Introduction
Oral lichen planus (OLP) is an inflammatory disease
that affects oral mucosa, with a prevalence of 0.5 to 2.0% among
the general population. This disease is more common in middle-aged
patients (30–60 years) and affects more females than males
(1). The pathogenesis of OPL remains
unclear, and it is generally considered to be an immunologically
mediated process, including cytotoxic T cells activation and mast
cell degranulation, thus demonstrating a hypersensitivity reaction
(2). In oral mucosa of OLP,
CD8+ T cells infiltrate in epithelium-connective tissue
interface and promote apoptosis of oral epithelial cells at the
basal layer (3). A large clinical
investigation among 7,806 patients showed that OLP has a malignant
transformation rate of about 1.09% that develop oral squamous cell
carcinoma (OSCC) (4). The mechanisms
underlying carcinogenesis of OLP include epithelial cell
proliferation and apoptosis, deregulation of oncogenes and
tumor-suppressor genes, oxidative stress and inflammatory process
(5). Currently histology is the main
method to evaluate the clinical severity and potential risk of
malignant transformation of OLP. New biomarkers are highly needed
to indicate the transformation potential of OLP, thus will greatly
improving the accurate evaluation of OLP.
Periostin is a soluble and secreted extracellular
matrix protein and plays various roles in embryonic development,
injury, tooth and bone formation (6). Periostin expression is upregulated in
various tumor types, such as breast, lung, ovarian, prostate,
gastric, colon and pancreatic cancers (7). Furthermore, periostin participates in
various biologic processes of tumorigenesis, including cell
survival, proliferation, adhesion, angiogenesis,
epithelial-mesenchymal transition (EMT), tumor invasion and
metastasis (8). However, there were
no studies about the role of periostin in OLP, as well as the
associations between periostin and clinicopathological parameters
of OLP.
In this study, periostin protein level in OLP was
measured by immunohistochemistry (IHC) and enzyme-linked
immunosorbent assay (ELISA), and the correlations between periostin
and clinicopathological process of OLP were analyzed. Our study may
provide periostin as a new biomarker for the early diagnosis and
treatment of OLP.
Materials and methods
Subjects
A total of 117 consecutive OLP patients were
included in the First Hospital of Lanzhou University (Lanzhou,
China) between July 2012 and December 2015, and their tissue
specimens and serums were obtained. Before sampling, the patients
had not received any treatment. The OLP was diagnosed based on
clinical appearance and paraffin-embedded tissue specimens stained
with hematoxylin and eosin (H&E) by a pathologist. Any OLP
patients with history of systemic disease or inflammatory disease,
oral candida infection, oral lichenoid reactions, pregnancy or
using antibiotic within one month before diagnosis were excluded.
The samples of oral mucosa and serum were obtained from a study
group of 45 males and 72 females, with ages ranging from 28 to 72
years (median age, 52 years). The clinicopathological data
including age, gender and clinical type were obtained. There were
110 normal oral mucosal tissue and their serums serving as the
control group, which were harvested from patients with alveolar
bone cyst who underwent paraneoplastic plastic surgery and had no
any systemic disease. This study was approved by the ethics
committee of the First Hospital of Lanzhou University. Informed
consent was signed by the patients or their relatives.
IHC
The periostin protein levels in tissue specimens of
OLP and controls were determined by IHC staining using
avidin-biotin-peroxidase complex (ABC) method. All formalin-fixed
and paraffin-embedded oral tissue specimens were processed for 5
µm-thick sections. The sections were dewaxed in xylene and
rehydrated with descending alcohol gradient, and then were
incubated with 0.3% hydrogen peroxidase for 25 min to block the
endogenous peroxidase activity. The sections were heated with 700 W
microwave oven for 15 min for antigen retrieval, and then incubated
with rabbit anti-periostin primary antibody (1:150; ab92460; Abcam,
Shanghai, China) at room temperature for 2 h, followed by
incubation with biotinylated secondary antibody (mouse anti-rabbit
IgG; 1:500 dilution; Zhongshan Jinqiao Biology & Technology
Co., Ltd., Beijing, China). The 3,3′-diaminobenzidine
tetra-hydrochloride (liquid DAB+; Dako, Glostrup, Denmark) and
hematoxylin were used to visualize the antigen-antibody reactions.
Finally, the sections were dehydrated and mounted. The sections of
negative controls were incubated with phosphate-buffered saline
other than primary antibody.
Evaluation of immunohistochemical
staining
Two experienced pathologists who were blinded to the
clinical data independently evaluated the stained slices. Five
visual fields were randomly selected for evaluation in each
section. The expression of periostin for each case was evaluated
based on the percentage of positive cell and staining intensity.
Grading of stain-positive cells was as follows: 0, stain-positive
cells <5%; 1, stain-positive cells 5–25%; 2, stain-positive
cells 26–50%; 3, stain-positive cells >50%. Grading of staining
intensity was as follows: 0, negative; 1, light yellow; 2,
brownish-yellow; 3, brown. The total score=Grading of
stain-positive cells × Grading of staining intensity. For
statistical analyses, the cases that were found to have total score
of ≥4 and <4 were considered high expression and low expression
of periostin, respectively (9).
