Introduction
Uterine leiomyoma, the most common benign tumor type
of the female reproductive tract, has a reported prevalence of
20–50% in women of reproductive age (1,2). It is
associated with high rates of gynecological morbidity, including
dysmenorrhea infertility, menometrorrhagia and pelvic pain
(3). Numerous factors are likely to
influence fibroid growth, and uterine muscle cells may be
particularly vulnerable to growth abnormalities. In premenopausal
women, the uterus is regulated in part by estrogen, a hormone
broadly associated with cell growth (4,5). Uterine
leiomyoma is common; >80% of African American females and 70% of
white females develop uterine fibroids detectable on ultrasound in
the USA (6). The risk of uterine
leiomyoma is influenced by age, ethnicity, caffeine intake, number
of pregnancies, endogenous hormone levels, obesity and genetic
factors (7).
Environmental endocrine disruptors comprise of
numerous natural and synthetic compounds that have the potential to
interfere with the normal endocrine system of animals, including
humans; they include environmental estrogens, which mimic the
action of natural estrogen in the body (8). Uterine leiomyoma is a gynecological
disease characterized by estrogen dependence (9); however, the association between
exposure to environmental endocrine disruptors and the disease
remains unclear.
Di-(2-ethylhexyl) phthalate (DEHP) is the most
common environmental endocrine disruptor (10–12).
DEHP is widely used in consumer products, including food packaging,
medical devices and toys, to improve the flexibility and durability
of polyvinyl chloride-based plastics. DEHP is not covalently bound
to the plastic matrix and maybe released from its substrate into
the environment (13). As a
widespread environmental pollutant and an endocrine disruptor, DEHP
is a serious concern due to its potential toxic effects, including
reproductive toxicity (14),
neurotoxicity (15) and
carcinogenicity (16). Recent
epidemiological evidence suggests that women have an increased
exposure profile to phthalates compared with men, as they are
present in a number of beauty products, including skin lotions,
perfumes and nail products, which raises concerns about their
potential health hazards (17,18). In
animals, the reproductive and developmental toxicities are exerted
through similar mechanisms in the two sexes, but the toxicity seems
to occur at an older age in females compared with males (18).
Hypoxia inducible factor-1 (HIF-1) is a
heterodimeric transcription factor comprising a basic
helix-loop-helix/PAS domain, and includes the subunits HIF-1α and
aryl hydrocarbon receptor nuclear translocator (also known as
HIF-1β) (19). The availability of
HIF-1 is determined primarily by HIF-1α, which is regulated at the
protein level in an oxygen-sensitive manner (20). HIF-1α has been demonstrated to
mediate angiogenesis, cell proliferation, apoptosis and migration
(21). By contrast, HIF-1β is stably
expressed (20). HIF-1 has been
reported to enhance cyclooxygenase-2 (COX-2) expression by
interacting with functional hypoxia response elements in the COX-2
promoter region (22). COX proteins
(COX-1 and COX-2) catalyze the synthesis of prostaglandins from
arachidonic acid. While COX-1 is expressed constitutively in most
of the tissues and appears to be responsible for housekeeping
functions, COX-2 is transcriptionally induced by pro-inflammatory
stimuli (23). COX-2 is upregulated
in numerous types of malignancy (24,25) and
favors malignant growth by stimulating proliferation and
angiogenesis (26) via multiple
pathways, including the mitogen-activated protein kinase and NF-κB
pathways, in different cell types (27).
To the best of our knowledge, the mechanisms
underlying DEHP action in human leiomyoma cells have not been
studied previously. In the present study, the effect of phthalate
exposure on the pathogenesis of uterine leiomyoma was investigated.
It was identified that DEHP enhances proliferative activity and
blocks apoptosis of leiomyoma cells, and induces the expression of
HIF-1α and COX-2.
Materials and methods
Chemicals
DEHP was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany).
Human uterine leiomyoma cells
Human uterine leiomyoma cells (GM10964) were
purchased from the Coriell Institute for Medical Research (Camden,
NJ, USA) and maintained in minimum essential medium (MEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with MEM vitamins
solution (100X; cat. no. 1112005), MEM amino acids (50X; cat. no.
11130036), MEM non-essential amino acids (100X; cat. no. 11140050)
and L-glutamine (2 mM; all Gibco; Thermo Fisher Scientific, Inc.)
at 37°C in 95% humidity with 5% carbon dioxide, as described
previously (28).
