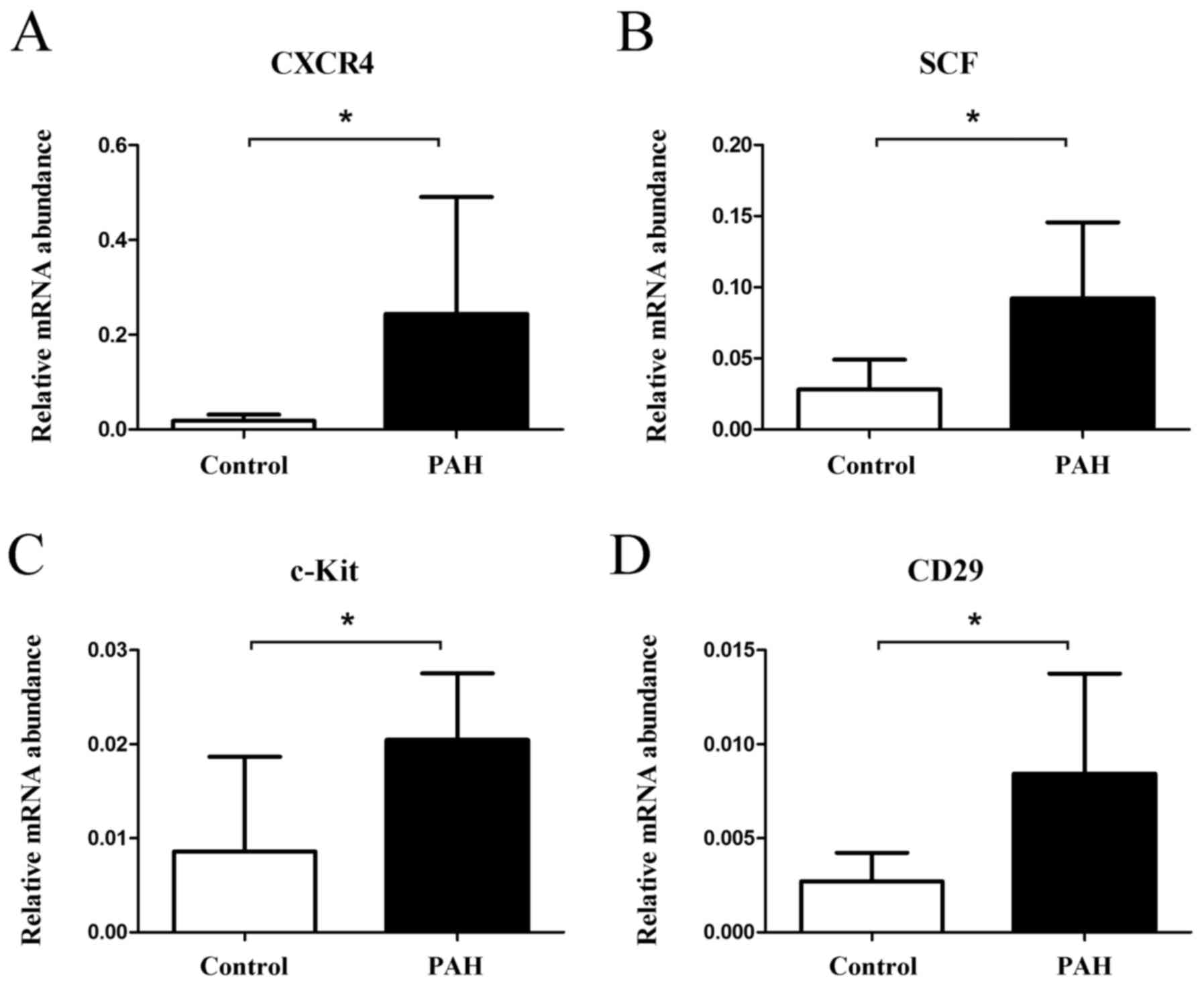
Expression of stem cell markers in
RT-qPCR
Rats in the PAH group exhibited significantly higher
gene expression levels of CXCR4 (relative expression 0.2438±0.2463
in the PAH group and 0.0183±0.0131 in the control group, P=0.036,
Fig. 4A), stem cell factor (SCF)
(0.0922±0.0535 in the PAH group and 0.0281±0.0210 in the control
group, P=0.012, Fig. 4B), c-Kit
(0.0205±0.0071 in the PAH group and 0.0086±0.0101 in the control
group, P=0.016, Fig. 4C), and CD29
(0.0084±0.0053 in the PAH group and 0.0027±0.0015 in the control
group, P=0.019, Fig. 4D).