Measurement of serum periostin,
interleukin (IL)-6, tumour necrosis factor-α (TNF-α), interferon-γ
(IFN-γ), IL-4 and thymic stromal lymphopoietin (TSLP)
concentration
Venous blood samples were collected from OLP
subjects and controls, and centrifugation was performed within 30
min at 1,000 × g for 15 min. The serum was separated and stored in
aliquots at −70°C until measurement. ELISA kits (R&D Systems,
Inc., Minneapolis, MN, USA) were applied to measure the serum
concentrations of periostin (DY3548), IL-6 (DY206), TNF-α (DY210),
IFN-γ (DY285), IL-4 (DY204-05) and TSLP (DY1398). The absorbance at
OD450 wavelength was measured by an ELISA plate reader (Ricso
RK201; Shenzhen Ricso Technology Co., Ltd., Shenzhen, China). All
samples were measured in triplicate to calculate the mean
concentration of each subject.
Counting of mast cells
Toluidine blue (1%) is used to evaluate mast cell
density by selectively staining mast cells of oral mucosa tissue.
In mast cells, the granules were stained with purplish red and the
nuclei with sky blue in colour. The number of mast cells was
counted under a an microscope (BX51; Olympus Corporation, Tokyo,
Japan; magnification, ×400). Mast cell density was expressed as the
mast cell number per square millimeter (MCs/mm2)
(10).
Statistical analysis
Data were statistically analyzed using SPSS 19.0
(SPSS, Inc., Chicago, IL, USA) software. Continuous variables were
expressed as medians and interquartile ranges. Categorical
variables were expressed as frequencies and percentages.
Differences between two or three groups were determined by
Wilcoxon-Mann-Whitney test in comparing continuous variables, or by
Chi-squared test or a Fisher's exact test in comparing categorical
variables. Spearman's rank correlation tests were used to analyze
correlations between serum periostin and other variables. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of periostin in normal and
OLP oral mucosa
The study group of 117 OLP patients included 45
males and 72 females, with ages ranging from 28 to 72 years (median
age, 52 years). There were 52 (44.4%) cases of reticular form, 41
(35.0%) cases of erosive form, and 24 (20.5%) cases of atrophic
form. The clinical type of OLP was significantly associated with
serum cytokine levels, and atrophic form had the highest levels of
serum IL-6, TNF-α, IL-4, TSLP, oral tissue mast cell density, and
the lowest level of serum IFN-γ/IL-4 ratio (P<0.05; Table I).
 | Table I.Characteristics of the study
population. |
Table I.
Characteristics of the study
population.
| Characteristic | OLP (n=117) | Reticular (n=52) | Erosive (n=41) | Atrophic (n=24) | P-value |
|---|
| Age, years
(range)a | 52 (48–55) | 52 (47–56) | 52 (48.5–54.5) | 51 (48–55) | 0.918 |
| Male (%)b | 45 (38.5) | 21 (40.4) | 18 (43.9) | 6 (25.0) | 0.296 |
| IL-6, ng/l
(range)a | 47.4 (42.7–53.3) | 44.0 (38.4–50.4) | 48.3 (43.2–52.8) | 59.2 (47.2–66.1) | <0.001 |
| TNF-α, ng/l
(range)a | 54.4 (47.6–61.7) | 52.6 (45.5–57.1) | 54.9 (50.1–60.6) | 62.7 (53.6–69.9) | <0.001 |
