Introduction
Myocardial ischemia (MI) is one of the most severe
cardiovascular diseases, which poses a serious threat to human
health (1) and is expected to become
the most common cause of mortality worldwide by 2030 (2). At present, 7.3 million people succumb
to ischemic heart disease each year globally, which accounts for
12.8% of all mortalities due to disease (3). Acute myocardial ischemia (AMI) is
usually caused by coronary atherosclerotic heart disease (4). A number of risk factors induce the
formation of atherosclerosis in the coronary arteries, including
aging, high blood pressure, high cholesterol, diabetes, smoking,
lack of physical activity and obesity (5,6). This
leads to narrowing or obstruction of the lumen, which in turn,
causes MI, hypoxia and myocardial cell death, which is responsible
for cardiac dysfunction (4). A
number of studies have demonstrated that changes in coagulation and
fibrinolysis stimulate the formation and development of
atherosclerotic and thrombotic diseases. Coronary heart disease,
particularly acute coronary syndrome, is closely associated with an
increase in blood coagulation activity and a decrease in
fibrinolytic activity (7,8). The treatment for MI primarily includes
lifestyle changes, drug treatment and surgery. Lifestyle changes
include maintaining a healthy diet and mental health. Drugs may be
used to reduce blood lipid content, inhibit platelet aggregation
and control angina pectoris, while surgical measures include
coronary artery bypass grafting, atrioventricular valvuloplasty or
replacement and ventricular reduction (9).
Plasminogen activator inhibitor-1 (PAI-1) is a
serine protease inhibitor that inactivates tissue (t)- and
urokinase-PA, inhibits intravascular fibrinolysis, causes changes
in blood rheology and aggravates ischemic injury (10). In addition, PAI-1 is associated with
the abnormal activation of platelets (11,12) and
is a risk factor for the onset and development of ischemic heart
and brain diseases (13,14).
Previous studies performing miRNA expression
profiling have demonstrated that microRNA (miRNA) molecules serve
important roles in the pathology of MI (15,16) and
miRNAs regulate the expression of various genes by direct targeting
mRNA (17,18). miRNAs that exist in the blood are
also reliable biological markers associated with a number of
diseases, including MI (19,20). It has been reported that miR-30b
regulates the proliferation and apoptosis of gastric cancer cells
by targeting PAI-1 (21). However,
to the best of our knowledge, it remains unknown whether miR-30b
regulates PAI-1 in MI. Therefore, in the present study, the
expression of miR-30b and PAI-1 in the blood of patients with AMI
and in the blood and myocardial tissue of mice with AMI was
determined. The current study also aimed to understand the
mechanism by which AMI occurs.
Materials and methods
Patients
A total of 36 patients aged 65.6±11.8 years old
(range, 46–82 years) with AMI receiving treatment at the Luoyang
Central Hospital Affiliated to Zhengzhou University (Luoyang,
China) between August 2012 and January 2017 were included in the
present study. Out of the 36 patients, there were 26 males and 10
females. A total of 21 patients received immediate treatment by
percutaneous coronary intervention (PCI) and 15 patients received
selective PCI at another time. A control group consisting of 28
healthy subjects (16 males and 12 females) aged 61.15±8.6 years old
(range, 50–70 years old) were included in the current study.
Peripheral blood was collected from patients with AMI within 6 h of
MI onset and healthy subjects on the day of physical examination.
Serum was isolated from peripheral blood by centrifugation at 4°C
at 1,000 × g for 10 min. The present study was approved by the
Ethics Committee of Zhengzhou University (Henan, China) and written
informed consent was obtained from all participants or their
families.
Animals
A total of 60 male BALB/C mice (4 weeks old;
weighing 18–22 g) were purchased from Chongqing Tengxin
Biotechnology Co., Ltd. (http://www.cqtx123.com/; Chongqing, China) with a
numbered certificate [SCXK(Yu) 2015–0012]. For 1 week prior to the
experiments, mice had ad libitum access to food and water.
The animals were maintained at 24±2°C and 55±5% humidity in cages
with a 12 h light/dark cycle. The Reduction, Replacement and
Refinement animal welfare principle (22) was followed during the experiments.
All mice were evenly divided into two groups (each, n=30): A
control group and an AMI model group.
Following 1 week adaptive feeding, all mice received
intraperitoneal injection of urethane (1,300 mg/kg) to induce
anesthesia. Mice were kept in a supine position and needle
electrodes were inserted into the subcutaneous layers of the limbs.
An animal twelve-lead electrocardiograph (ECG-1350P; Nihon Kohden,
Tokyo, Japan) was used to record a lead II electrocardiogram of
normal mice (10 mm on the chart represented standard voltage 1 mV;
chart speed, 50 ram/s). Mice in the AMI group were
intraperitoneally injected with pituitrin (20 U/kg; Shanghai
Pharma, Shanghai, China) to construct AMI mouse model. Mice in the
control group were intraperitoneally injected with an equal volume
of saline. After 30 min, the lead II electrocardiogram of mice in
the control and AMI groups was recorded again. Changes in J point
voltage on the electrocardiogram prior to and following ischemia
were observed and the J point shift (mV) of each group was recorded
using the PR segment as a baseline. A total of 30 min following
construction of AMI mouse model, blood was collected from the eyes
of mice under anesthetic in the control and AMI groups. The blood
was then centrifuged at 1,200 × g for 15 min at 4°C to obtain
serum. Subsequently, mice were sacrificed by decapitation,
myocardial tissues were collected and stored in liquid nitrogen.
All animal experiments were conducted according to the ethical
guidelines of Zhengzhou University (Henan, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Myocardial tissues (100 mg) were ground using liquid
nitrogen and mixed with 1 ml TRIzol (10606ES60; Shanghai Yeasen
Biotechnology, Co., Shanghai, China) for lysis. Serum samples (100
µl) were directly mixed with 1 ml TRIzol for lysis. Total RNA was
then extracted using the phenol chloroform method as previously
described (23). The concentration
and quality of RNA was assessed using ultraviolet spectrophotometry
(Nanodrop ND2000, Thermo Scientific, Inc., Wilmington, DE, USA).
Reverse transcription of mRNA was performed using TIANScript II
cDNA First Strand Synthesis kit (Tiangen Biotech Co., Ltd.,
Beijing, China) and reverse transcription of miRNA was performed
using an miRcute miRNA cDNA First Strand Synthesis kit (Tiangen
Biotech, Co., Ltd.). cDNA was stored at −20°C. A SuperReal PreMix
(SYBR Green) RT-qPCR kit (Tiangen Biotech, Co., Ltd.) was used to
detect the expression of PAI-1 mRNA. The sequences of the primers
used to detect human PAI-1 were: PAI-1, forward,
5′-AATGACTGGGTGAAGACACACACA-3′ and reverse,
5′-TTCCACTGGCCGTTGAAGTAGA-3′; β-actin, forward,
5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. The sequences of the primers used
to detect mouse PAI-1 were as follows: PAI-1, forward,
5′-AGGGCTTCATGCCCCACTTCTTCA-3′ and reverse,
5′-AGTAGAGGGCATTCACCAGCACCA-3′; GAPDH, forward,
5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse,
5′-GTCCACCACCCTGTTGCTGTAG-3′. The qPCR reaction system (20 µl) to
detect PAI-1 consisted of 10 µl RT-qPCR-Mix, 0.5 µl upstream
primer, 0.5 µl downstream primer, 2 µl cDNA and 7 µl
ddH2O. The qPCR conditions were as follows: Initial
denaturation at 95°C for 2 min; 40 cycles of denaturation at 95°C
for 25 sec, annealing at 55°C for 30 sec and elongation at 72°C for
30 sec; final extension at 72°C for 30 sec. qPCR was performed
using an iQ5 Real-Time PCR system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The 2−ΔΔCq method (24) was used to calculate the relative
expression of human PAI-1 mRNA against β-actin and the relative
expression of mouse PAI-1 mRNA against GAPDH. Each sample was
tested in triplicate.