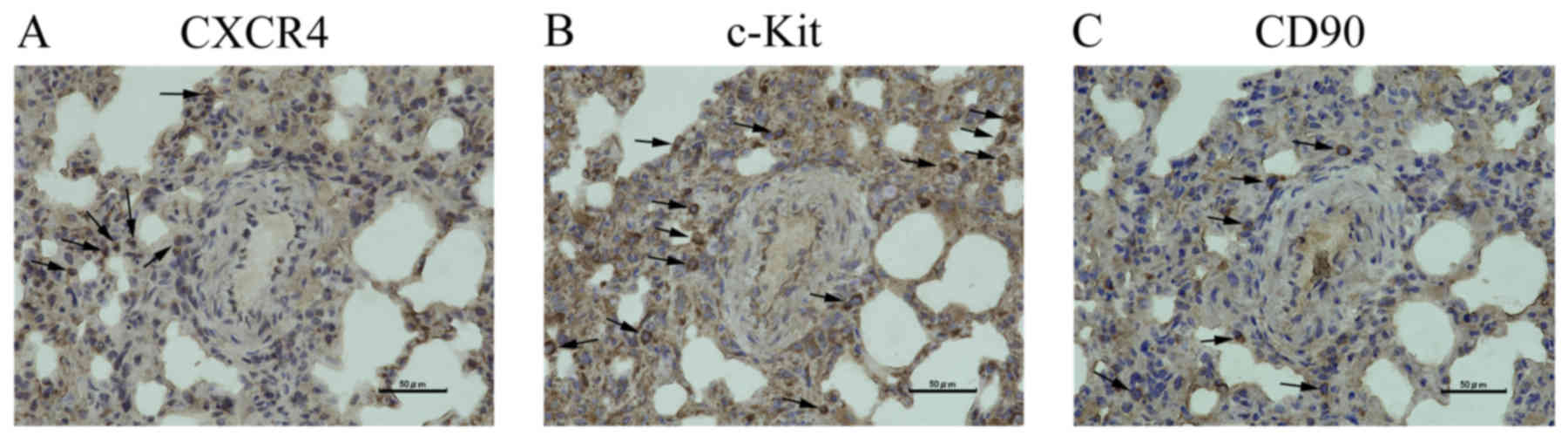
Protein expression of CXCR4 and other
stem cell markers in immunohistochemical staining
To further investigate the expression of CXCR4 and
its implications, immunohistochemical staining was performed. Few
CXCR4-positive cell clusters were observed in lung tissue of the
control group (Fig. 5A). However,
there was a marked increase in CXCR4 expression in the PAH group
(Fig. 5B), with 10.39±1.73
CXCR4-positive cell clusters observed in the PAH group compared
with 6.83±1.20 in the control group (P=0.015, Fig. 5C). Additionally, compared to the
control group (Fig. 5D),
c-Kit-positive cells around the PAs were greater in number in the
PAH group (Fig. 5E): 31.61±6.10
c-Kit-positive cells in the PAH group and 14.43±3.24 in the control
group (P=0.003, Fig. 5F).
c-Kit-positive cells were also larger in size in the PAH group.
Compared with the control group (Fig.
5G), the number of CD90-positive cells around the PAs was much
greater in the PAH group (Fig. 5H):
7.13±2.13 in the PAH group and 2.85±0.68 in the control group
(P=0.009, Fig. 5I).