| IFN-γ, ng/l
(range)a | 27.2 (24.8–30.5) | 27.2 (24.7–30.0) | 27.6 (26.0–31.0) | 25.5 (23.9–29.5) | 0.241 |
| IL-4, ng/l
(range)a | 26.5 (24.1–28.8) | 25.0 (21.9–27.6) | 26.7 (24.5–28.7) | 28.3 (26.2–31.2) | 0.001 |
| IFN-γ/IL-4
(range)a | 1.005
(1.065–1.144) | 1.085
(1.032–1.201) | 1.076
(1.016–1.143) | 0.964
(0.813–1.024) | <0.001 |
| Mast cell,
MCs/mm2 (range)a | 26 (24–29) | 25 (23–26) | 28 (26–29) | 30 (27–32) | <0.001 |
| TSLP, ng/l
(range)a | 204.9
(185.3–230.9) | 196.1
(179.8–217.0) | 211.3
(189.9–229.9) | 232.5
(191.6–246.7) | 0.001 |
| Periostin
expressionb (%) |
|
|
|
| 0.012 |
| High | 56 (47.9) | 18 (34.6) | 21 (51.2) | 17 (70.8) |
| Low | 61 (52.1) | 34 (65.4) | 20 (48.8) | 7 (29.2) |
There were 110 healthy controls including 37 males
and 73 females, with the ages ranging from 27 to 67 years (median
age, 52 years). No significant differences were found in age and
gender between control group and OLP group. The protein expression
of periostin was determined by IHC in oral epithelium tissues and
by ELISA in serums of OLP patients and controls. The periostin
protein level was evaluated based on the combination of positive
cell percentage and staining intensity, and there were 56 (47.9%)
OLP cases showing high periostin expression, which was
significantly higher than that of controls (12 cases, 10.9%)
(P<0.001). Furthermore, the serum periostin level was
significantly higher in OLP group (Median 86.9 µg/l) than control
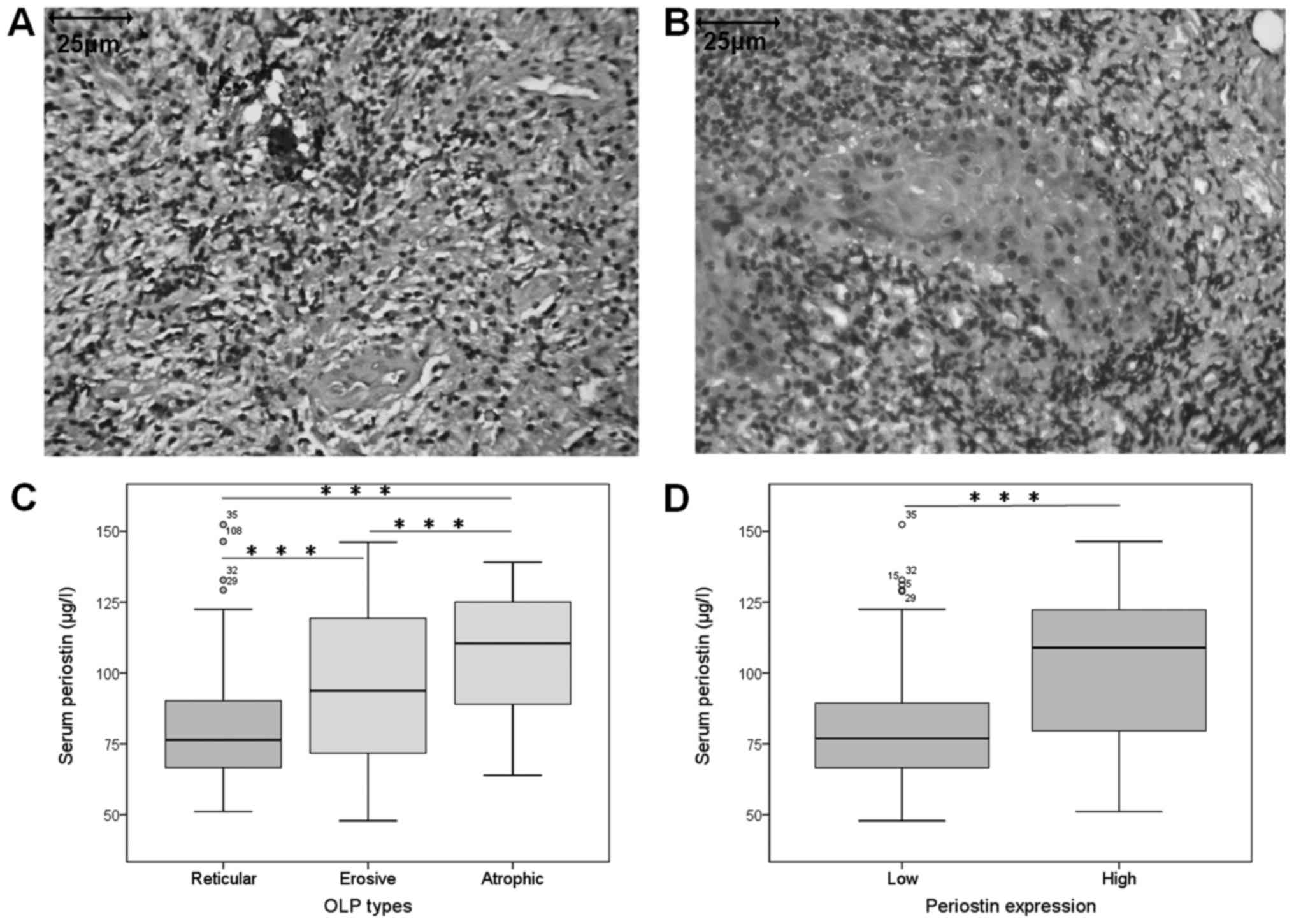
group (Median 37.4 µg/l) (P<0.001) (Table II). In most cases of OLP, strong
periostin staining was found in the oral epithelium, and little or
no periostin staining was found in oral epithelium of most controls
(Fig. 1A and B), Therefore, in the
later study, we divided OLP subjects into high periostin group
(n=56) and low periostin group (n=61) according the results of
IHC.
 | Table II.Comparison between healthy controls
and oral lichen planus in tissue and serum periostin
expressions. |
Table II.
Comparison between healthy controls
and oral lichen planus in tissue and serum periostin
expressions.
| Characteristic | Healthy controls
(n=110) | Oral lichen planus
(n=117) | P-value |
|---|
| Age, years
(range)a | 52 (49–55) | 52 (48–55) | 0.624 |
| Male
(%)b | 37 (33.6) | 45 (38.5) | 0.491 |
| Tissue periostin
(%)b | 12 (10.9) | 56 (47.9) | <0.001 |
| Serum periostin,
ng/l (range)a | 37.4
(32.9–48.4) | 86.9
(70.7–115.2) | <0.001 |
The periostin expression was significantly
associated with OLP clinical type. Atrophic form had the highest
percentage (17/24) of high tissue periostin expression among all
three OLP clinical types (P<0.05; Table I). Moreover, serum periostin levels
in erosive form and atrophic form were significantly higher compare
with that in reticular form (P<0.001; Fig. 1C). There was significant correlation
between tissue periostin expression and serum periostin level,
which was supported by significantly higher serum periostin level
in high periostin group compared with low periostin group (Fig. 1D).
