Introduction
Human osteosarcoma (OS) is the most common primary
malignant bone tumour that mainly occurs in children and
adolescents. Conventional therapeutic approaches include treatment
of disseminated disease with multi-agent cytotoxic chemotherapy,
and local control of the primary lesion by chemotherapy or surgery.
However, the 5-year survival rate of OS patients is only 60–70%,
and there have been no improvements in this rate in the last 30
years (1,2), especially for patients showing
metastasis at diagnosis (3).
Consequently, there is a critical need to identify novel diagnostic
markers and effective therapeutic targets for OS.
Eukaryotic translation initiation factor 3 (EIF3) is
the largest of the translation initiation factors, comprising 13
non-identical protein subunits with a mass that is approximately
50% less than that of the 40S ribosomal subunit. One member of this
family, EIF3H, plays a central role in translation initiation in
higher eukaryotes and is located on chromosome 8q23 (4), a region frequently amplified in many
tumour types (5,6). Recently, expression of the EIF3H
gene was shown to be significantly upregulated in many human
cancers (7,8). Knockdown of EIF3H could decrease
cell viability through both cell cycle arrest and apoptosis
induction and inhibited the formation of colonies in
anchorage-independent conditions in breast cancer cells (7). Cappuzzo et al examined 54
metastatic NSCLC patients treated with gefitinib and found 10 cases
(18.5%) showed amplification of EIF3H (8). These results indicated that EIF3H could
play an important role in the growth and malignant phenotypes of
cancer cells. However, the participation of EIF3H in human
OS development and progression has been scarcely studied, and
therefore its function in human OS is poorly understood.
To fill this knowledge gap and evaluate EIF3H
as a candidate therapeutic or diagnostic target, we synthesized a
sequence-specific interfering short hairpin RNA (shRNA) lentivirus
targeting the EIF3H gene in OS cell lines to evaluate
whether EIF3H could affect OS cell proliferation in
vitro. MTT, cell cycle and apoptosis assay were selected to
assess cell proliferation. In order to further verified this
effect.
We investigated the potential role of EIF3H in OS by
knocking down its expression in two OS cell lines, and evaluated
the effects in vivo using an animal model.
Materials and methods
Cell culture
293T cells and the human OS cell lines Saos-2, U2OS
and MG-63 were purchased from the Cell Bank of the Chinese Academy
of Science (Shanghai, China). All cells were cultured in Dulbecco's
minimum essential medium (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) with 10% fetal bovine serum (Biowest, Riverside,
MO, USA, 100 units/ml penicillin, and 100 mg/ml streptomycin at
37°C in a 5% CO2 atmosphere incubator.
Construction of the shRNA interference
lentiviral vector
Two RNA interference sequences targeting
EIF3H mRNA were designed and synthesized according to the
EIF3H mRNA sequence in GenBank (NCBI accession no.
NM_003756): EIF3H siRNA s1, 5′-GCAACTCTTGGAAGAAATATA-3′;
EIF3H siRNA s2, 5′-CCCAAGGATCTCTCTCACTAA-3′. A random
sequence was also designed to serve as a negative control (shCon).
Sequences in the form of shRNA were inserted to a shRNA cloning and
expression lentivirus vector containing green fluorescent protein
(GFP)-tagged (SBI, Palo Alto, CA, USA). The lentivirus particles
were produced in 293T cells transfected with the shRNA vector
(shEIF3H) or the control (shCon) vector together with pHelper
plasmids SHP001 (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany).
Infection of cells by the
lentivirus
Saos-2 and U2OS cells were seeded in 6-well plates
at approximately 3×104 cells/well and 2.5×104
cells/well, respectively, and maintained in a 5% CO2
incubator at 37°C until reaching approximately 30% confluence.
Saos-2 cells and U2OS cells were infected with the shEIF3H or shCon
vectors at a multiplicity of infection of 40 and 20, respectively,
according to the virus titre of the sequence. The medium was
replaced after culturing for 16 h. Expression of the GFP reporter
gene in lentivirus-infected cells was observed under a fluorescence
microscope at 120 h post-infection.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was prepared from the human OS cell lines
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). First-strand cDNA was synthesized from total RNA
with M-MLV reverse transcriptase (Promega, Madison, WI, USA)
according to the manufacturer's instructions. RT-qPCR was performed
using the SYBR-Green mix (Thermo Fisher Scientific, Inc.) on the
Bio-Rad CFX96 sequence detection system. The primer sequences for
the human EIF3H gene were: Forward
5′-GTGCTTTTGGGTCTGGTTGT-3′ and reverse 5′-ATACCAGCCCACGTGAAGAT-3′.
The EIF3H gene expression levels obtained were normalized to
the mRNA expression levels of actin, amplified with the following
primers: Forward 5′-GTGGACATCCGCAAAGAC-3′ and reverse
5′-AAAGGGTGTAACGCAACTA-3′. The reaction conditions were as follows:
a predenaturation step of 1 min at 95°C followed by 40 cycles at
95°C for 5 sec and 60°C for 20 sec. The melting curve was
established under the following conditions: 95°C for 15 sec, 55°C
for 30 sec, and 95°C for 15 sec. Each reaction was repeated three
times per sample. The relative expression level of EIF3H was
calculated using the comparative quantification cycle (Cq) method
2−ΔΔCq (9).