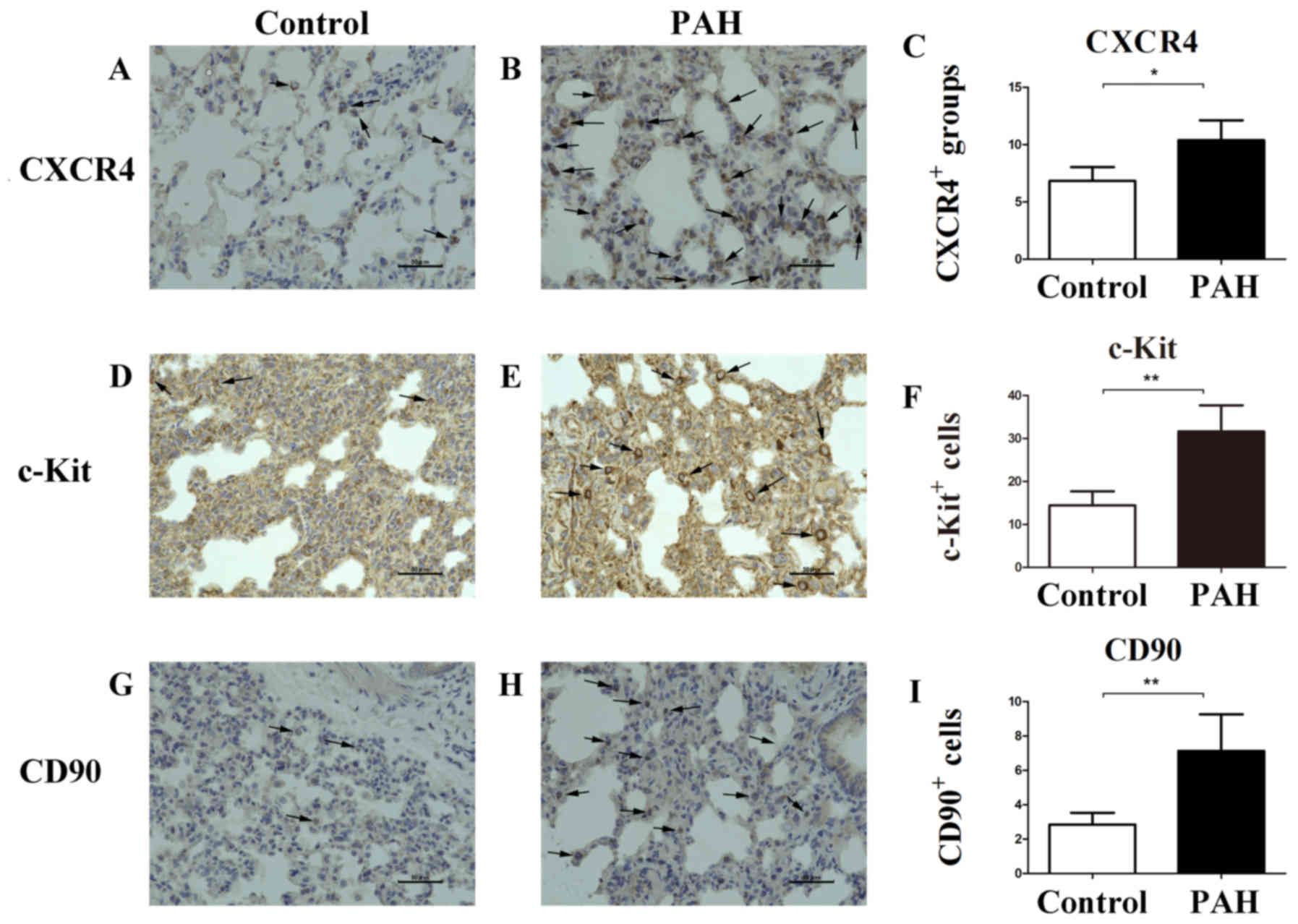 | Figure 5.Immunohistochemical evaluation of
CXCR4, c-Kit, and CD90 stem cell marker proteins increased in PAH.