Association between periostin
expression and proinflammatory cytokine levels
To further investigate the clinical significance of
periostin in OLP, the correlations between periostin expression and
proinflammatory cytokine levels in 117 patients were analyzed.
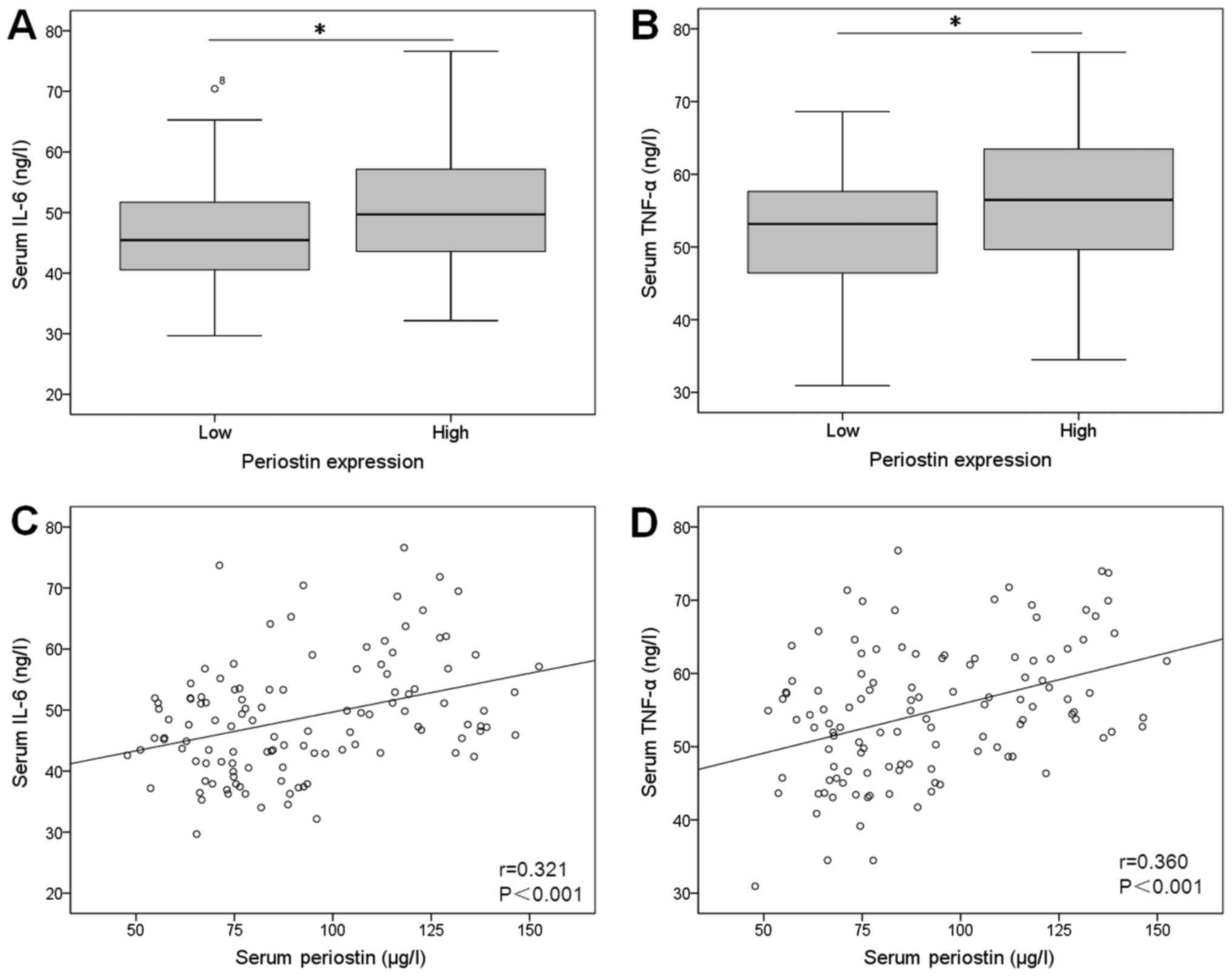
Serum IL-6 and TNF-α levels were significantly higher in high
periostin group compared with low expression group (Fig. 2A and B). Furthermore, serum periostin
level was correlated positively to IL-6 (r=0.321, P<0.001) and
TNF-α (r=0.360, P<0.001; Fig. 2C and
D).