The expression of miR-30b was determined using the
miRcute miRNA RT-PCR kit (Tiangen Biotech, Co., Ltd), using U6 as
internal reference. The sequences of the primers used to detect
human miR-30b were: Forward, 5′-CGCGCTGTAAACATCCTACAC-3′ and
reverse, 5′-GTGCAGGGTCCGAGGT-3′; U6, forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The PCR reaction system (20 µl) for
human miR-30b determination was composed of 10 µl RT-qPCR-Mix, 0.5
µl upstream primer, 0.5 µl downstream primer, 2 µl cDNA and 7 µl
ddH2O. PCR conditions for human miR-30b determination
were: Initial denaturation at 95°C for 5 min; 40 cycles of
denaturation 95°C for 10 sec, annealing at 60°C for 20 sec and
elongation at 72°C for 10 sec; final extension at 72°C for 5 min.
qPCR was performed using the iQ5 Real-Time PCR system.
The sequences used to detect mouse miR-30b were,
forward, 5′-GCGCCTGTAAACATCCTACAC-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′; U6, forward, 5′-GCTTCGGCAGCACATATACTAA-3′
and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. The PCR reaction system
(25 µl) for mouse miR-30b determination was composed of 12.5 µl
SYBR Premix EXTaq™ (Takara Biotechnology Co., Ltd., Dalian, China),
0.5 µl upstream primer, 0.5 µl downstream primer, 1 µl cDNA and
10.5 µl ddH2O. qPCR conditions for mouse miR-30b
determination were: Initial denaturation at 95°C for 30 sec; 45
cycles of denaturation at 95°C denaturation for 5 sec and annealing
at 60°C for 30 sec; elongation at 72°C for 45 sec; final extension
at 72°C for 5 min. qPCR was performed using an iQ5 Real-Time PCR
system. The 2−ΔΔCq method was used to calculate the
relative expression of miR-30b against U6. Each sample was tested
in triplicate.
Enzyme-linked immunosorbent assay
(ELISA)
Sera were examined using human and mouse PAI-1 ELISA
kits [cat. nos. ab108891 (human) and ab197752 (mouse); Abcam,
Cambridge, UK] according to the manufacturer's protocol. In
microplates, standards (50 µl), samples (10 µl sample liquid and 40
µl diluent) and blank were set into predefined wells. In the wells
for standards and samples, horseradish peroxidase-labelled
conjugates (100 µl) were added before sealing the plates for
incubation at 37°C for 1 h. The plates were washed 5 times and then
substrates A (50 µl) and B (50 µl) were added to each well.
Following incubation at 37°C for 15 min, stop solution (50 µl) was
added to each well and the absorbance of each well was measured at
450 nm.
Western blotting
Precooled radioimmunoprecipitation assay lysis
buffer (600 µl; 50 mM Tris-base, 1 mM EDTA, 150 mM NaCl, 0.1%
sodium dodecyl sulfate, 1% Triton X-100, 1% sodium deoxycholate;
Beyotime Institute of Biotechnology, Haimen, China) was used to
lyse the samples. Following lysis for 50 min on ice, the mixture
was centrifuged at 18,000 × g and 4°C for 5 min. Protein
concentration of the supernatant was determined using a
bicinchoninic acid protein concentration determination kit
[RTP7102; Real-Times (Beijing) Biotechnology Co., Ltd., Beijing,
China]. Protein samples (20 µg) were then mixed with SDS loading
buffer prior to denaturation in a boiling water bath for 5 min. The
samples were then subjected to 10% SDS-PAGE. Resolved proteins were
transferred to polyvinylidene difluoride membranes on ice (100 V, 2
h) and blocked with 5% skimmed milk at room temperature for 1 h.
Subsequently, membranes were incubated with rabbit anti-mouse PAI-1
polyclonal (1:1,000; cat. no. ab7205; Abcam) and rabbit anti-mouse
β-actin (1:5,000; cat. no. ab8227; Abcam) primary antibodies at 4°C
overnight. Following three washes with phosphate-buffered saline
with Tween 20 (15 min/wash), membranes were incubated with goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(1:3,000; cat. no. ab6721; Abcam) for 1 h at room temperature prior
to three washes with phosphate-buffered saline with Tween 20 (15
min/wash). The membrane was developed using an enhanced
chemiluminescence detection kit (Abcam). Image lab v3.0 software
(Bio-Rad Laboratories, Inc.) was used to acquire and analyze
imaging signals. The relative content of PAI-1 protein was
expressed as the ratio of PAI-1/β-actin.
Bioinformatics
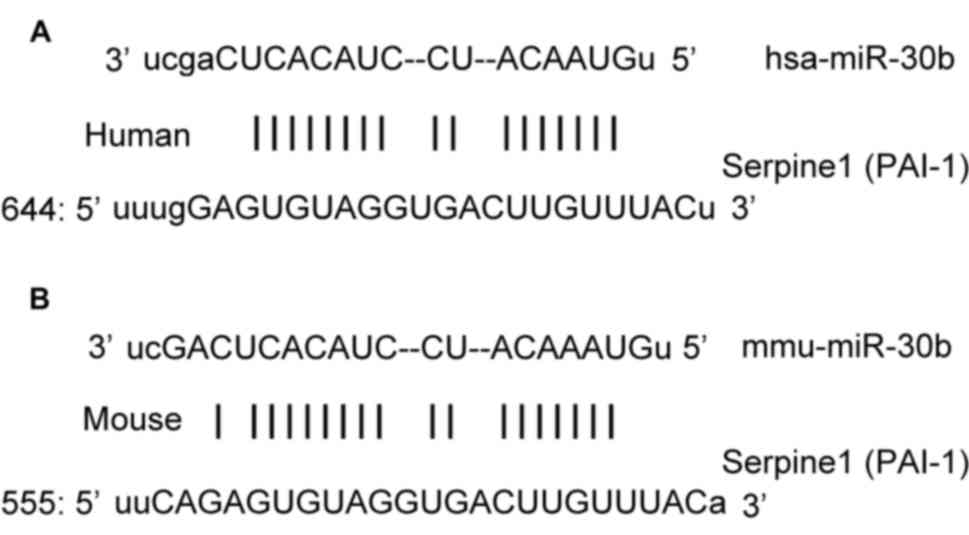
Bioinformatics prediction is a powerful tool to
study the function of miRNAs. To determine the regulatory mechanism
of PAI-1, miRanda (http://www.microrna.org/microrna/home.do), TargetScan
(http://www.targetscan.org), PiTa
(http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/)
and PICTA (http://pictar.mdc-berlin.de/) were used to predict the
miRNA molecules that may regulate PAI-1. The results indicated that
miR-30b was potentially able to regulate PAI-1 (Fig. 1).