(A, B and C) Only a few CXCR4-positive cells were observed on
vascular cells in the lungs of the control group, but increased
CXCR4 expression in PAH group was observed within the lumen and
vascular wall of the PAs (arrows). (D, E and F) The number and size
of c-Kit-positive cells around PAs were greater in the PAH group
(arrows). (G, H and I) The number of CD90-positive cells around PAs
was larger in the PAH group (arrows). Scale bar, 50 µm.
**P<0.01, *P<0.05. PAH, pulmonary arterial hypertension;
CXCR4, C-X-C chemokine receptor type 4; PAs, pulmonary
arteries. |
Serial sections were cut from PAH rats to compare
the localization of CXCR4 (Fig. 6A),
c-Kit (Fig. 6B), and CD90 (Fig. 6C). Differences in the localization of
these proteins were observed; protein expression did not
overlap.
Discussion
The main finding of this study is that the
expression of CXCR4 and other markers of stem/progenitor cells is
significantly higher in PAH rats than in normal rats. Thus, CXCR4
and stem cells such as MSCs may play an important role in pulmonary
vascular remodeling. These results provide basic evidence for
further studies of treatment with CXCR4 inhibitors for PAH, which
may benefit clinical PAH patients.
Methods for establishing PAH models are
well-developed. Because the MCT and CH models are regarded as
models of mild pulmonary hypertension (19), a combination of two treatments, such
as MCT and CH (15), MCT and
pneumonectomy (20), or Sugen and CH
(21), are commonly used to create a
severe disease model that mimics human PAH. In the present study,
we used a combination of MCT and CH for experimental simplicity;
this method requires only a single subcutaneous injection of MCT
and induction of CH, which is straightforward and results in a more
severe disease model, which may be more similar to clinical PAH,
than a single treatment. We initially developed 6-week models of
PAH, but most of the rats died after 5 weeks; therefore, 5-week
models were used instead. Significant differences in RVSP and
Fulton index between the PAH and control groups were observed.
Additionally, the %MT values obtained from α-SMA
immunohistochemical staining showed proliferation of smooth muscle
cells in the PAH model, and the VOS (which reflects PCNA-positive
proliferating cells) showed pulmonary arteriolar occlusion in the
PAH model, as we have described previously (10). Increased expression of MCP1, IL-6,
TNFα, CXCR1, and CXCR2 mRNA in the PAH model indicated chronic
inflammation. These results suggest that we successfully
established a PAH model with pulmonary vascular remodeling.
RT-qPCR experiments showed that the expression of
CXCR4 and other markers of stem/progenitor cells was significantly
higher in PAH rats than in control rats. These results indicate
that CXCR4 or stem/progenitor cells play a role in the pathogenesis
of PAH. CXCR4 has been shown to be expressed in cancer cells and to
contribute to the proliferation and survival of tumor cells
(13). We previously showed that
valproic acid, an epigenetic modifier, was effective for treating
PAH in similar rat models (10). We
predicted that CXCR4 plays an important role in the chemotaxis,
proliferation, and survival of smooth muscle cells, endothelial
cells, and other cells in lung tissue, leading to pulmonary
arteriolar occlusion or pulmonary vascular remodeling.
The observation that the expression of
stem/progenitor cell markers was higher in the PAH group has
several interesting implications. Firstly, c-Kit has been detected
on the surface of lung stem cells, which can repopulate airways and
vessels (22), and on the surface of
lung vascular endothelial stem cells, which generate functional
blood vessels (23). SCF, which is
the ligand of c-Kit, has also been demonstrated to play an
essential role in regulating cell proliferation (24). In our study, c-Kit-positive cells
were significantly more numerous and SCF gene expression was
significantly enhanced in the PAH group compared with the control
group. These c-Kit-positive stem-like cells surrounding blood
vessels may differentiate into mature cells and proliferate,
eventually contributing to pulmonary vascular remodeling. Secondly,
CD90, which is an MSC marker (25),
and CD29, which is an MSC and fibroblast marker, were more highly
expressed in the PAH group than in the control group, indicating
that MSCs are involved in the pathogenesis of PAH; these MSCs can
also differentiate into mature cells, proliferate, and contribute
to pulmonary vascular remodeling. Thirdly, the serial sections
revealed that the localization of CXCR4, c-Kit, and CD90 expression
differed, which may be because they have different functions or
because a subset of MSCs differentiated along smooth muscle or
endothelial lineages as part of the PAH injury-repair response.