Association between the periostin
expression and IFN-γ/IL-4 ratio
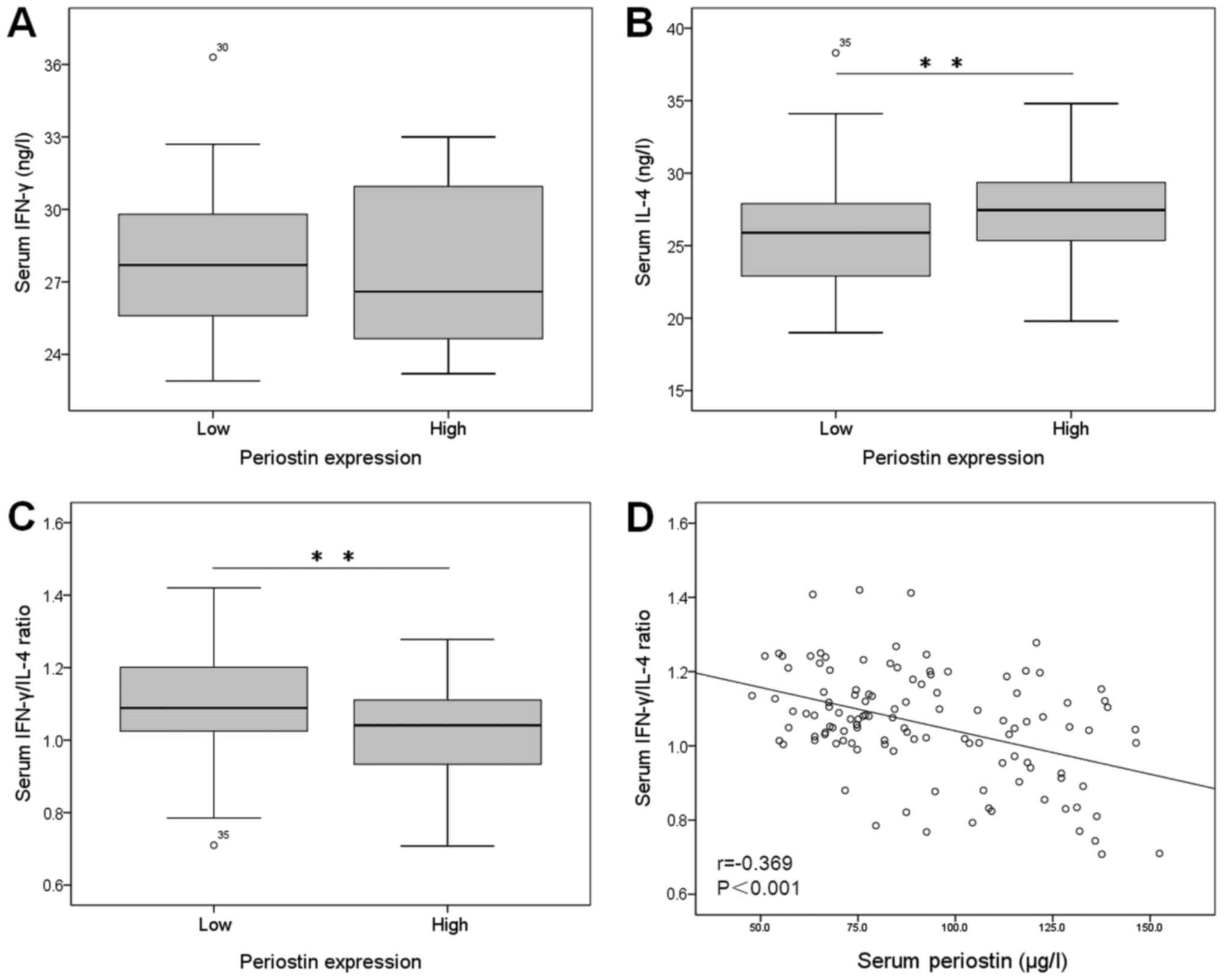
The serum IFN-γ concentrations showed no
statistically significant between periostin-high group and
periostin-low group (P=0.750; Fig.
3A). The serum IL-4 concentrations were significant higher in
patients with periostin-high than in patients with periostin-low
(P=0.005; Fig. 3B). The serum
IFN-γ/IL-4 ratio was significantly lower in OLP patients with
periostin-high than in those with periostin-low (P=0.003; Fig. 3C). Furthermore, we found negetive
correlation between the serum periostin level and serum IFN-γ/IL-4
ratio (r=0.369, P<0.001; Fig.
3D).