Automatic biochemical analysis
The activities of catalase (CAT; cat. no. A007-1-1),
glutathione peroxidase (GSH-Px; cat. no. A005) and superoxide
dismutase (SOD; cat. no. A001-2-1) were determined using the
appropriate kits (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) following the manufacturer's protocol. Following
the addition of all reagents, absorbance was measured continuously
over a 5 min period. The activities of CAT, GSH-Px and SOD were
determined by the increase rate of average absorbance per minute in
the linear segment. The results were automatically calculated using
an AU5800 automatic biochemical analyzer (Beckman Coulter, Inc.,
Brea, CA, USA).
Dual luciferase reporter assay
The results of bioinformatics were used to
chemically synthesize the wild-type (WT) and mutant seed regions of
miR-30b in the 3′-untranslated region (UTR) of PAI-1 gene in
vitro. SpeI and HindIII restriction sites were added and
UTRs were then cloned into pMIR-REPORT luciferase reporter plasmids
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Plasmids (0.8
µg) containing WT or mutant 3′-UTR DNA sequences were
co-transfected with agomiR-30b (100 nM; Sangon Biotech, Co., Ltd.,
Shanghai, China) into 293T cells using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Following 24 h
cultivation, activity was assessed using a dual luciferase reporter
assay kit (Promega Corporation, Madison, WI, USA), following the
manufacturer's protocol and fluorescence intensity was measured
using a GloMax 20/20 luminometer (Promega Corporation). Using
Renilla luciferase fluorescence activity as an internal
reference, the fluorescence values of each group of cells were
measured.
Statistical analysis
The results were analyzed using SPSS 18.0
statistical software (SPSS, Inc., Chicago, IL, USA). All data are
expressed as the mean ± standard deviations. Data were tested for
normality. Multigroup measurement data were analyzed using one-way
analysis of variance. In cases of homogeneity of variance, the
Least Significant Difference and Student-Newman-Keuls methods were
used as a post-hoc test; in cases of heterogeneity of variance,
Tamhane's T2 or Dunnett's T3 method was used. P<0.05 was
determined to indicate a statistically significant difference.
Results
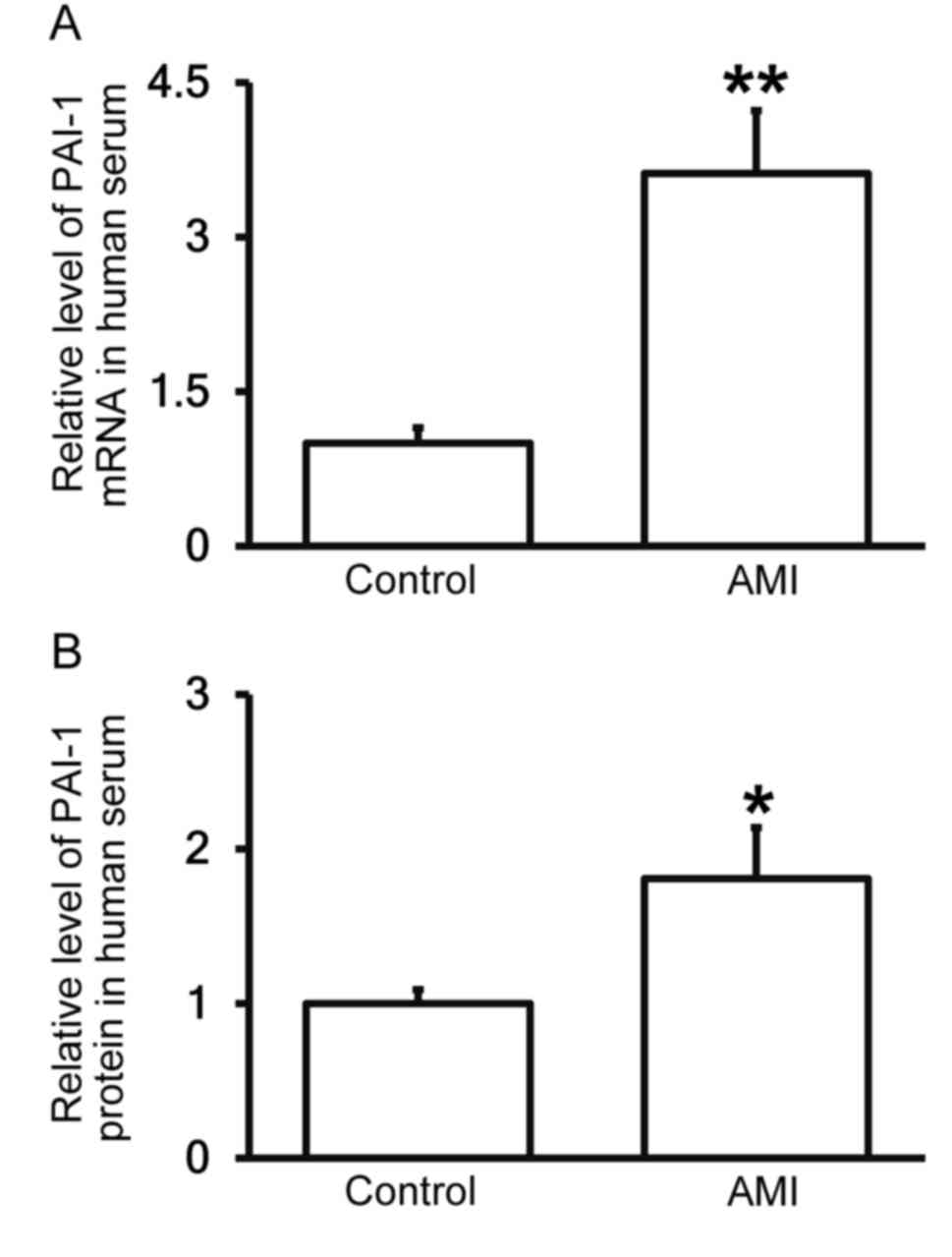
Patients with AMI exhibit elevated
PAI-1 expression in the peripheral blood
To measure the expression of PAI-1 mRNA and protein
in the serum of patients with AMI compared with healthy controls,
RT-qPCR and ELISA were performed. The results demonstrated that
levels of PAI-1 mRNA and protein in the serum of patients with AMI
were significantly higher than in healthy controls (P<0.01 and
P<0.05; Fig. 2A and B,
respectively), confirming that patients with AMI exhibit elevated
PAI-1 expression in the peripheral blood.