Interestingly, Farkas et al found that the
number of c-Kit+ vWF+ cells and the
expression of CXCL12 began decreasing after 21 days in a Sugen and
CH-induced rat model of PAH (14).
Based on these data and the present study, we propose that in the
first three weeks of progression in rat models of PAH, stem cells
emerge and begin to proliferate, constituting a protective and
compensatory mechanism against PAH, such that the rat does not
initially show symptoms of heart failure. However, as pulmonary
arterial pressure continues to increase, the compensatory mechanism
becomes insufficient, symptoms begin to occur, and stem cells stop
growing and begin to decline in number; however, pulmonary vascular
remodeling may have already occurred because of the stem cells and
through other mechanisms. Hence, if PAH rats or patients could be
treated with a CXCR4 inhibitor while the compensatory mechanism is
still effective, fewer CXCR4-positive cells would emerge and
pulmonary vascular remodeling may be less severe.
Our experimental results and previous studies
(26,27) suggest that CXCR4 is involved in PAH
development; thus, CXCR4 inhibitors may be a potential treatment
for PAH. Although one small-molecule CXCR4 inhibitor has already
been reported to prevent pulmonary arterial muscularization in a
PAH model (14,28), the role of CXCR4 in the pathogenesis
and progression of PAH and the potential of other CXCR4 inhibitors
such as LY2510924 or T134 to prevent PAH remain unclear. CXCR4
inhibitors currently in clinical trials are used predominantly for
various cancers (29,30) and HIV therapy (31). The use of CXCR4 inhibitors in PAH
treatment may decrease the number of CXCR4-positive stem cells,
decreasing the number of mature cells such as smooth muscle cells
and endothelial cells, thereby reducing proliferation, vascular
occlusion, and vascular remodeling. CXCR4 inhibitors may therefore
offer an alternative to the three major drug classes currently in
clinical use for PAH treatment and to organ transplantation,
providing new options for PAH patients.
The present study has some limitations. Firstly,
animal models are less complex than clinical patients, and it is
difficult to establish congenital or hereditary PAH models.
Secondly, although we confirmed the expression of CXCR4 and other
proteins, we did not confirm the efficacy of inhibitors of these
proteins in PAH treatment. Thirdly, CXCR4 has a complex biological
function; CXCR4-expressing cells can be recruited to
SDF-1-expressing cells in injured areas, assisting injury repair
(32). Therefore, further studies of
CXCR4 are necessary. Finally, the precise role of stem/progenitor
cells in PAH should be examined in more detail.
Acknowledgements
The authors acknowledge Mr. Kenji Yoshihara and Mr.
Hiroaki Nagao at Tokyo Women's Medical University for their
technical assistance.
Funding
The authors would like to acknowledge the China
Scholarship Council (grand no. 201708050127) for financially
supporting the studies of TZ as a foreign student.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZ and TN designed the concept and conducted the
experiments. TZ constructed the animal models, performed RVSP
measurements and wrote the paper. NK performed data analysis and
modified the paper. EH performed reverse transcription-quantitative
polymerase chain reaction. YF performed the immunohistochemical
staining. TN was also involved in mechanism analysis.