Western blot analysis
Cell lysates were prepared from Saos-2 and U2OS
cells using 2X sodium dodecyl sulphate (SDS) Sample Buffer [100 mM
Tris-HCl (pH 6.8), 10 mM ethylenediaminetetraacetic acid, 4% SDS,
and 10% glycine]. Lysates were clarified by centrifugation at
13,000 × g for 5 min at 4°C, and the total protein was quantified
by the bicinchoninic acid method and read at 560 nm. Protein
samples were fractionated on 12% SDS-polyacrylamide gel
electrophoresis gels and then transferred to polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with 5% non-fat milk for 1 h at room
temperature and incubated with rabbit monoclonal anti-EIF3H
antibody (1:500; Proteintech, Rosemont, IL, USA) followed by
incubation with the secondary antibody horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:5,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Bands were detected
using enhanced chemiluminescence (ECL-PLUS/kit; Amersham Pharmacia
Biotech, Tokyo, Japan) reagents. Anti-GAPDH antibody (1:500,000;
Santa Cruz Biotechnology, Inc.) was used as the loading
control.
Cell proliferation assays
The effect of EIF3H knockdown on cell
proliferation was measured using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
M2128; Sigma-Aldrich; Merck KGaA) plus acidic isopropanol. In
brief, Saos-2 and U2OS cells were respectively seeded in 6-well
plates at a density of 2×103 cells/well. At 24, 48, 72,
96, and 120 h after viral infection, MTT plus acidic isopropanol
solution was added to each well and the plates were incubated at
37°C for 1 h. Absorbance values were determined at 450 nm on a
microplate reader (Epoch; BioTek, Winooski, VT, USA).
Colony formation assays
For colony formation assays, Saos-2 and U2OS cells
were plated on 6-well plates at 400 and 600 cells/well and cultured
for 7 and 8 days, respectively, in a 5% CO2 incubator at
37°C (Thermo Fisher Scientific, Inc.). The colonies formed were
washed with phosphate-buffered saline (PBS), fixed with methanol,
and finally stained with 0.1% crystal violet solution (C0121;
Beyotime Institute of Biotechnology, Haimen, China). The number of
colonies containing 50 or more cells was counted under an inverted
microscope (CKX41; Olympus, Tokyo, Japan). Each assay was repeated
in triplicate.
Cell cycle analysis
For cell cycle analysis, Saos-2 and U2OS cells
infected with shEIF3H or shCon were seeded in 6-cm dishes at
6×104 cells/dish and 8×104 cells/dish,
respectively. After being cultured for 5 days, or until the cells
reached approximately 80% confluence, the cells were stained with
propidium iodide (Beyotime Institute of Biotechnology), and the
cell cycle distribution was assayed on a Gallios flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA). The percentages of cells
infected with ShEFI3H or shCon at the G0/G1, S, and G2/M phases
were determined and compared. The experiments were performed in
triplicate.
Flow cytometric analysis of
apoptosis
The quantification of apoptotic cells was determined
by flow cytometry using the Annexin V/7-AAD double staining kit
(KGA1026; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
according to the manufacturer's instructions. In brief, Saos-2
cells and U2OS cells infected with ShRNA or shCon were seeded in
6-cm dishes at 6×104 cells/dish and 1.5×105
cells/dish, respectively. When the cells reached approximately 80%
confluence, they were harvested, washed twice with PBS, and
suspended in 450 µl binding buffer. Annexin V was added at room
temperature, let to stand for 15 min for staining without light,
and then resuspended in 450 µl binding buffer. The cells were then
stained with 7-AAD in the dark. Cell apoptosis was analysed on a
Gallios flow cytometer (Beckman Coulter, Inc.).