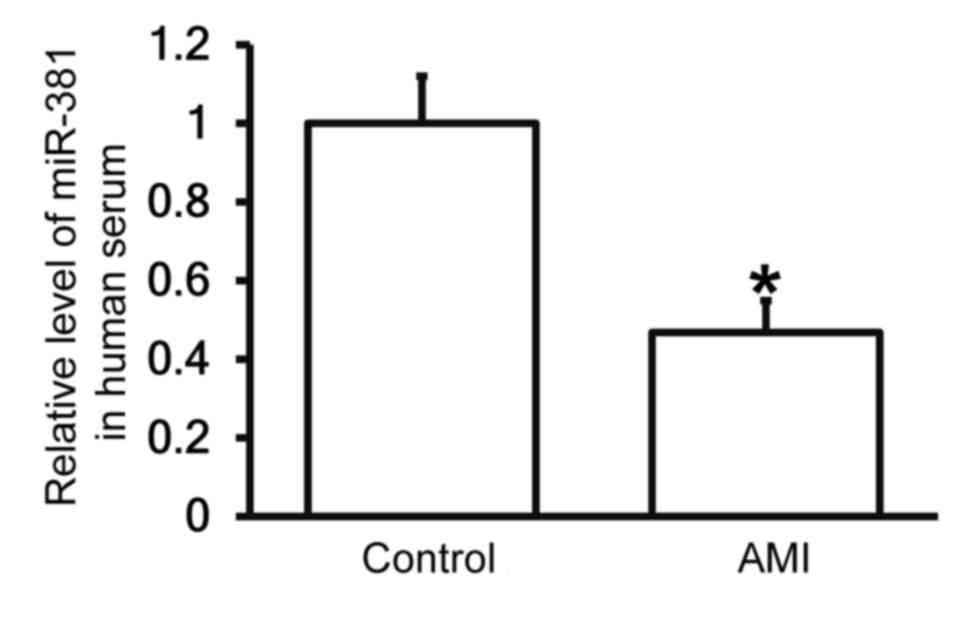
Patients with AMI exhibit reduced
miR-30b levels in the peripheral blood
To measure the expression of miR-30b in the
peripheral blood of patients and healthy controls, RT-qPCR was
performed. The results demonstrated that the expression of miR-30b
in the serum of patients with AMI was significantly decreased
compared with the control group (P<0.05; Fig. 3), indicating that patients AMI
exhibit decreased miR-30b expression.
The J point voltage in
electrocardiogram is enhanced and the activities of SOD, CAT and
GSH-Px are decreased in mice with AMI
To determine changes in the associated physiological
and biochemical indexes in the mouse model of AMI,
electrocardiography and an automatic biochemical analyzer were
used. The results indicated that the J point voltage in mice with
AMI was significantly higher than mice in the negative control
group (P<0.05; Table I). In
addition, the activity of SOD, CAT and GSH-Px in the peripheral
blood of mice with AMI was significantly increased compared with
mice in the negative control group (all P<0.05). These results
indicate that AMI may enhance the J point voltage and decrease SOD,
CAT and GSH-Px activity.
 | Table I.Changes in levels of physiological
and biochemical indexes in the mouse model of AMI. |
Table I.
Changes in levels of physiological
and biochemical indexes in the mouse model of AMI.
| Groups | N | J point voltage
(mV) | SOD (mkat/ml) | CAT (mkat/ml) | GSH-Px
(mkat/ml) |
|---|
| Negative
control | 30 |
0.016±0.03 |
3.96±0.53 |
6.28±0.96 |
0.31±0.85 |
| AMI model | 30 |
0.25±0.15a |
2.16±0.56a |
3.18±0.63a |
0.15±0.61a |
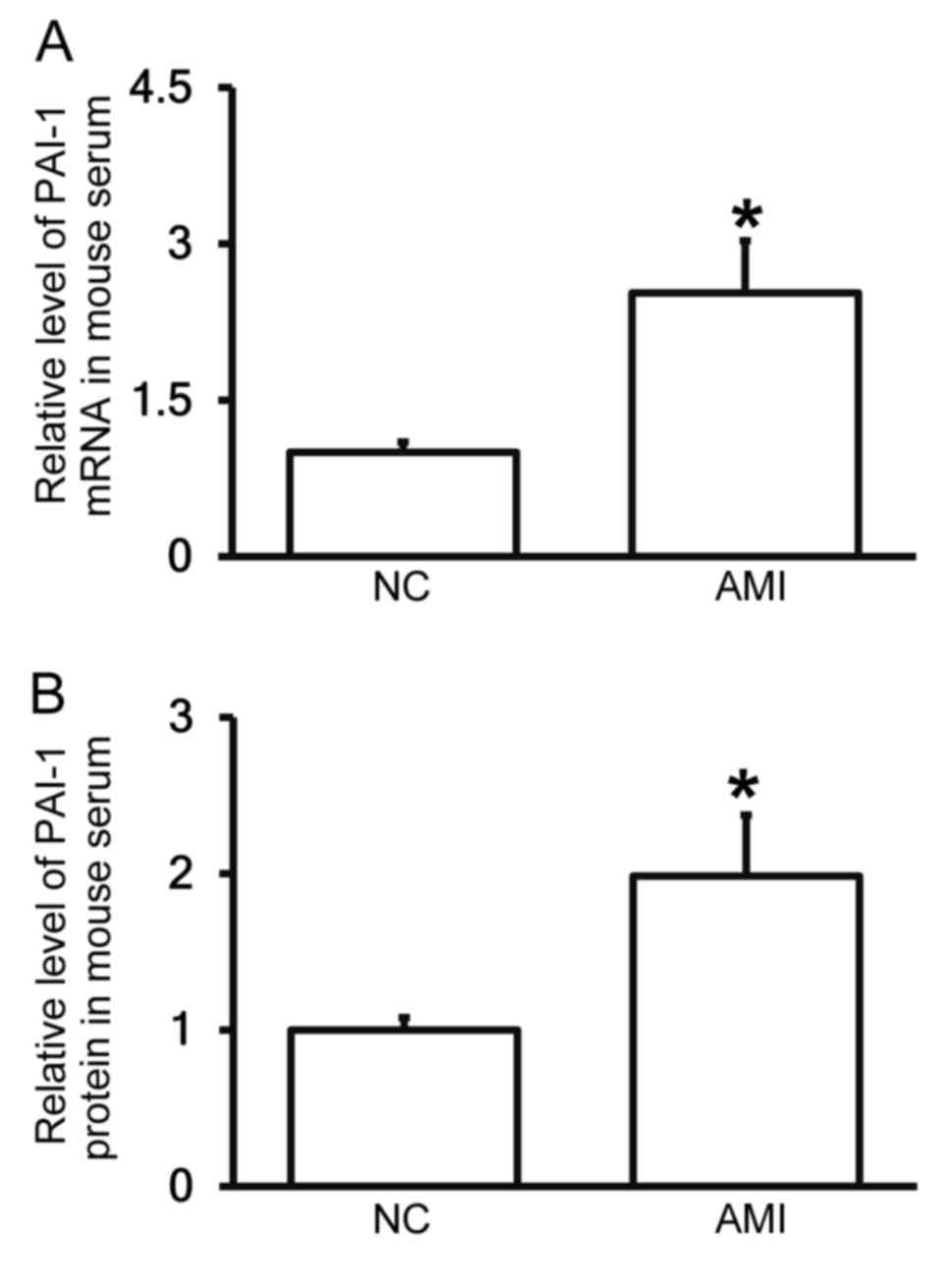
AMI model mice exhibit increased PAI-1
levels in the peripheral blood
To measure the expression of PAI-1 mRNA and protein
in the serum of mice, RT-qPCR and ELISA were performed. The results
demonstrated that levels of PAI-1 mRNA and protein in the serum of
mice with AMI were significantly higher than in the negative
control group (each P<0.05; Fig. 4A
and B). These results suggest that PAI-1 levels are increased
in the blood following AMI and are consistent with the results from
patients with AMI.