Ethics approval and consent to
participate
All experiments were conducted according to a
protocol approved by the Institutional Animal Experiment Committee
of the Tokyo Women's Medical University (AE16-117).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhou L, Chen Z, Vanderslice P, So SP, Ruan
KH, Willerson JT and Dixon RA: Endothelial-like progenitor cells
engineered to produce prostacyclin rescue monocrotaline-induced
pulmonary arterial hypertension and provide right ventricle
benefits. Circulation. 128:982–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng GS, Zhang YS, Zhang TT, He L and
Wang XY: Bone marrow-derived mesenchymal stem cells modified with
IGFBP-3 inhibit the proliferation of pulmonary artery smooth muscle
cells. Int J Mol Med. 39:223–230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Farber HW and Loscalzo J: Pulmonary
arterial hypertension. N Engl J Med. 351:1655–1665. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huertas A, Perros F, Tu L, Cohen-Kaminsky
S, Montani D, Dorfmüller P, Guignabert C and Humbert M: Immune
dysregulation and endothelial dysfunction in pulmonary arterial
hypertension: A complex interplay. Circulation. 129:1332–1340.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tuder RM, Archer SL, Dorfmüller P, Erzurum
SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R,
Stenmark KR and Morrell NW: Relevant issues in the pathology and
pathobiology of pulmonary hypertension. J Am Coll Cardiol. 62 25
Suppl:D4–D12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ranchoux B, Antigny F, Rucker-Martin C,
Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F,
Planté S, et al: Endothelial-to-mesenchymal transition in pulmonary
hypertension. Circulation. 131:1006–1018. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang JX, Pan YY, Zhao YY and Wang XX:
Endothelial progenitor cell-based therapy for pulmonary arterial
hypertension. Cell Transplant. 22:1325–1336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen H, Strappe P, Chen S and Wang LX:
Endothelial progenitor cells and pulmonary arterial hypertension.
Heart Lung Circ. 23:595–601. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang XX, Zhang FR, Shang YP, Zhu JH, Xie
XD, Tao QM, Zhu JH and Chen JZ: Transplantation of autologous
endothelial progenitor cells may be beneficial in patients with
idiopathic pulmonary arterial hypertension: A pilot randomized
controlled trial. J Am Coll Cardiol. 49:1566–1571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lan B, Hayama E, Kawaguchi N, Furutani Y
and Nakanishi T: Therapeutic efficacy of valproic acid in a
combined monocrotaline and chronic hypoxia rat model of severe
pulmonary hypertension. PLoS One. 10:e01172112015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teicher BA and Fricker SP: CXCL12
(SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 16:2927–2931.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller RJ, Banisadr G and Bhattacharyya
BJ: CXCR4 signaling in the regulation of stem cell migration and
development. J Neuroimmunol. 198:31–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng SB, Zhang X, Paul D, Kays LM, Gough
W, Stewart J, Uhlik MT, Chen Q, Hui YH, Zamek-Gliszczynski MJ, et
al: Identification of LY2510924, a novel cyclic peptide CXCR4
antagonist that exhibits antitumor activities in solid tumor and
breast cancer metastatic models. Mol Cancer Ther. 14:480–490. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farkas D, Kraskauskas D, Drake JI,
Alhussaini AA, Kraskauskiene V, Bogaard HJ, Cool CD, Voelkel NF and
Farkas L: CXCR4 inhibition ameliorates severe obliterative
pulmonary hypertension and accumulation of C-kit+ cells
in rats. PLoS One. 9:e898102014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morimatsu Y, Sakashita N, Komohara Y,
Ohnishi K, Masuda H, Dahan D, Takeya M, Guibert C and Marthan R:
Development and characterization of an animal model of severe
pulmonary arterial hypertension. J Vasc Res. 49:33–42. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rondelet B, Kerbaul F, Motte S, van
Beneden R, Remmelink M, Brimioulle S, McEntee K, Wauthy P, Salmon
I, Ketelslegers JM and Naeije R: Bosentan for the prevention of
overcirculation-induced experimental pulmonary arterial
hypertension. Circulation. 107:1329–1335. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishimura T, Vaszar LT, Faul JL, Zhao G,
Berry GJ, Shi L, Qiu D, Benson G, Pearl RG and Kao PN: Simvastatin
rescues rats from fatal pulmonary hypertension by inducing
apoptosis of neointimal smooth muscle cells. Circulation.
108:1640–1645. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bahmanpour S, Khozani Talaei T, Fard Zarei
N, Jaberipour M, Hosseini A and Esmaeilpour T: A comparison of the
multiple oocyte maturation gene expression patterns between the
newborn and adult mouse ovary. Iran J Reprod Med. 11:815–822.