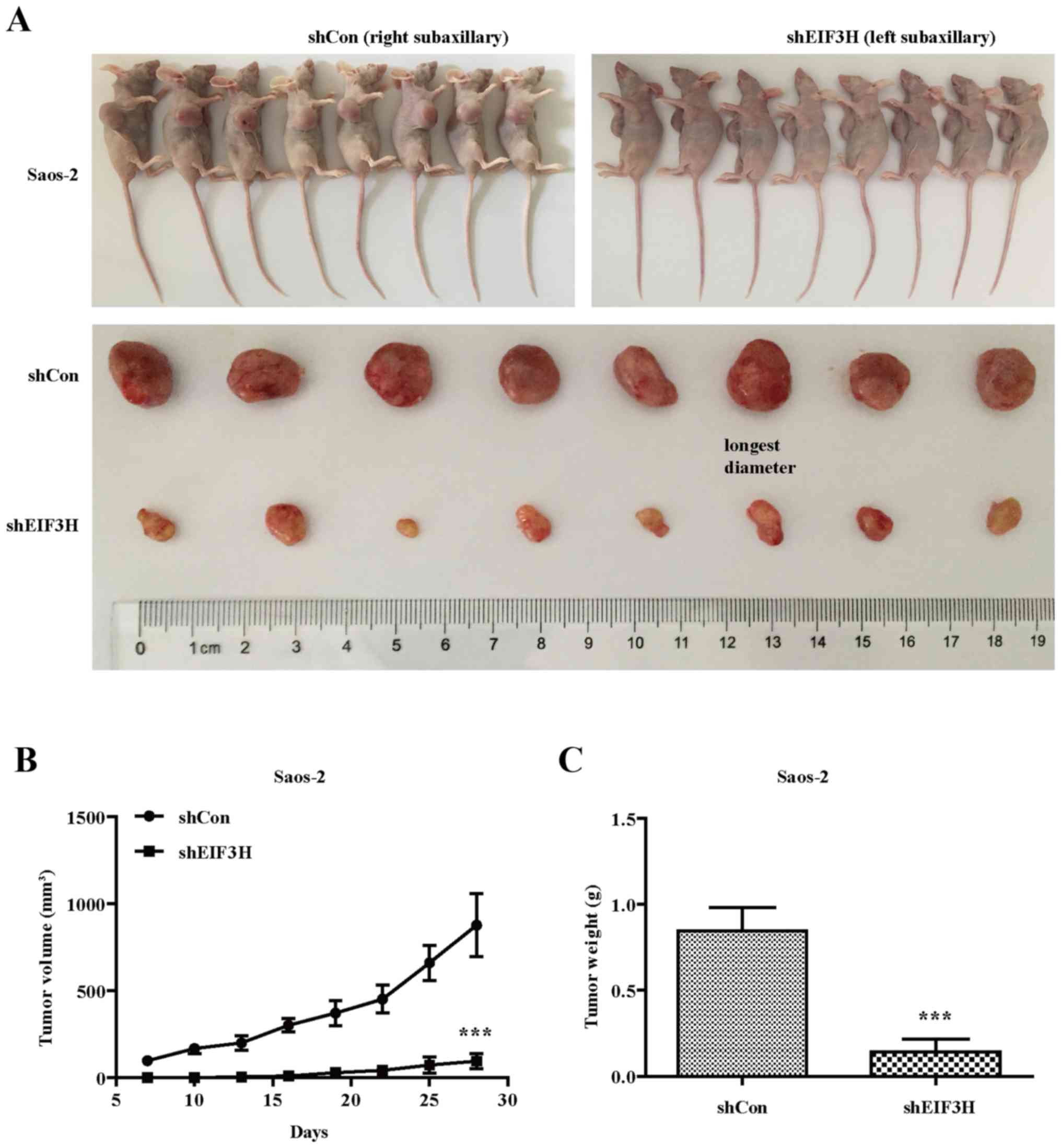
Xenograft tumorigenicity assay
Male BALB/c-Nude mice, 6–9 weeks old, were purchased
from SLRC Laboratory Animal Company (Shanghai, China), and were
housed under pathogen-free conditions in the barrier animal
facility. For in vivo tumorigenicity experiments, Saos-2
cells stably infected with shCon or shEIF3H were collected,
resuspended in PBS, and injected subcutaneously into the right or
left subaxillary region of each mouse (2×104 per mouse),
respectively. From the 7th day on, tumour xenografts were measured
with callipers every 3 days, and tumour volume was calculated using
the following formula: (length × width2) × 0.5. At the
end of the experiments (day 28 post inoculation), the mice were
anaesthetized with intraperitoneal injection of 100 mg/kg
pentobarbital sodium, killed by cervical dislocation, and tumour
xenografts were recovered and weighed. All animal experiments were
approved by the Committee on the Ethics of Affiliated Hospital of
Zunyi Medical College.