Cell viability assay
Cell viability was analyzed using a MTS assay
(CellTiter 96®AQueous Cell Proliferation Assay kit;
Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol.
Briefly, the cells were cultured for 24 and 48 h and
20 µl/well MTS solution was added to the samples in 100 µl culture
medium. The cells were subsequently incubated at 37°C for 4 h and
the absorbance was measured using a microplate reader at 490
nm.
Concentration of DEHP
In previous case-control studies, serum
concentrations of DEHP were reported to range from 1. 5 to 6. 2 µM
(29,30). The concentrations of DEHP used in the
present study were determined using a concentration-response curve.
Briefly, leiomyoma cells were exposed for 48 h to increasing
concentrations of DEHP (from 0.0 to 6.0 µM). After 48 h, cell
survival was analyzed by MTT assay according to the aforementioned
method (data not shown). Concentrations of DEHP >1. 5 µM
significantly decreased the percentage of live cells in the MTT
assay. Therefore, for the majority of experiments, concentrations
of DEHP ranging from 0.0 to 1.0 µM were selected. Concentrations of
0.01 and 1 µM DEHP were regarded as ‘low’ and ‘high’ levels of DEHP
exposure, respectively.
TUNEL assay
In order to confirm apoptosis by identifying
apoptotic bodies in human uterine leiomyoma cells, a TUNEL assay
was performed using an in situ cell death detection kit
(cat. no. 1684795; Roche Diagnostics GmbH, Mannheim, Germany).
Briefly, cells (5×104/per well) were fixed using 4%
formaldehyde for 40 min at room temperature. This was followed by
multiple rinses in PBS and permeabilization in 0.2% Triton X-100
solution on ice for 5 min. Subsequently, 50 µl TUNEL reaction
mixture was added on coverslips before being incubated for 60 min
at 37°C in a dark, humidified chamber. Finally, the coverslips were
incubated with 4′,6-diamidino-2-phenylindole (DAPI, 2 µg/ml;
Sigma-Aldrich; Merck KGaA) for 20 min at room temperature and
mounted with VECTASHIELD Antifade Mounting medium (cat. no. H-1000;
Vector Laboratories Ltd., Peterborough, UK). The coverslips were
examined with an LSM 510 confocal microscope (Zeiss GmbH, Jena,
Germany) and counted in three fields of view. Data were expressed
as the ratio of TUNEL-positive cells to total nuclei.
Annexin V staining and flow
cytometry
To determine the apoptosis rate, cells were
incubated in culture medium containing 0, 0.1 and 1 µM DEHP for 48
h and stained with Annexin V-fluorescein isothiocyanate (FITC),
according to the manufacturer's protocol (Molecular Probes; Thermo
Fisher Scientific, Inc.). Approximately 1×105 cells were
harvested and washed with phosphate-buffered saline. Cells were
then resuspended in 100 µl Annexin V binding buffer (10 mM HEPES,
140 mM NaCl and 2.5 mM CaCl2, pH 7.4), incubated with 5
µl of Annexin V-FITC for 15 min at room temperature, and
counterstained with propidium iodide (PI; final concentration, 1
µg/ml) for 10 min at room temperature. Following the incubation
period, the cells were diluted with 190 µl Annexin V binding
buffer. Cells were analyzed by flow cytometry using a
Becton-Dickinson FACScan flow cytometer with Cell Quest 3.
1software (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
The cells (1×106/per ml) were resuspended
in a radioimmunoprecipitation buffer (50 mM Tris; pH 8.0; Cell
Signaling Technology, Inc., Danvers, MA, USA) containing a protease
inhibitor cocktail (cOmplete™ Mini Protease Inhibitor Tablet; Roche
Diagnostics GmbH). The protein concentration was measured in the
supernatant using a Pierce BCA Protein Assay kit (cat. no. 23225;
Thermo Fisher Scientific, Inc.). A total of 40 µg of protein was
loaded per lane, separated by 10% SDS-PAGE and transferred onto
nitrocellulose membranes (cat. no. LC2009; Thermo Fisher
Scientific, Inc.). Following transfer the membranes were blocked
for 2 h at room temperature with 5% skimmed milk in Tris buffered
saline-Tween-20 (TBST; 20 mM Tris, 500 nM NaCl, 0.1% Tween-20; pH
7.5). The membranes were incubated with primary antibodies against
proliferating cell nuclear antigen (PCNA; cat. no. 13110; 1:1,000),
B-cell lymphoma 2 (Bcl-2; cat. no. 2872; 1:1000), HIF-1α (cat. no.