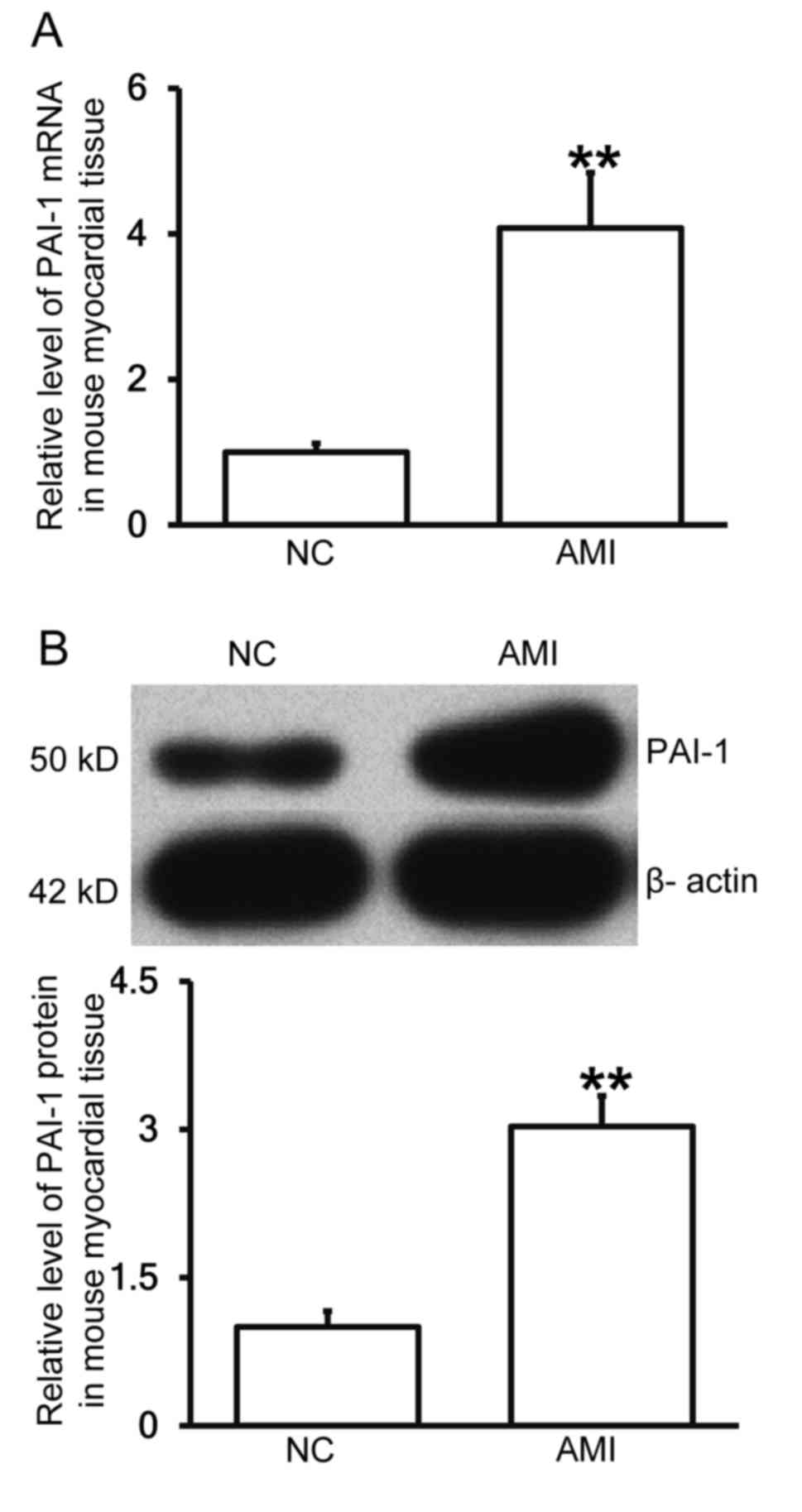
Mice with AMI exhibit increased PAI-1
expression in myocardial tissue
To determine the levels of PAI-1 mRNA and protein in
myocardial tissue, RT-qPCR and western blotting were performed. The
results demonstrated that levels of PAI-1 mRNA and protein in the
myocardial tissues of mice with AMI were significantly higher than
those in the negative control group (each P<0.01; Fig. 5A and B), indicating that mice with
AMI exhibit increased PAI-1 expression.
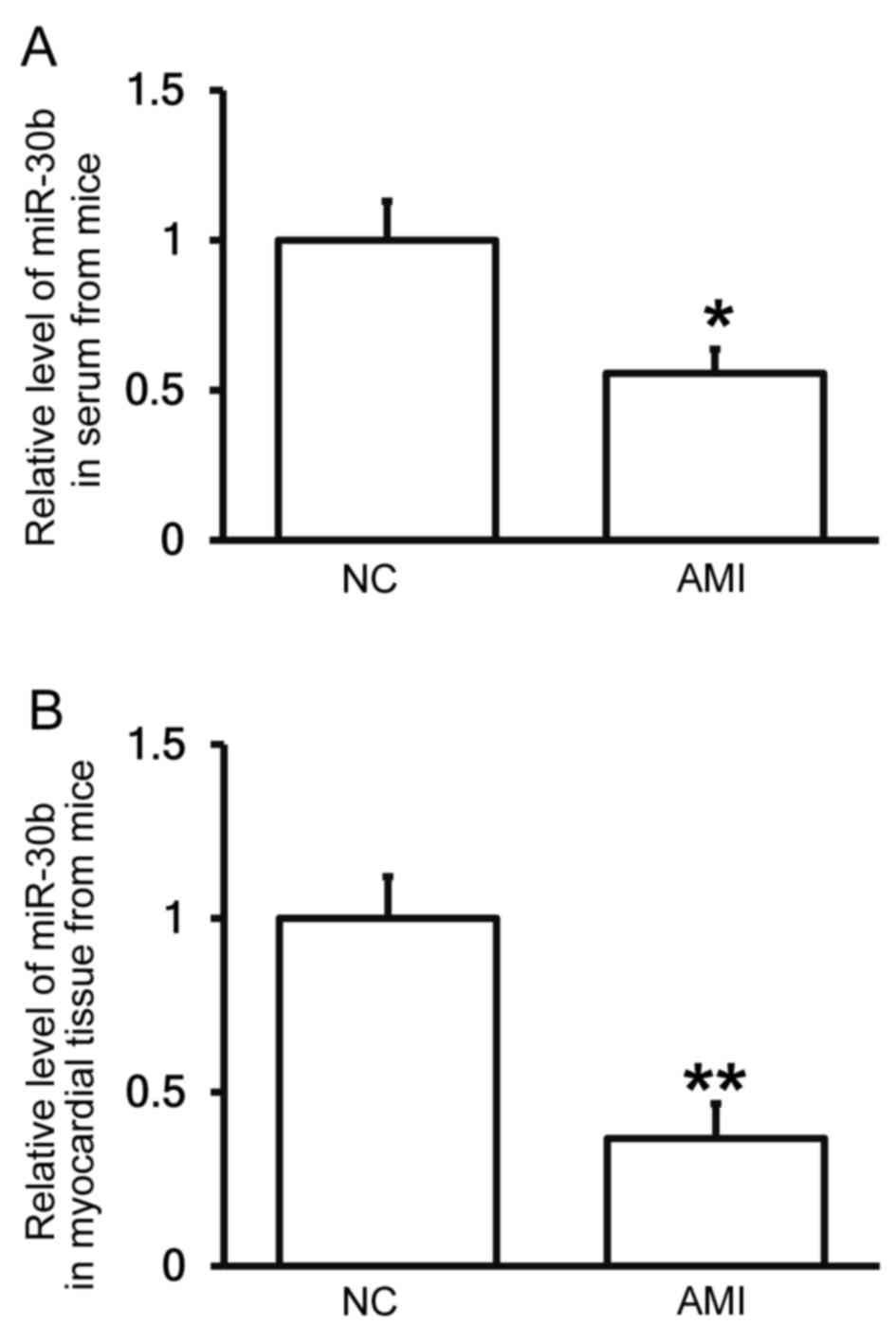
Mice with AMI exhibit decreased
miR-30b levels in the peripheral blood and myocardial tissues
To determine the expression of miR-30b in the
peripheral blood and myocardial tissue of mice, RT-qPCR was
conducted. The results demonstrated that the expression of miR-30b
in the serum and myocardial tissues of mice with AMI was
significantly reduced compared with the negative control group
(P<0.05 and P<0.01; Fig. 6A and
B, respectively), indicating that mice with AMI exhibit
decreased miR-30b levels.