Statistical analysis
Student's t-test was performed using GraphPad Prism
5.0 software. Data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Infection efficiency of Saos-2 and
U2OS cells with lentiviral vectors
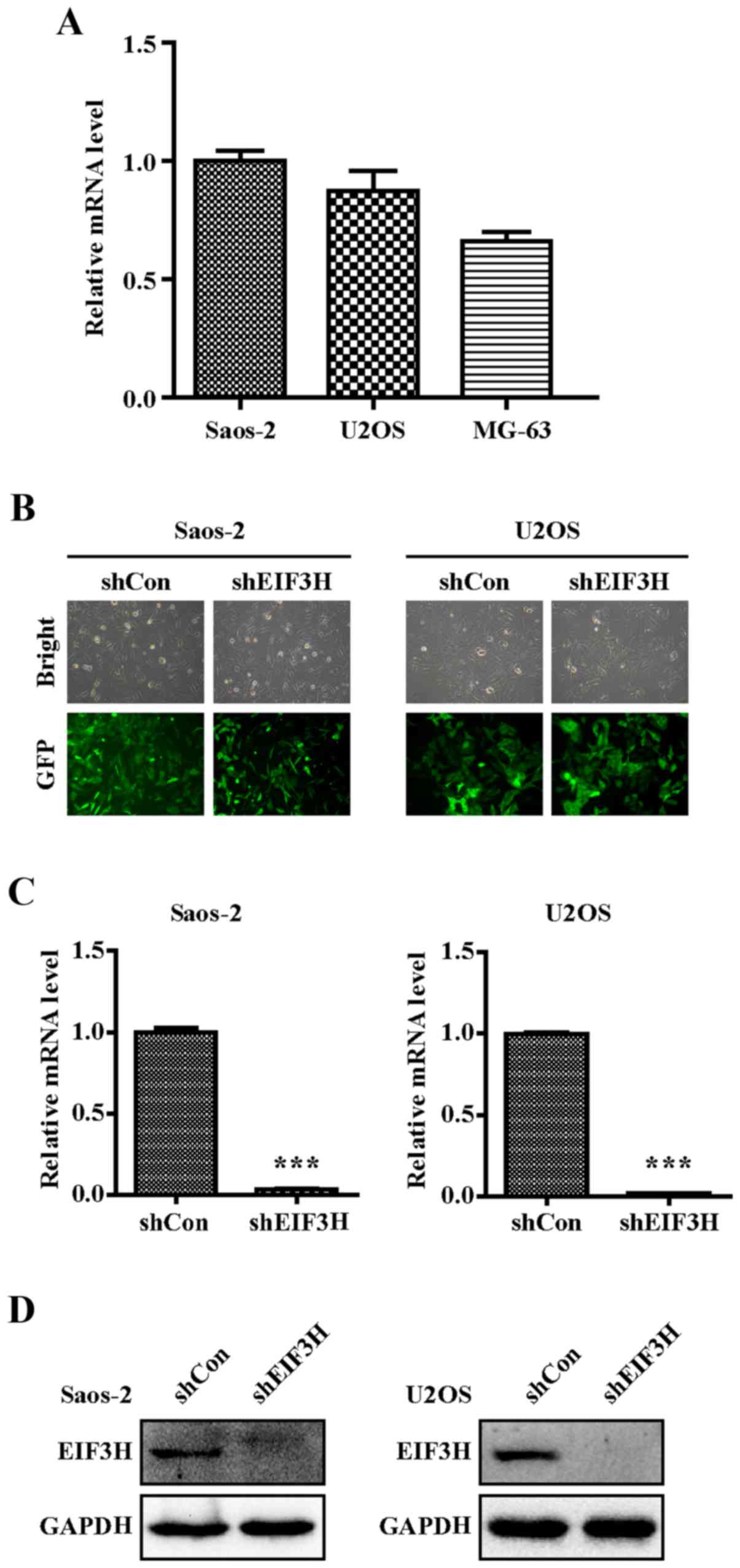
Expression of EIF3H in four human OS cell lines were
detected and results showed higher expression in Saos-2 and U2OS
(Fig. 1A), which were selected for
the next experiment. At 120 h post-infection with shEIF3H and
shCon, both Saos-2 and U2OS cells showed strong expression of GFP,
with an infection efficiency of over 80% in both cell lines
(Fig. 1B), indicating that the
lentiviral vector was successfully constructed to establish two
stable cell lines.
shRNA effectively knocked down EIF3H
mRNA expression
RT-qPCR analysis showed a 95% reduction in the
EIF3H mRNA levels in both OS cell lines infected with