Association between the periostin
expression and mast cell count
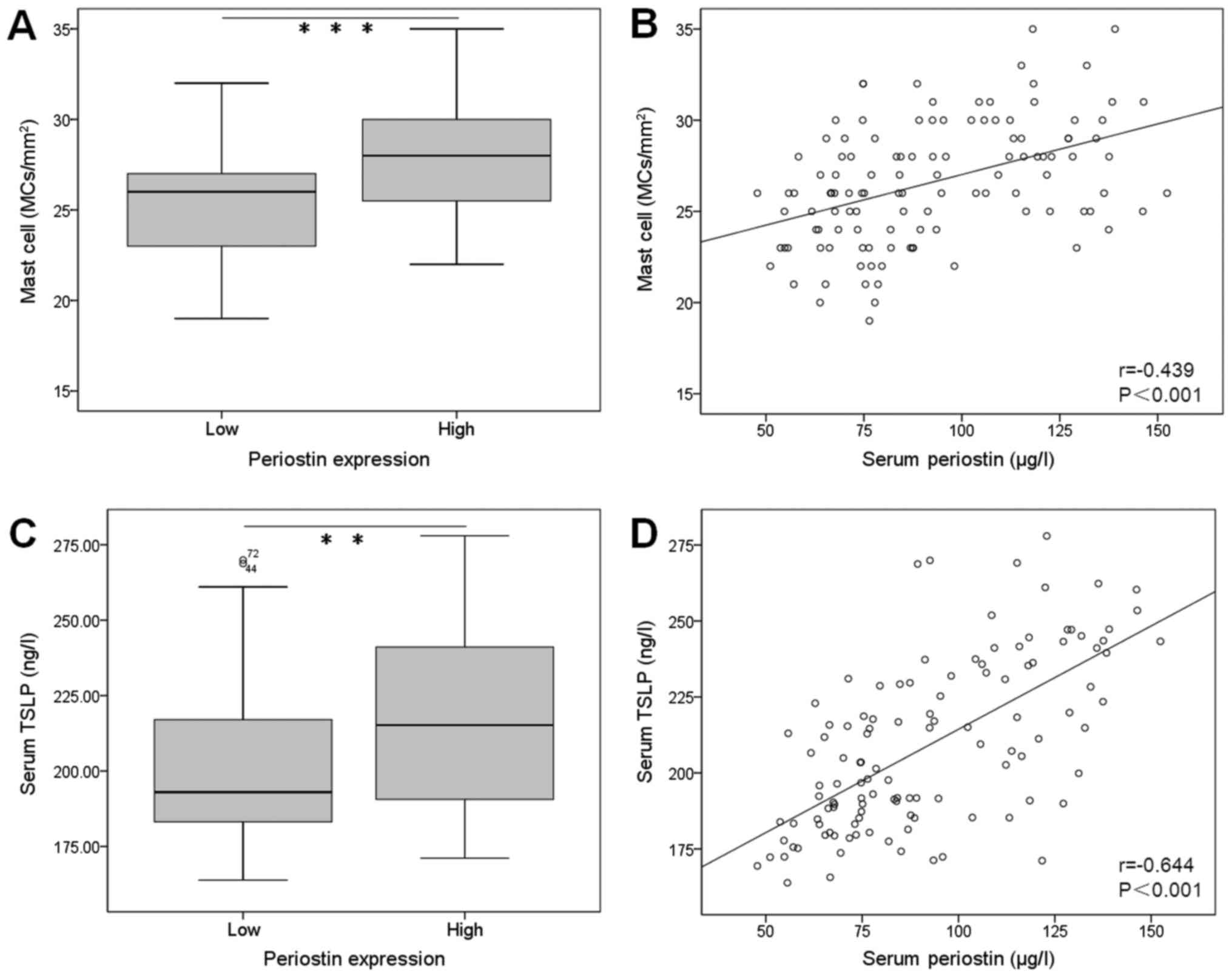
Total number of mast cells stained with TB showed
significantly higher in oral tissue of patients with periostin-high
(median=28 MCs/mm2) than that with periostin-low
(median=26 MCs/mm2) (Fig.
4A). Positive correlation was observed between the serum
periostin level and mast cell density in oral tissue of OLP
subjects (r=0.439, P<0.001; Fig.
4B).
Association between the periostin
expression and serum TSLP
The serum concentration of TSLP was significantly
higher in patients with periostin-high than those with
periostin-low (P<0.01; Fig. 4C).
There was positive correlation between the serum periostin level
and TSLP in OLP subjects (r=0.644, P<0.001; Fig. 4D).
Discussion
In this study, we measured periostin expression in
117 OLP subjects and found that periostin protein levels were
significantly higher in oral mucosa and serum compared with those
in controls. The high periostin expression was positively
correlated with serum IL-6, TNF-α, TSLP and tissue mast cell
density, and negatively correlated with IFN-γ/IL-4 ratio. Our study
provides a new biomarker for clinical status and malignant
potential of OLP.
Our study showed that high expression of periostin
was detected in more cases of OLP specimens than those of normal
oral mucosa specimens. Other report showed that periostin
expression was higher in OSCC tumor tissue compared with control
tissue (11), and periostin protein
level was significantly correlated with OSCC invasion and
metastasis (12). This indicates
that periostin has cancerous potential and might play roles in
malignant transformation of OLP. In fact, our results showed that
periostin expression was significantly higher in OLP with erosive
and atrophic forms, two OLP clinical types with the highest
malignant transformation rate (13).
Furthermore, serum periostin levels were also significantly higher
in OLP subjects than in controls, and positive correlation was
observed between tissue periostin and serum periostin levels. These
indicate that the serum periostin might come from oral mucosa, as
periostin is a soluble and secreted extracellular matrix protein.
Therefore, serum periostin may act as a biomarker for evaluating
clinical status and malignant transformation potential of OLP
patients.
Our results showed the association between periostin
expression and inflammatory response, as evidenced by increased
serum IL-6 and TNF-α level in periostin high group compared with
periostin low group, and our results are in accordance with other
report (14). Chronic inflammation
is an important mechanism of OLP and it participates in the
development and progression to OSCC (15). Aberrant production of cytokines may
lead to immune deficiency or autoimmunity, which are involved in
the mechanisms of OLP (16).
Periostin is also a protein involved in inflammatory response and
could produce tumor-promotive microenvironments (17). Treatment with TNF-α could
significantly increase in periostin expression in intestinal
epithelial cells (18), and this
indicates that inflammation can promote the production of
periostin. Furthermore, periostin is also a promotor of
inflammation and can activating NF-κB signaling, thereby increasing
IL-6 production (19). Therefore, a
positive feedback loop may exist in OLP that induce the interaction
between periostin and proinflammatory cytokines.