14179; 1:1,000), COX-2 (cat. no. 12282; 1:1,000) or β-actin (cat.
no. 4970; 1:5,000; all Cell Signaling Technology, Inc.) at 4°C.
Following three washes with TBST, the membranes were incubated with
secondary horseradish peroxidase-conjugated anti-IgG antibodies
(cat. no. 65-6120; 1:5,000; Invitrogen; Thermo Fisher Scientific,
Inc.) for 2 h at room temperature and visualized using a Pierce
enhanced chemiluminescence substrate (Thermo Fisher Scientific,
Inc.). Densitometric quantification of the protein density bands
was achieved using ImageJ software (version 1.29×, National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
The Kolmogorov-Smirnov test was performed to
evaluate whether data were normally distributed. If this was the
case, continuous variables were compared using two-sample Student's
t-tests or, with three groups, analysis of variance followed by
Fisher's least significant difference post-hoc test for pairwise
comparisons. If data were not normally distributed, the variables
were compared using the Mann-Whitney U test or the Kruskal-Wallis
test followed by the Mann-Whitney U test with Bonferroni
correction, depending on whether two or three groups were
considered, respectively. Statistical analysis was performed on
SPSS 14.0 software (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation of separate experiments
(n=3). P<0.05 was considered to indicate a statistically
significant difference.
Results
Viability of human leiomyoma cells
following DEHP exposure
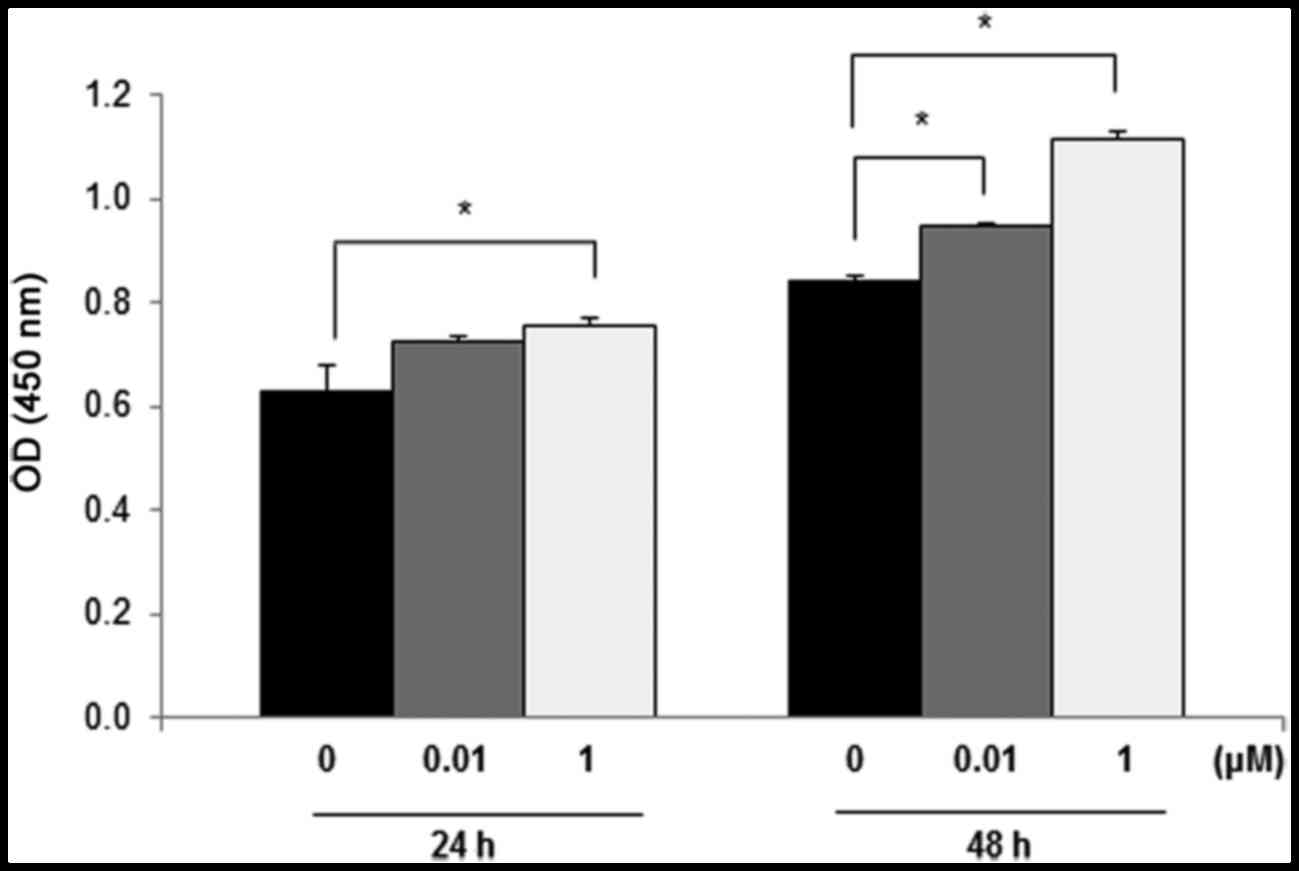
To assess the influence of DEHP exposure on cell
viability, an MTT assay was conducted using leiomyoma cells treated
for 24 or 48 h with DEHP (0, 0.01 and 1 µM). Exposure of leiomyoma
cells to 0.01 or 1 µM DEHP for 24 and 48 h led to higher viability
compared with the untreated controls. Viability of leiomyoma cells
was significantly higher after 24 h exposure to 1 µM DEHP
(P<0.05) and 48 h exposure to 0.01 or 1 µM DEHP (both P<0.05)
compared with control cells (Fig.
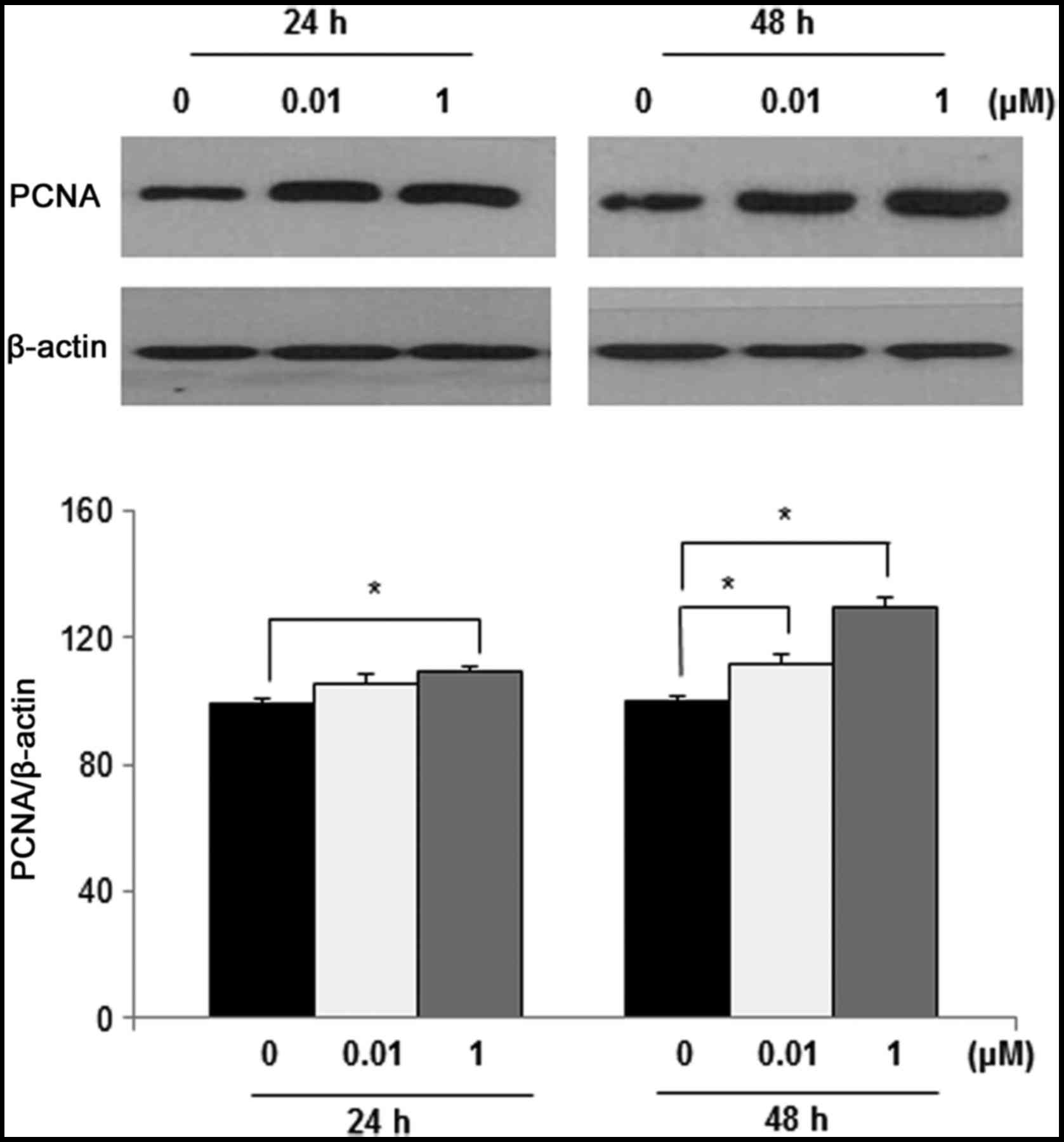
1). To further examine the effects of DEHP on cell
proliferation, PCNA protein levels were determined by western blot
analysis. PCNA expression was significantly higher in leiomyoma
cells following treatment with 1 µM DEHP for 24 h (P<0.05) and