shEIF3H compared to those infected with shCon (P<0.001; Fig. 1C). Western blot analysis further
confirmed the efficacy of gene silencing, given a significant
decrease in the relative protein expression level of EIF3H in the
shEIF3H groups for both cell types (Fig.
1D).
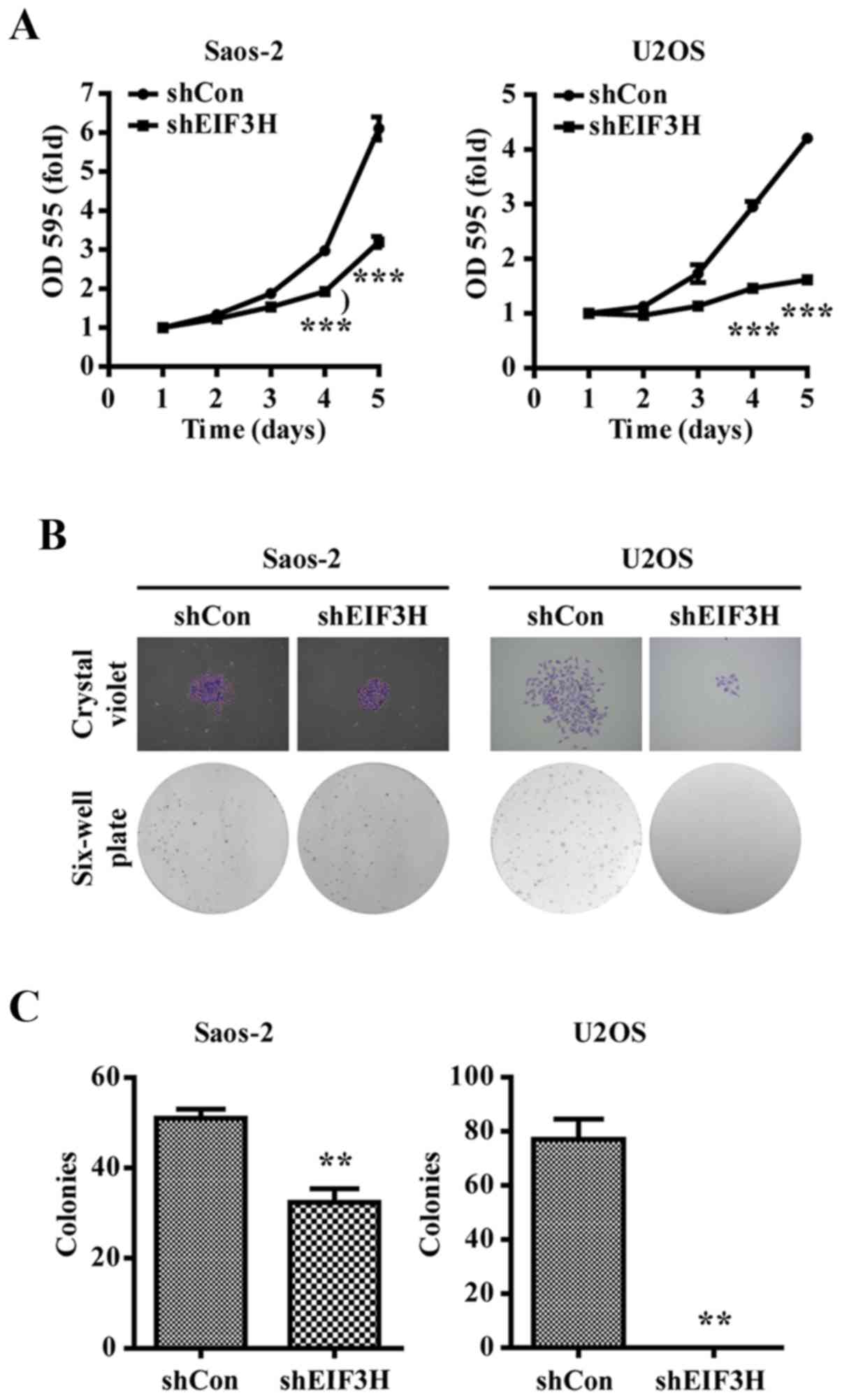
EIF3H knockdown inhibited OS cell
proliferation and colony formation
As shown in Fig. 2A,
knockdown of endogenous EIF3H significantly inhibited the
proliferation of Saos-2 and U2OS cells at 24, 48, 72, 96 and 120 h
after viral infection.
Moreover, the number cells forming colonies was
visibly reduced in the EIF3H knockdown groups compared to that in
the shCon-infected groups, with a significant difference
(P<0.01; Fig. 2B and C).
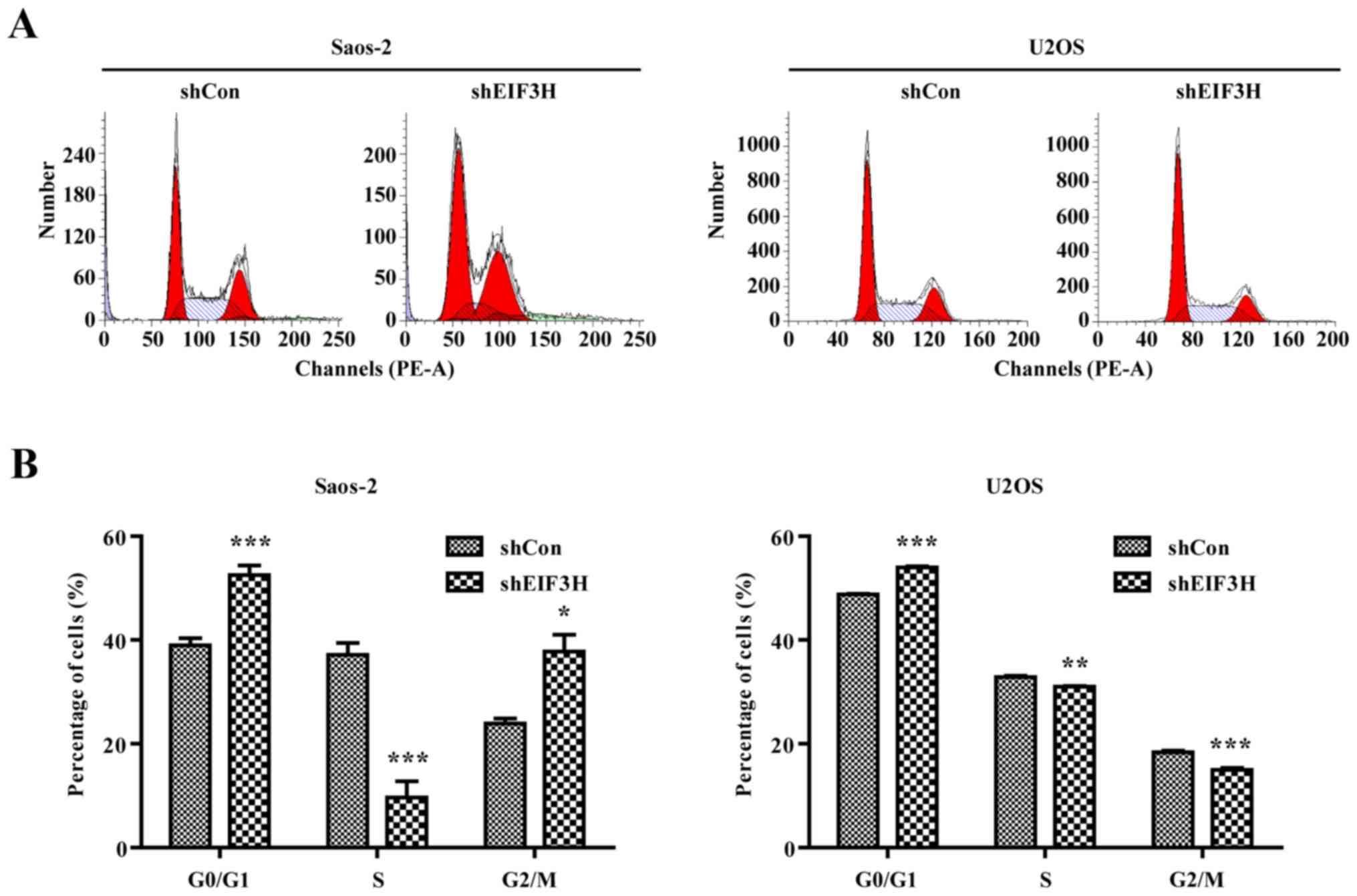
Knockdown of EIF3H led to cell cycle
arrest and promoted apoptosis in OS cells
To investigate the potential mechanism of the