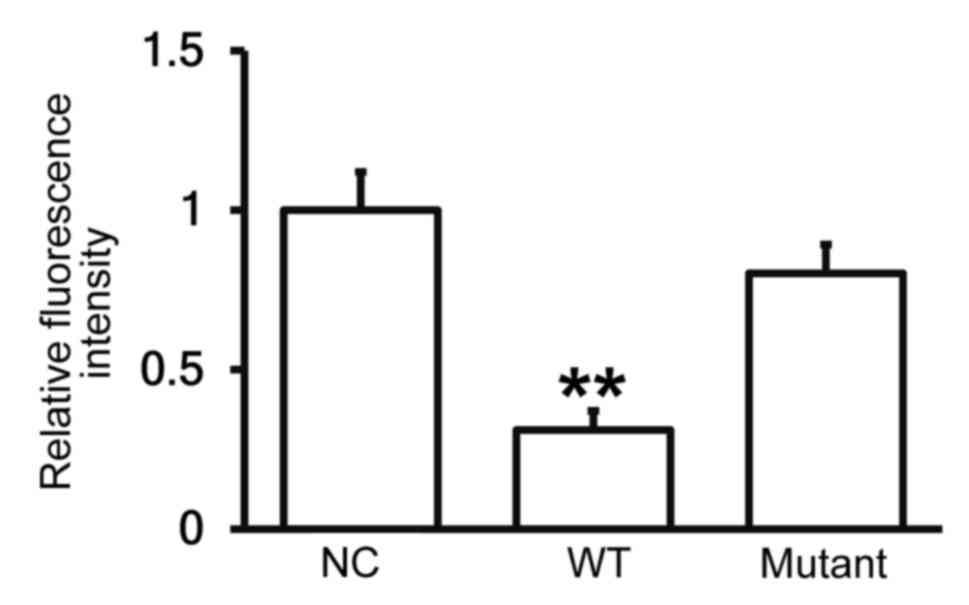
miR-30b is able to bind to the 3′-UTR
seeding region of PAI-1 mRNA to regulate its expression
To identify the interaction between miR-30b and the
3′-UTR of human PAI-1 mRNA, a dual luciferase reporter assay was
performed. The fluorescence value of cells co-transfected with
agomiR-30b and pMIR-REPORT-WT luciferase reporter plasmids was
significantly lower than that of the negative control group
(P<0.01; Fig. 7). By contrast,
the fluorescence value of cells co-transfected with agomiR-30b and
pMIR-REPORT-mutant luciferase reporter plasmid did not differ
significantly to that of the negative control group (Fig. 7). These results indicate that miR-30b
is able to bind to the 3′-UTR seeding region of PAI-1 mRNA, thus
regulating its expression.
Discussion
MI is a pathological condition in which blood
perfusion of the heart is decreased, the oxygen supply to the heart
is reduced and myocardium metabolism is deregulated (25). Due to the increased availability of
high-energy, processed food and the adoption of more sedentary
lifestyles (5), the prevalence of MI
in China is increasing (26). MI is
usually caused by vasculopathy and an insufficient blood supply to
the heart (27,28). Vasculopathy is associated with
abnormal changes in blood coagulation and fibrinolysis and is a
primary cause of thrombosis (29,30). tPA
and PAI-1 are important active substances in the fibrinolytic
system and PAI-1 inhibits the action of tPA. It has been reported
that PAI-1 and tPA are closely associated with ischemic
cardiovascular disease (31,32). PAI-1 is also closely associated with
vascular disease and participates in the accumulation of
extracellular matrix, as well as the proliferation and migration of
smooth muscle cells. It also stimulates the binding of low-density
lipoprotein to vascular smooth muscle cells (33,34).
PAI-1 deposits in the extracellular matrix facilitate the formation
of atherosclerotic plaques, thicken the vascular basement membrane
and stiffen the vascular walls, thus promoting the onset and
development of vasculopathy and therefore atherosclerosis (35–37).
PAI-1 is considered to be closely associated with vascular disease
(38); therefore, it is important to
determine the regulatory mechanism of action of PAI-1.
The present study identified the relevant factors in
AMI in the peripheral blood. Subsequently, an AMI mouse model was
constructed using pituitrin. The animal model of MI induced by
pituitrin has been widely used in the screening of anti-MI drugs
(39,40) and the progression of AMI and the
pathological changes that occur in this particular mouse model are
very similar to what occurs in patients with AMI. The results of
the current study demonstrated that the expression of PAI-1 in the
serum of patients with AMI is upregulated, indicating that abnormal
changes in coagulation and fibrinolysis occur in such patients. The
results of the electrocardiogram confirmed the successful
construction of the AMI mouse model.
Maintaining the balance of free radicals in the
blood is dependent on the activity of the free radical-scavenging
enzymes SOD, CAT and GSH-Px (41–43).
When tissues and cells are ischemic and anoxic, the function of the
scavenging system is impaired, the activities of these enzymes
decrease and they react with the proteins and nucleic acids in the
cells. This causes a cascade of changes in cell structure and
function that eventually damages myocardial cells (41–43). In
the current study, upregulation of PAI-1 expression in the blood
and myocardium of mice was also observed, further indicating that
PAI-1 serves an important role in AMI.
miRNAs are posttranscriptional regulators. It has
been reported that miRNAs participate in various cardiac
physiological and pathological processes, including cardiac
development, cardiac hypertrophy, heart failure and vascular
proliferation (44). Wang et
al (45) demonstrated that
miR-142-3p protects against damage in the cardiomyocytes induced by
hypoxia/reoxygenation by targeting the high mobility group box 1
gene. Singh et al (46)
reported that miR-200c directly regulates the expression of dual
specificity protein phosphatase 1, downregulates the activity of
the mitogen-activated protein kinase signaling pathway and promotes
cardiomyocyte hypertrophy. Based on the results of a study
investigating the regulation of PAI-1 by miRNA (47), the present study used bioinformatics
to identify the miRNA that regulates PAI-1 expression; the results
indicated that miR-30b regulates PAI-1. Previous studies have
demonstrated that miR-30b affects the proliferation and apoptosis
of coronary artery endothelial cells via integrin subunit α 4,
phospholipase γ 1 (48), caspase 3
(49) and Bcl-2 (50). Furthermore, myocardial miR-30b serves
anti-apoptotic and protective roles during the pathological process
of heart ischemia reperfusion (51,52).