0.01 and 1 µM DEHP for 48 h (both P<0.05) compared with control
cells (Fig. 2).
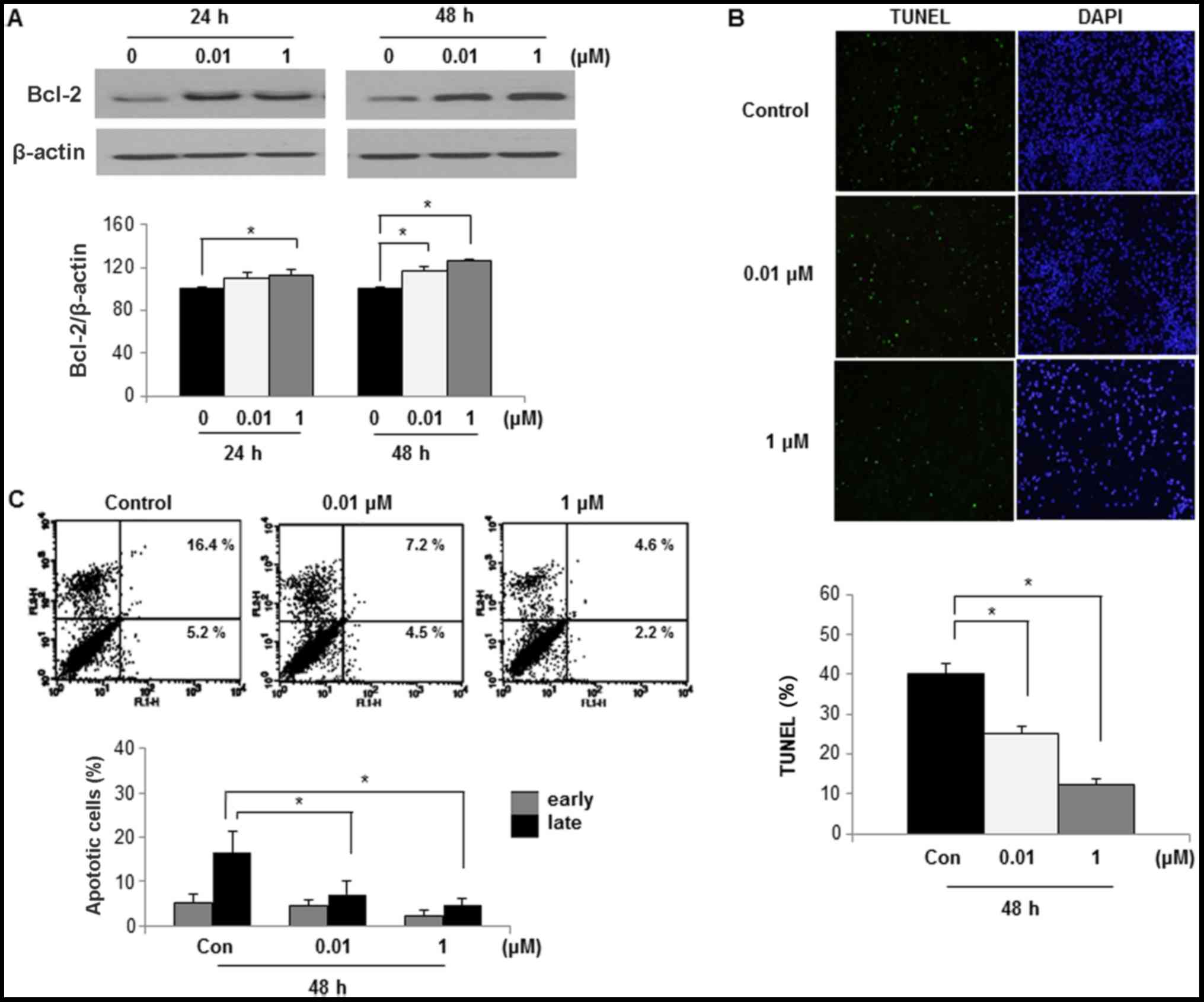
Effects of DEHP on the apoptosis of
human leiomyoma cells
To examine if the increased viability could be
attributed to an anti-apoptotic pathway, Bcl-2 expression levels
were determined by western blotting. Bcl-2 expression was
significantly higher in leiomyoma cells after 24 h exposure to 1 µM
DEHP (P<0.05) and 48 h exposure to 0.01 or 1 µM DEHP (both
P<0.05), as compared with control cells (Fig. 3A).
To assess if DEHP caused apoptosis, apoptotic cells
were detected by TUNEL assay. The rate of apoptosis was assessed in
cells treated with 0, 0.01 and 1 µM DEHP for 48 h by confocal
microscopy. The number of apoptotic cells was significantly lower
after 48 h exposure to 0.01 or 1 µM DEHP compared with the control
group (both P<0.05; Fig. 3B).
To assess if DEHP had an anti-apoptotic effect, the
percentage of apoptotic cells was determined by Annexin V and PI
staining. The rate of apoptosis was assessed in leiomyoma cells
treated with 0, 0.01 and 1 µM DEHP for 48 h by flow cytometry
analysis. The rate of late apoptosis in the control group was