inhibition of proliferation in the two OS cell lines, we assessed
the effect of EIF3H knockdown on the cell cycle of OS cells
by flow cytometry analysis. Both Saos-2 and U2OS cells with
suppressed EIF3H expression significantly accumulated in the G0/G1
phase, whereas the percentages of cells in the S phase were
significantly decreased compared to controls (P<0.05; Fig. 3A and B). These results indicated that
EIF3H knockdown contributed to induction of G0/G1 arrest in
OS cells.
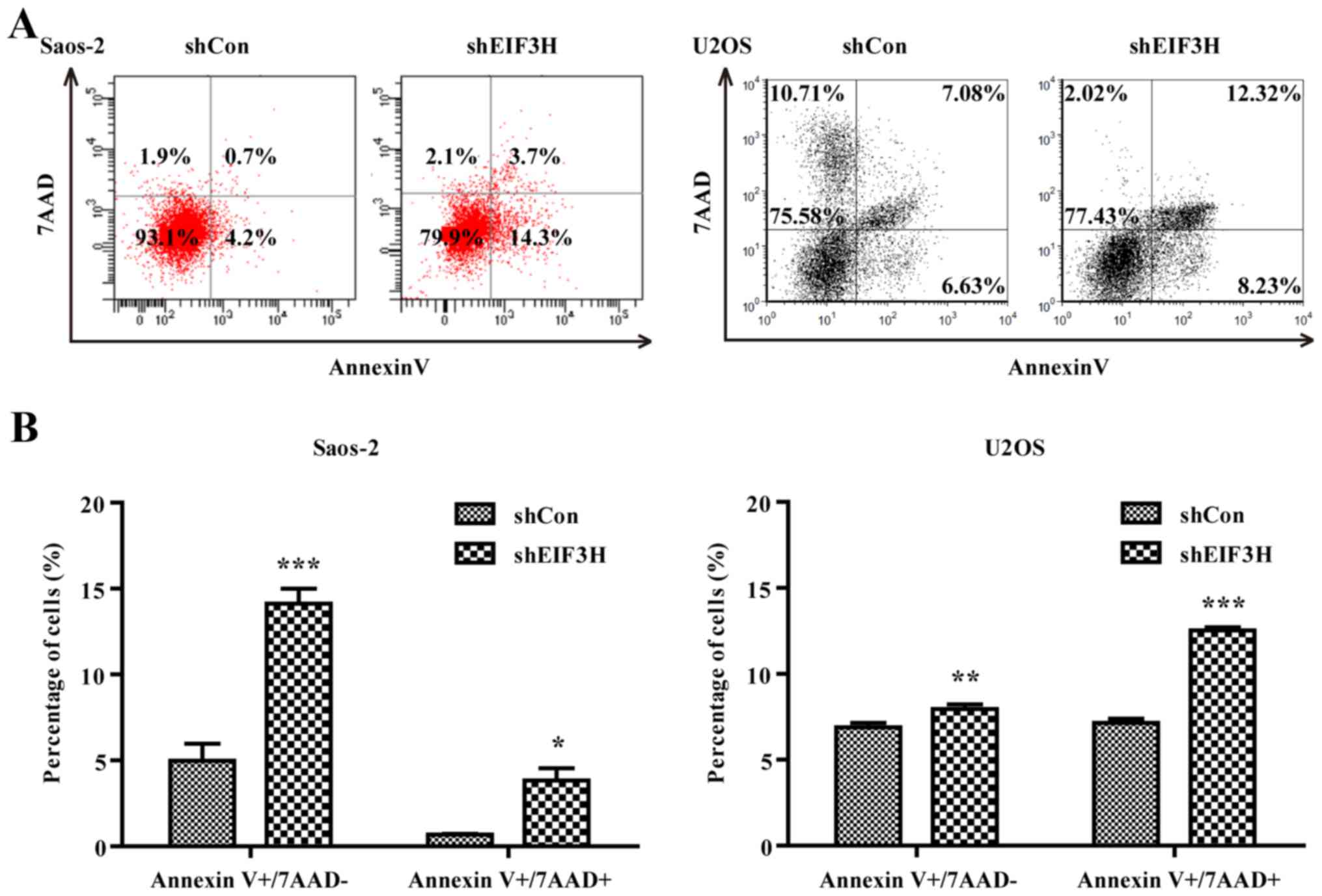
As shown in Fig. 4A and
B, EIF3H knockdown also increased the rate of apoptosis
in the two OS cell lines based on flow cytometry using Annexin V
and 7-AAD double-staining at both the early and late phases of
apoptosis (Saos2 cells: 14.13 and 3.83% vs. 4.97 and 0.67% in
controls, respectively, P<0.05; U2OS cells: 7.96 and 12.53% vs.