Our results showed the serum IFN-γ/IL-4 ratio was
significantly lower in periostin-high group compared with
periostin-low group, and a negative correlation was observed
between serum periostin and IFN-γ/IL-4 ratio. In mucosal immune
response, CD4+ T lymphocytes can differentiate into two
functionally distinct populations with opposite effects, T helper 1
(Th1) cells and Th2 cells. IFN-γ and IL-4 are two principal
cytokines which are produced from Th1 and Th2 cells respectively,
thus maintaining Th1/Th2 balance (20). OLP patient showed a lower IFN-γ/IL-4
ratio in both serum and saliva, especially in
erythematous/ulcerative group compared with the reticular group
(21). This indicates OLP is a Th2
cytokine-predominant disorder and Th2 immune imbalance may lead to
immunosuppression and increase the risk for malignant
transformation. In allergic skin inflammation mouse model, Th2
cytokines IL-4 and IL-13 could stimulate periostin production,
thereby inducing proinflammatory cytokines and accelerating
Th2-type immune responses (22).
Therefore, in our study, periostin may mediate the inflammatory
response induced by Th2 immune imbalance.
We found OLP patients with high periostin expression
showed increased total number of mast cells in oral mucosa tissue
than patients with low periostin, and the mast cell density was
positively correlated to serum periostin levels. Mast cell count
was higher in oral mucosa of OLP compared to that of healthy
controls (23). Currently, the
association between mast cell and periostin remains unclear. A
report showed that mast cell count was higher in oral submucous
fibrosis (OSMF) than in normal buccal mucosa (24). OSMF is a premalignant mucosal disease
with inflammation and progressive fibrosis. As an inducer of
fibrosis in various tissues, periostin might mediate the fibrosis
process of OSMF (25), and the
potential regulatory roles of periostin in mast cells of OLP
deserve further investigation (26).
Our results showed the serum TSLP was significantly
higher in periostin-high group compared with periostin-low group,
and a positive correlation was found between the serum TSLP and
periostin levels. TSLP is a cytokine that can activate dendritic
cells to induce Th2-mediated inflammation (27). In OLP subjects, the TSLP-positive
cells in oral epithelium and the TSLP serum level were
significantly increased than in normal controls (28). The pathogenesis of TSLP in OLP may be
associated with activating Th2 cytokine production and enhanced
mast cell proliferation (29).
Periostin can contribute chronic inflammation by stimulating TSLP
production in lesional skin of cutaneous T-cell lymphoma (30). Therefore, in our study the high serum
TSLP may induced by overexpressed periostin in oral epithelium of
OLP, and the detailed mechanism underlying TSLP production by
periostin in OLP deserve further investigation.
In conclusion, the expression of periostin is
significantly elevated in oral epithelium and serum of OLP
patients, which indicates higher malignant potential of OLP. The
high periostin expression in OLP is positively correlated with
serum IL-6, TNF-α, TSLP and tissue mast cell density. Periostin may
cause chronic inflammation, stimulating TSLP production and mast
cell proliferation, and direct the autoimmune response towards Th2
immune imbalance. Periostin might promote malignant transformation
of OLP, which required verification in future studies with larger
sample size. More investigation is needed to elucidate the
mechanisms of periostin in OLP canceration processes by in
vitro and in vivo experimental studies.
Acknowledgements
Not applicable.
Funding
This study was supported in part by grants from the
Gansu Natural Science Foundation (2008GS01177).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
ZRZ designed the study and wrote the manuscript. LYC
and HYQ performed all the experiments and collected the data. SHS
analyzed the data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the First Hospital of Lanzhou University. Informed consent was
signed by the patients or their relatives.
Consent for publication
Written informed consent was obtained from the
patients or their relatives for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alrashdan MS, Cirillo N and McCullough M:
Oral lichen planus: A literature review and update. Arch Dermatol
Res. 308:539–551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krupaa RJ, Sankari SL, Masthan KM and
Rajesh E: Oral lichen planus: An overview. J Pharm Bioallied Sci. 7
Suppl 1:S158–S161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eversole LR: Immunopathogenesis of oral
lichen planus and recurrent aphthous stomatitis. Semin Cutan Med
Surg. 16:284–294. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fitzpatrick SG, Hirsch SA and Gordon SC:
The malignant transformation of oral lichen planus and oral
lichenoid lesions: A systematic review. J Am Dent Assoc. 145:45–56.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kurago ZB: Etiology and pathogenesis of
oral lichen planus: An overview. Oral Surg Oral Med Oral Pathol
Oral Radiol. 122:72–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rios H, Koushik SV, Wang H, Wang J, Zhou
HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, et
al: Periostin null mice exhibit dwarfism, incisor enamel defects,
and an early-onset periodontal disease-like phenotype. Mol Cell
Biol. 25:11131–11144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ratajczak-Wielgomas K and Dziegiel P: The
role of periostin in neoplastic processes. Folia Histochem
Cytobiol. 53:120–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia W, Wang W, Ji CS, Niu JY, Lv YJ, Zhou
HC and Hu B: Coexpression of periostin and EGFR in patients with
esophageal squamous cell carcinoma and their prognostic
significance. Onco Targets Ther. 9:5133–5142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Madhuri AR, Alka KD and Ramakanth N: Mast
cells are increased in leukoplakia, oral submucous fibrosis, oral
lichen planus and oral squamous cell carcinoma. JOMFP. 11:18–22.