2013.PubMed/NCBI
|
|
19
|
Stenmark KR, Meyrick B, Galie N, Mooi WJ
and McMurtry IF: Animal models of pulmonary arterial hypertension:
The hope for etiological discovery and pharmacological cure. Am J
Physiol Lung Cell Mol Physiol. 297:L1013–L1032. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okada K, Tanaka Y, Bernstein M, Zhang W,
Patterson GA and Botney MD: Pulmonary hemodynamics modify the rat
pulmonary artery response to injury. A neointimal model of
pulmonary hypertension. Am J Pathol. 151:1019–1025. 1997.PubMed/NCBI
|
|
21
|
Abe K, Toba M, Alzoubi A, Ito M, Fagan KA,
Cool CD, Voelkel NF, McMurtry IF and Oka M: Formation of plexiform
lesions in experimental severe pulmonary arterial hypertension.
Circulation. 121:2747–2754. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kajstura J, Rota M, Hall SR, Hosoda T,
D'Amario D, Sanada F, Zheng H, Ogórek B, Rondon-Clavo C,
Ferreira-Martins J, et al: Evidence for human lung stem cells. N
Engl J Med. 364:1795–1806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang S, Wei J, Pentinmikko N, Leinonen H
and Salven P: Generation of functional blood vessels from a single
c-kit+ adult vascular endothelial stem cell. PLoS Biol.
10:e10014072012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim JY, Choi JS, Song SH, Im JE, Kim JM,
Kim K, Kwon S, Shin HK, Joo CK, Lee BH and Suh W: Stem cell factor
is a potent endothelial permeability factor. Arterioscler Thromb
Vasc Biol. 34:1459–1467. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ridzuan N, Al Abbar A, Yip WK, Maqbool M
and Ramasamy R: Characterization and expression of senescence
marker in prolonged passages of rat bone marrow-derived mesenchymal
stem cells. Stem Cells Int. 2016:84872642016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang X, Wu P, Huang F, Xu M, Chen M,
Huang K, Li GP, Xu M, Yao D and Wang L: Baicalin attenuates chronic
hypoxia-induced pulmonary hypertension via adenosine A2A
receptor-induced SDF-1/CXCR4/PI3K/AKT signaling. J Biomed Sci.
24:522017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Favre S, Gambini E, Nigro P, Scopece A,
Bianciardi P, Caretti A, Pompilio G, Corno AF, Vassalli G, von
Segesser LK, et al: Sildenafil attenuates hypoxic pulmonary
remodelling by inhibiting bone marrow progenitor cells. J Cell Mol
Med. 21:871–880. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Young KC, Torres E, Hatzistergos KE, Hehre
D, Suguihara C and Hare JM: Inhibition of the SDF-1/CXCR4 axis
attenuates neonatal hypoxia-induced pulmonary hypertension. Circ
Res. 104:1293–1301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ling X, Spaeth E, Chen Y, Shi Y, Zhang W,
Schober W, Hail N Jr, Konopleva M and Andreeff M: The CXCR4
antagonist AMD3465 regulates oncogenic signaling and invasiveness
in vitro and prevents breast cancer growth and metastasis in vivo.
PLoS One. 8:e584262013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galsky MD, Vogelzang NJ, Conkling P,
Raddad E, Polzer J, Roberson S, Stille JR, Saleh M and Thornton D:
A phase I trial of LY2510924, a CXCR4 peptide antagonist, in
patients with advanced cancer. Clin Cancer Res. 20:3581–3588. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arakaki R, Tamamura H, Premanathan M,
Kanbara K, Ramanan S, Mochizuki K, Baba M, Fujii N and Nakashima H:
T134, a small-molecule CXCR4 inhibitor, has no cross-drug
resistance with AMD3100, a CXCR4 antagonist with a different
structure. J Virol. 73:1719–1723. 1999.PubMed/NCBI
|
|
32
|
Yang JX, Zhang N, Wang HW, Gao P, Yang QP
and Wen QP: CXCR4 receptor overexpression in mesenchymal stem cells
facilitates treatment of acute lung injury in rats. J Biol Chem.
290:1994–2006. 2015. View Article : Google Scholar : PubMed/NCBI
|