6.89 and 7.14% in controls, respectively, P<0.01).
EIF3H knockdown inhibited tumour
growth in nude mice
Saos-2 cells infected with shEIF3H developed
significantly smaller and reduced weighted tumors in mice compared
to those infected with shCon (Fig.
5A), indicating that EIF3H knockdown also inhibited the
growth of OS cells in vivo.
Discussion
OS is a malignant tumour that has become a global
health issue. Although advances have been made in OS diagnosis and
treatment, patient prognosis remains poor. We have demonstrated a
clear role of EIF3H in the growth of OS cells and tumour
development, suggesting a new candidate therapeutic target.
Over the past decade, the contribution of EIF3 to
malignant transformation and progression has been established, and
a previous study demonstrated that EIF3H expression was
up-regulated in 18% of breast cancers and 30% of prostate cancers
(10). Earlier studies also
indicated that EIF3H was essential for maintaining the
malignant state in cells (11). Zhu
et al (12) reported that
knockdown of EIF3H expression in hepatocellular carcinoma
cells promoted apoptosis, and inhibited cell growth, colony
formation, migration, as well as tumour growth in nude mice. In
another study, reduction of EIF3H levels reduced cell proliferation
and anchorage-independent growth in soft agar in breast and
prostate cancer cell lines (13).
However, the roles of EIF3H in human OS cells have thus far
remained unclear, and there has been minimal research conducted on
the effects of EIF3H in OS initiation and progression.
ShRNA-mediated gene silencing has proven to be a
powerful tool to investigate the roles of cancer-related genes.
Mahmood et al (7)
demonstrated that EIF3H knockdown with specific small
interfering RNA induced cell cycle arrest and apoptosis in breast
tumour cells. We confirmed that an RNA interference strategy could
effectively reduce the protein and gene expression of EIF3H in both
OS cell lines as confirmed by western blot and RT-qPCR.
Moreover, knockdown of EIF3H markedly
inhibited the growth and colony formation, resulted in G1 arrest,
and induced apoptosis in Saos-2 and U2OS cells. The in vivo
tumorigenicity experiments showed that EIF3H knockdown
further inhibited the growth of xenograft OS tumours in
vivo. Collectively, these results suggest that EIF3H may play
an important role in OS, and that an EIF3H knockdown
approach may be a potential therapy for the treatment of OS.
Therefore, targeting EIF3H may provide a new tool for the clinical
prevention and treatment of human OS.
Although we did not determine the mechanism by which
high levels of EIF3H influence cell growth, previous studies have
shown that dysregulation of protein synthesis is implicated in
oncogenesis through influencing the mRNA levels of proteins
involved in cell proliferation, which are translated with
activation of the protein synthesis apparatus (14,15).
Therefore, changing the translational apparatus elements or
activity, particularly the initiation factors, may be an efficient
strategy to modify protein synthesis (16,17),
because the initiation phase is the rate-limiting step for the
translation of most mRNAs (18).
When EIF3H is overactivated, the translation of mRNAs related to
malignancy would be disproportionately enhanced to contribute to
malignant activity (19–21). Zhu and colleagues (12) identified that the transforming growth
factor-beta and mitogen-activated protein kinase pathways are
potentially targeted by EIF3H using microarray analysis. This
mechanism along with others are worthy of further detailed
investigation to establish a new therapeutic strategy for
cancer.
In conclusion, our study provides the first
demonstration that knockdown of EIF3H using shRNA technology
could inhibit the growth and colony formation of two OS cell lines
and further suppress the development of xenograft tumours. These
findings suggest that knocking down EIF3H expression could become a
novel therapeutic strategy for OS prevention and treatment.
Acknowledgements
The authors are thankful for the financial support
from the Brainstorm Project on Social Development by Guizhou
Province [no. QiankeheSY(2015)3046]. We would like to thank Editage
(www.editage.co.kr) for English language
editing.