2007.
|
|
11
|
Choi P, Jordan CD, Mendez E, Houck J, Yueh
B, Farwell DG, Futran N and Chen C: Examination of oral cancer
biomarkers by tissue microarray analysis. Arch Otolaryngol Head
Neck Surg. 134:539–546. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siriwardena BS, Kudo Y, Ogawa I, Kitagawa
M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M and Takata T:
Periostin is frequently overexpressed and enhances invasion and
angiogenesis in oral cancer. Br J Cancer. 95:1396–1403. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agha-Hosseini F, Sheykhbahaei N and
SadrZadeh-Afshar MS: Evaluation of potential risk factors that
contribute to malignant transformation of oral lichen planus: A
literature review. J Contemp Dent Pract. 17:692–701. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaur J and Jacobs R: Proinflammatory
cytokine levels in oral lichen planus, oral leukoplakia, andoral
submucous fibrosis. J Korean Assoc Oral Maxillofac Surg.
41:171–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Messadi DV, Wu H and Hu S: Oral
lichen planus is a unique disease model for studying chronic
inflammation and oral cancer. Med Hypotheses. 75:492–494. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu R, Zhang J, Sun W, Du G and Zhou G:
Inflammation-related cytokines in oral lichen planus: An overview.
J Oral Pathol Med. 44:1–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu AY, Zheng H and Ouyang G: Periostin, a
multifunctional matricellular protein in inflammatory and tumor
microenvironments. Matrix Biol. 37:150–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koh SJ, Choi Y, Kim BG, Lee KL, Kim DW,
Kim JH, Kim JW and Kim JS: Matricellular protein periostin mediates
intestinal inflammation through the activation of nuclear factor κB
signaling. PLoS One. 11:e01496522016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taniguchi K, Arima K, Masuoka M, Ohta S,
Shiraishi H, Ontsuka K, Suzuki S, Inamitsu M, Yamamoto KI, Simmons
O, et al: Periostin controls keratinocyte proliferation and
differentiation by interacting with the paracrine IL-1α/IL-6 loop.
J Invest Dermatol. 134:1295–1304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neurath MF, Finotto S and Glimcher LH: The
role of Th1/Th2 polarization in mucosal immunity. Nat Med.
8:567–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu WZ, He MJ, Long L, Mu DL, Xu MS, Xing
X, Zeng X, Liao G, Dan HX and Chen QM: Interferon-γ and
interleukin-4 detected in serum and saliva from patients with oral
lichen planus. Int J Oral Sci. 6:22–26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masuoka M, Shiraishi H, Ohta S, Suzuki S,
Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, et al:
Periostin promotes chronic allergic inflammation in response to Th2
cytokines. J Clin Invest. 122:2590–2600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma R, Sircar K, Singh S and Rastogi V:
Role of mast cells in pathogenesis of oral lichen planus. J Oral
Maxillofac Pathol. 15:267–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pujari R and Vidya N: Mast cell density in
oral submucous fibrosis: A possible role in pathogenesis. Int J
Health Sci (Qassim). 7:23–29. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Y, Liu W, Xiao H, Maitikabili A, Lin
Q, Wu T, Huang Z, Liu F, Luo Q and Ouyang G: Matricellular protein
periostin contributes to hepatic inflammation and fibrosis. Am J
Pathol. 185:786–797. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim DW, Kulka M, Jo A, Eun KM, Arizmendi
N, Tancowny BP, Hong SN, Lee JP, Jin HR, Lockey RF, et al:
Cross-talk between human mast cells and epithelial cells by
IgE-mediated periostin production in eosinophilic nasal polyps. J
Allergy Clin Immunol. 139:1692–1695.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ziegler SF, Roan F, Bell BD, Stoklasek TA,
Kitajima M and Han H: The biology of thymic stromal lymphopoietin
(TSLP). Adv Pharmacol. 66:129–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun M, Tan W, Liu S, Liu G, Zhang X, Wang
N, Qu X and Wei F: In situ expression and serum level of thymic
stromal lymphopoietin in oral lichen planus. J Oral Pathol Med.
43:740–745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Akasaki S, Matsushita K, Kato Y, Fukuoka
A, Iwasaki N, Nakahira M, Fujieda S, Yasuda K and Yoshimoto T:
Murine allergic rhinitis and nasal Th2 activation are mediated via
TSLP- and IL-33-signaling pathways. Int Immunol. 28:65–76.
2016.PubMed/NCBI
|
|
30
|
Takahashi N, Sugaya M, Suga H, Oka T,
Kawaguchi M, Miyagaki T, Fujita H and Sato S: Thymic stromal
chemokine TSLP acts through Th2 cytokine production to induce
cutaneous T-cell lymphoma. Cancer Res. 76:6241–6252. 2016.
View Article : Google Scholar : PubMed/NCBI
|