These studies suggest that miR-30b may be closely associated with
the onset and development of heart disease in humans. The results
of the present study indicate that the expression of miR-30b in the
blood of patients with AMI is significantly decreased. Furthermore,
the expression of PAI-1 is abnormally high in the blood of patients
with AMI. This suggests that the downregulation of miR-30b may
stimulate the upregulation of PAI-1. The current study obtained
similar results regarding miR-30b and PAI-1 expression in the blood
and myocardial tissues of mice with AMI, indicating that miR-30b
negatively regulates PAI-1 in other species as well as humans.
Furthermore, the results of the dual luciferase reporter assay
indicated that miR-30b regulates the expression of PAI-1 by
directly targeting the 3′-UTR of PAI-1 mRNA.
In conclusion, the results of the present study
demonstrate that the expression of miR-30b is reduced and the
expression of PAI-1 is increased following AMI and that these
changes alter the expression of t-PA and u-PA proteins. Therefore,
miR-30b serves an important role in AMI and may be developed as a
potential biomarker in the diagnosis and treatment of AMI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Author contributions
BL conceived and designed the study, performed
experiments and collected and analyzed the data. JH collected and
analyzed data. XC conceived and designed the study, drafted and
revised the manuscript and gave final approval of version to be
published. The final version of the manuscript has been read and
approved by all authors.
Ethical approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Zhengzhou University. Written
informed consent was obtained from all patients or their
families.
Consent for publication
Written informed consent was obtained from all
participants or their families for the publication of their
data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nabel EG and Braunwald E: A tale of
coronary artery disease and myocardial infarction. N Engl J Med.
366:54–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mathers CD and Loncar D: Projections of
global mortality and burden of disease from 2002 to 2030. PLoS Med.
3:e4422006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ and
Chen Y: Protective role of melatonin in cardiac
ischemia-reperfusion injury: From pathogenesis to targeted therapy.
J Pineal Res. 64:e124712018. View Article : Google Scholar
|
|
4
|
Fuke Y, Yasutsune T and Sakamoto M: Aortic
valve replacement after coronary artery bypass grafting with the in
situ right gastroepiploic artery to the occluded right coronary
artery using a temporary vein graft for cardioplegia. Surg Case
Rep. 3:562017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Orth-Gomér K, Deter HC, Grün AS,
Herrmann-Lingen C, Albus C, Bosbach A, Ladwig KH, Ronel J, Söllner
W, de Zwaan M, et al: Socioeconomic factors in coronary artery
disease-results from the SPIRR-CAD study. J Psychosom Res.
105:125–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kundi H, Kiziltunc E, Korkmaz A, Cicek G,
Ornek E and Ileri M: A novel risk scoring system to predict
cardiovascular death in patients with acute myocardial infarction:
CHA2DS2-VASc-CF score. Clin Appl Thromb Hemost. 24:273–278. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folsom AR, Aleksic N, Park E, Salomaa V,
Juneja H and Wu KK: Prospective study of fibrinolytic factors and
incident coronary heart disease: The atherosclerosis risk in
communities (ARIC) study. Arterioscler Thromb Vasc Biol.
21:611–617. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenfeld ME: An overview of the evolution
of the atherosclerotic plaque: From fatty streak to plaque rupture
and thrombosis. Z Kardiol. 89 Suppl 7:S2–S6. 2000. View Article : Google Scholar
|
|
9
|
Moran AE, Forouzanfar MH, Roth GA, Mensah
GA, Ezzati M, Murray CJ and Naghavi M: Temporal trends in ischemic
heart disease mortality in 21 world regions, 1980 to 2010: The
global burden of disease 2010 study. Circulation. 129:1483–1492.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hua Y, Xi G, Keep RF, Wu J, Jiang Y and
Hoff JT: Plasminogen activator inhibitor-1 induction after
experimental intracerebral hemorrhage. J Cereb Blood Flow Metab.
22:55–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pieters M, Barnard SA, Loots DT and Rijken
DC: The effects of residual platelets in plasma on plasminogen
activator inhibitor-1 and plasminogen activator inhibitor-1-related
assays. PloS One. 12:e01712712017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng ZY, Shan WG, Wang SF, Hu MM and Chen
Y: Effects of astaxanthin on blood coagulation, fibrinolysis and
platelet aggregation in hyperlipidemic rats. Pharm Biol.
55:663–672. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eitzman DT, Westrick RJ, Xu Z, Tyson J and
Ginsburg D: Plasminogen activator inhibitor-1 deficiency protects
against atherosclerosis progression in the mouse carotid artery.
Blood. 96:4212–4215. 2000.PubMed/NCBI
|
|
14
|
Kohler HP and Grant PJ:
Plasminogen-activator inhibitor type 1 and coronary artery disease.
N Engl J Med. 342:1792–1801. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang TY, Tsai WC, Huang TS, Su SH, Chang
CY, Ma HY, Wu CH, Yang CY, Lin CH, Huang PH, et al: Dysregulation
of endothelial colony-forming cell function by a negative feedback
loop of circulating miR-146a and −146b in cardiovascular disease
patients. PLoS One. 12:e01815622017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Satoh M, Nasu T, Takahashi Y, Osaki T,
Hitomi S, Morino Y and Nakamura M: Expression of miR-23a induces
telomere shortening and is associated with poor clinical outcomes
in patients with coronary artery disease. Clin Sci (Lond).
131:2007–2017. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inoue K: MicroRNA function in animal
development. Tanpakushitsu Kakusan Koso. 52:197–204. 2007.(In
Japanese). PubMed/NCBI
|
|
18
|
Looney AM, Ahearne CE, Hallberg B, Boylan
GB and Murray DM: Downstream mRNA target analysis in neonatal
hypoxic-ischaemic encephalopathy identifies novel marker of severe
injury: A proof of concept paper. Mol Neurobiol. 54:8420–8428.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li S, Lee C, Song J, Lu C, Liu J, Cui Y,
Liang H, Cao C, Zhang F and Chen H: Circulating microRNAs as
potential biomarkers for coronary plaque rupture. Oncotarget.
8:48145–48156. 2017.PubMed/NCBI
|
|
20
|
Wang Q, Ma J, Jiang Z, Wu F, Ping J and
Ming L: Identification of microRNAs as diagnostic biomarkers for
acute myocardial infarction in Asian populations: A systematic
review and meta-analysis. Medicine (Baltimore). 96:e71732017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu ED, Li N, Li BS, Li W, Zhang WJ, Mao
XH, Guo G, Zou QM and Xiao B: miR-30b, down-regulated in gastric
cancer, promotes apoptosis and suppresses tumor growth by targeting
plasminogen activator inhibitor-1. PloS One. 9:e1060492014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clark MacArthur J: The 3Rs in research: A
contemporary approach to replacement, reduction and refinement. Br
J Nutr. 1–7. 2017. View Article : Google Scholar
|
|
23
|
Nwokeoji AO, Kilby PM, Portwood DE and
Dickman MJ: RNASwift: A rapid, versatile RNA extraction method free
from phenol and chloroform. Anal Biochem. 512:36–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang P, Wu X, Li G, He Q, Dai H, Ai C and
Shi J: Tumor necrosis factor-alpha gene polymorphisms and
susceptibility to ischemic heart disease: A systematic review and
meta-analysis. Medicine (Baltimore). 96:e65692017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao FY, Liu M, Chen BL, Cao S, Fan L, Liu
ZQ, Zhou HH, Zhang W and Zhou G: Effects of four novel genetic
polymorphisms on clopidogrel efficacy in Chinese acute coronary
syndromes patients. Gene. 623:63–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chistiakov DA, Grechko AV, Myasoedova VA,
Melnichenko AA and Orekhov AN: Impact of the cardiovascular
system-associated adipose tissue on atherosclerotic pathology.