Glossary
Abbreviations
Abbreviations:
|
EIF3H
|
eukaryotic translation initiation
factor 3H
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
PBS
|
phosphate-buffered saline
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
SDS
|
sodium dodecyl sulphate
|
|
shRNA
|
short hairpin RNA
|
|
OS
|
osteocarcinoma
|
|
GFP
|
green fluorescent protein
|
References
|
1
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3127. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gill J, Ahluwalia MK, Geller D and Gorlick
R: New targets and approaches in osteosarcoma. Pharmacol Ther.
137:89–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Masutani M, Sonenberg N, Yokoyama S and
Imataka H: Reconstitution reveals the functional core of mammalian
eIF3. EMBO J. 26:3373–3383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rooney PH, Murray GI, Stevenson DA, Haites
NE, Cassidy J and McLeod HL: Comparative genomic hybridization and
chromosomal instability in solid tumours. Br J Cancer. 80:862–873.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nupponen NN, Kakkola L, Koivisto P and
Visakorpi T: Genetic alterations in hormone-refractory recurrent
prostate carcinomas. Am J Pathol. 153:141–148. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mahmood SF, Gruel N, Chapeaublanc E,
Lescure A, Jones T, Reyal F, Vincent-Salomon A, Raynal V, Pierron
G, Perez F, et al: A siRNA screen identifies RAD21, EIF3H, CHRAC1
and TANC2 as driver genes within the 8q23, 8q24.3 and 17q23
amplicons in breast cancer with effects on cell growth, survival
and transformation. Carcinogenesis. 35:670–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cappuzzo F, Varella-Garcia M, Rossi E,
Gajapathy S, Valente M, Drabkin H and Gemmill R: MYC and EIF3H
Coamplification significantly improve response and survival of
non-small cell lung cancer patients (NSCLC) treated with gefitinib.
J Thorac Oncol. 4:472–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schefe JH, Lehmann KE, Buschmann IR, Unger
T and Funke-Kaiser H: Quantitative real-time RT-PCR data analysis:
Current concepts and the novel ‘gene expression's CT difference‘
formula. J Mol Med (Berl). 84:901–910. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nupponen NN, Porkka K, Kakkola L, Tanner
M, Persson K, Borg A, Isola J and Visakorpi T: Amplification and
overexpression of p40 subunit of eukaryotic translation initiation
factor 3 in breast and prostate cancer. Am J Pathol. 154:1777–1783.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daxinger L, Oey H, Apedaile A, Sutton J,
Ashe A and Whitelaw E: A forward genetic screen identifies
eukaryotic translation initiation factor 3, subunit H (eIF3h), as
an enhancer of variegation in the mouse. G3 (Bethesda).
2:1393–1396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu Q, Qiao GL, Zeng XC, Li Y, Yan JJ,
Duan R and Du ZY: Elevated expression of eukaryotic translation
initiation factor 3H is associated with proliferation, invasion and
tumorigenicity in human hepatocellular carcinoma. Oncotarget.
7:49888–49901. 2016.PubMed/NCBI
|
|
13
|
Savinainen KJ, Helenius MA, Lehtonen HJ
and Visakorpi T: Overexpression of EIFS3 promotes cancer cell
growth. Prostate. 66:1144–1150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Smit-McBride Z, Pan X, Rheinhardt
J and Hershey JW: An oncogenic role for the phosphorylated
h-subunit of human translation initiation factor eIF3. J Biol Chem.
283:24047–24060. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choudhuri A, Maitra U and Evans T:
Translation initiation factor eIF3h targets specific transcripts to
polysomes during embryogenesis. Proc Natl Acad Sci USA.
110:9818–9823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ingolia NT, Ghaemmaghami S, Newman JR and
Weissman JS: Genome-wide analysis in vivo of translation with
nucleotide resolution using ribosome profiling. Science.
324:218–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roy B, Vaughn JN, Kim BH, Zhou F,
Gilchrist MA and Von Arnim AG: The h subunit of eIF3 promotes
reinitiation competence during translation of mRNAs harboring
upstream open reading frames. RNA. 16:748–761. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Spilka R, Ernst C, Mehta AK and Haybaeck
J: Eukaryotic translation initiation factors in cancer development
and progression. Cancer Lett. 340:9–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Graff JR and Zimmer SG: Translational
control and metastatic progression: Enhanced activity of the mRNA
cap-binding protein eIF-4E selectively enhances translation of
metastasis-related mRNAs. Clin Exp Metastasis. 20:265–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zimmer SG, DeBenedetti A and Graff JR:
Translational control of malignancy: The mRNA cap-binding protein,
eIF-4E, as a central regulator of tumor formation, growth, invasion
and metastasis. Anticancer Res. 20:1343–1351. 2000.PubMed/NCBI
|
|
21
|
Kim BH, Cai X, Vaughn JN and von Arnim AG:
On the functions of the h subunit of eukaryotic initiation factor 3
in late stages of translation initiation. Genome Biol. 8:R602007.
View Article : Google Scholar : PubMed/NCBI
|