Atherosclerosis. 263:361–368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Al Badarin F, Aljizeeri A, Almasoudi F and
Al-Mallah MH: Assessment of myocardial blood flow and coronary flow
reserve with positron emission tomography in ischemic heart
disease: Current state and future directions. Heart Fail Rev.
22:441–453. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanabe N, Hiraoka E, Hoshino M, Deshpande
GA, Sawada K, Norisue Y, Tsukuda J and Suzuki T: Progressive
ischemic stroke due to thyroid storm-associated cerebral venous
thrombosis. Am J Case Rep. 18:194–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moreno JA, Gálvez MM, Cornudella R, Angós
JA, Romero MS and Gutiérrez M: Fibrinolytic system in patients with
myocardial infarction and other coronary disease risk factors.
Sangre (Barc). 39:111–116. 1994.(In Spanish). PubMed/NCBI
|
|
31
|
Iida K, Tani S, Atsumi W, Yagi T, Kawauchi
K, Matsumoto N and Hirayama A: Association of plasminogen activator
inhibitor-1 and low-density lipoprotein heterogeneity as a risk
factor of atherosclerotic cardiovascular disease with triglyceride
metabolic disorder: A pilot cross-sectional study. Coron Artery
Dis. 28:577–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shimizu T, Uematsu M, Yoshizaki T, Obata
JE, Nakamura T, Fujioka D, Watanabe K, Watanabe Y and Kugiyama K:
Myocardial production of plasminogen activator inhibitor-1 is
associated with coronary endothelial and ventricular dysfunction
after acute myocardial infarction. J Atheroscler Thromb.
23:557–566. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Denorme F, Wyseure T, Peeters M,
Vandeputte N, Gils A, Deckmyn H, Vanhoorelbeke K, Declerck PJ and
De Meyer SF: Inhibition of thrombin-activatable fibrinolysis
inhibitor and plasminogen activator inhibitor-1 reduces ischemic
brain damage in mice. Stroke. 47:2419–2422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Seferovic MD and Gupta MB: Increased
umbilical cord PAI-1 levels in placental insufficiency are
associated with fetal hypoxia and angiogenesis. Dis Markers.
2016:71241862016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ji Y, Meng QH and Wang ZG: Changes in the
coagulation and fibrinolytic system of patients with subarachnoid
hemorrhage. Neurol Med Chir (Tokyo). 54:457–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thögersen AM, Jansson JH, Boman K, Nilsson
TK, Weinehall L, Huhtasaari F and Hallmans G: High plasminogen
activator inhibitor and tissue plasminogen activator levels in
plasma precede a first acute myocardial infarction in both men and
women: Evidence for the fibrinolytic system as an independent
primary risk factor. Circulation. 98:2241–2247. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hamsten A, de Faire U, Walldius G, Dahlén
G, Szamosi A, Landou C, Blombäck M and Wiman B: Plasminogen
activator inhibitor in plasma: Risk factor for recurrent myocardial
infarction. Lancet. 2:3–9. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen R, Yan J, Liu P, Wang Z and Wang C:
Plasminogen activator inhibitor links obesity and thrombotic
cerebrovascular diseases: The roles of PAI-1 and obesity on stroke.
Metab Brain Dis. 32:667–673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li JY, Li Y, Gong HY, Zhao XB and Li LZ:
Protective effects of n-butanol extract of Potentilla anserina on
acute myocardial ischemic injury in mice. Zhong Xi Yi Jie He Xue
Bao. 7:48–52. 2009.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu XC, Wang X, Zheng H and Ma LP:
Protective effects of orientin on myocardial ischemia and hypoxia
in animal models. Nan Fang Yi Ke Da Xue Xue Bao. 27:1173–1175.
2007.(In Chinese). PubMed/NCBI
|
|
41
|
Maenpaa CJ, Shames BD, Van Why SK, Johnson
CP and Nilakantan V: Oxidant-mediated apoptosis in proximal tubular
epithelial cells following ATP depletion and recovery. Free Radic
Biol Med. 44:518–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim JK, Pedram A, Razandi M and Levin ER:
Estrogen prevents cardiomyocyte apoptosis through inhibition of
reactive oxygen species and differential regulation of p38 kinase
isoforms. J Biol Chem. 281:6760–6767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Castedo E, Segovia J, Escudero C,
Olmedilla B, Granado F, Blas C, Guardiola JM, Millán I, Pulpón LA
and Ugartea J: Ischemia-reperfusion injury during experimental
heart transplantation. Evaluation of trimetazidine's cytoprotective
effect. Rev Esp Cardiol. 58:941–950. 2005.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bardooli F, McAlindon E, Littlejohns B,
Suleiman MS, Bucciarelli-Ducci C and Baumbach A: TCT-184 Early
changes in circulating miRNA 133a are indicative of cardiac
remodelling after 3 months in patients presenting with acute ST
elevation myocardial infarction. J Am Coll Cardiol. 68:B75–B76.
2016. View Article : Google Scholar
|
|
45
|
Wang Y, Ouyang M, Wang Q and Jian Z:
MicroRNA-142-3p inhibits hypoxia/reoxygenationinduced apoptosis and
fibrosis of cardiomyocytes by targeting high mobility group box 1.
Int J Mol Med. 38:1377–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Singh GB, Raut SK, Khanna S, Kumar A,
Sharma S, Prasad R and Khullar M: MicroRNA-200c modulates DUSP-1
expression in diabetes-induced cardiac hypertrophy. Mol Cell
Biochem. 424:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li X, Gao Y, Meng Z, Zhang C and Qi Q:
Regulatory role of microRNA-30b and plasminogen activator
inhibitor-1 in the pathogenesis of cognitive impairment. Exp Ther
Med. 11:1993–1998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma F, Li T, Zhang H and Wu G: MiR-30s
family inhibit the proliferation and apoptosis in human coronary
artery endothelial cells through targeting the 3′UTR region of
ITGA4 and PLCG1. J Cardiovasc Pharmacol. 68:327–333. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li F, Chen Q, Song X, Zhou L and Zhang J:
MiR-30b Is involved in the homocysteine-induced apoptosis in human
coronary artery endothelial cells by regulating the expression of
caspase 3. Int J Mol Sci. 16:17682–17695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wei C, Li L and Gupta S: NF-κB-mediated
miR-30b regulation in cardiomyocytes cell death by targeting Bcl-2.
Mol Cell Biochem. 387:135–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Song CL, Liu B, Wang JP, Zhang BL, Zhang
JC, Zhao LY, Shi YF, Li YX, Wang G, Diao HY, et al: Anti-apoptotic
effect of microRNA-30b in early phase of rat myocardial
ischemia-reperfusion injury model. J Cell Biochem. 116:2610–2619.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang K, An T, Zhou LY, Liu CY, Zhang XJ,
Feng C and Li PF: E2F1-regulated miR-30b suppresses Cyclophilin D
and protects heart from ischemia/reperfusion injury and necrotic
cell death. Cell Death Differ. 22:743–754. 2015. View Article : Google Scholar : PubMed/NCBI
|