16.4±4.7%, indicating that apoptosis was the primary cell death
mechanism in leiomyoma cells in the control group. Compared with
the control group, the apoptosis rate of leiomyoma cells was
significantly inhibited when exposed to 0.1 µM DEHP (rate of late
apoptosis, 7.2±3.1%; P<0.05) or 1 µM DEHP (rate of late
apoptosis, 4.6±0.7%; P<0.05; Fig.
3C).
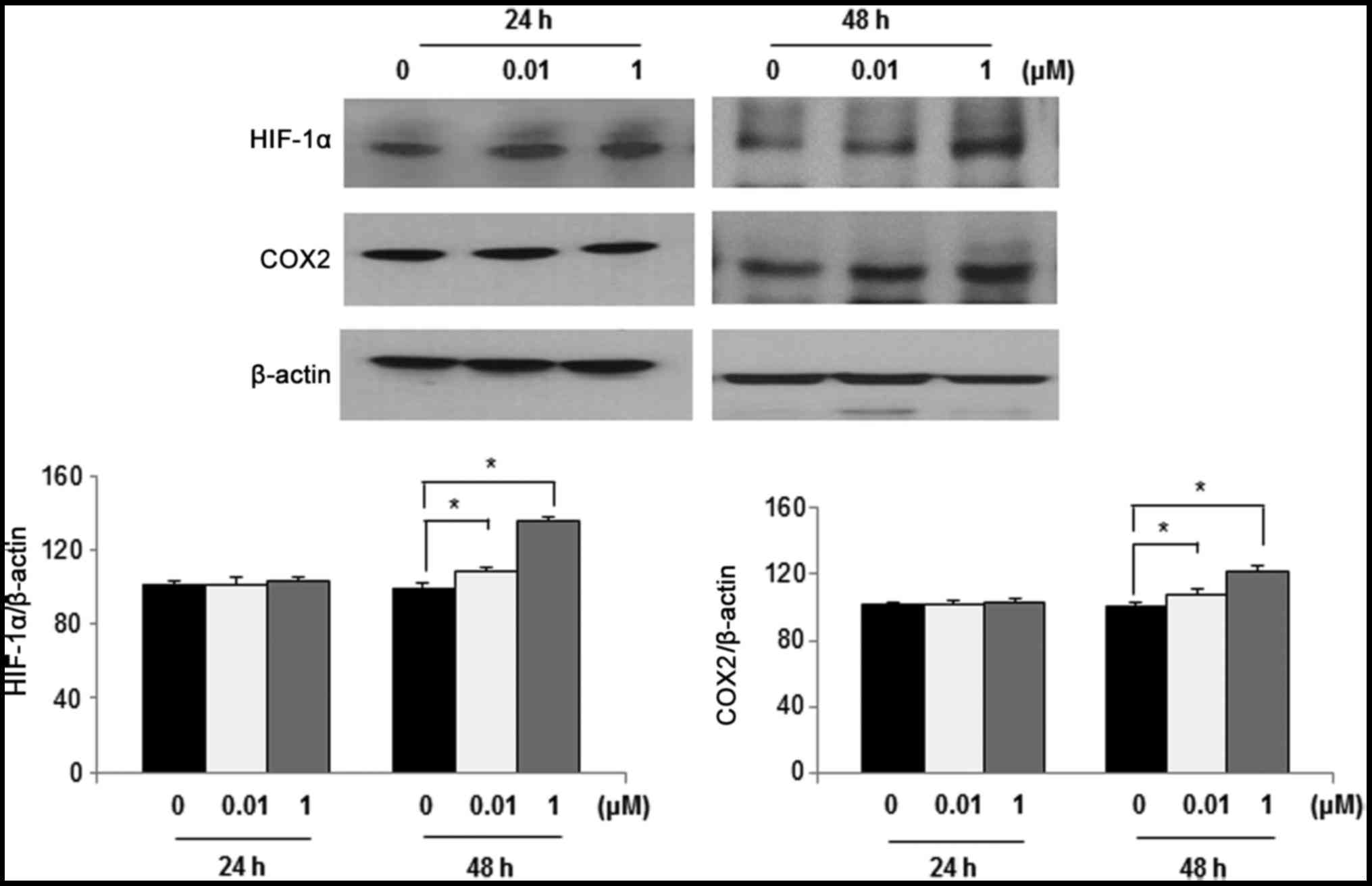
Effects of DEHP on HIF-1α and COX-2
expression in human leiomyoma cells
Western blot analysis was performed to determine the
effects of DEHP exposure on HIF-1α and COX-2 expression. In
leiomyoma cells, HIF-1α and COX-2 expression was significantly
increased following exposure to 1 µM DEHP for 48 h compared with
control cells (P<0.05). However, HIF-1α and COX-2 expression did
not significantly increase following exposure to DEHP for 24 h
compared with control cells (Fig.
4).
Discussion
The present study demonstrated that in vitro
DEHP treatment leads to increased viability, proliferation and
anti-apoptotic protein expression in human leiomyoma cells.
Furthermore, HIF-1α and COX-2 expression following DEHP treatment
was higher in human leiomyoma cells compared with control cells.
These in vitro results suggest that exposure to phthalates
may serve a function in the pathogenesis of uterine leiomyoma.
DEHP is the most common plasticizer in polyvinyl
chloride-containing plastics. Furthermore, DEHP is an endocrine
disruptor that is able to alter sexual differentiation and energy
metabolism (10–12). Given its chemical structure, DEHP
easily enters food, air and even the human body. Approximately 7.3%
of mono-ethylhexyl phthalate and 66.9% of oxidative metabolites are
excreted in urine by the human body; however, 25.8% of DEHP
bioaccumulates in the body (31). In
addition, the direct effects of DEHP are not well-characterized.
The toxicity potential of DEHP remains controversial. The presence
of DEHP has been reported in various human cell types (32–35).
DEHP could promote proliferation of breast cancer cells and induce
necrosis of keratinocytes (32,33).
DEHP exposure was observed to promote invasion of neuroblastoma
cells and increase the growth rate of hepatic carcinoma cells
(34,35). Furthermore, phthalates enhance
pro-inflammatory cytokine production in vitro (36) and may contribute to pro-inflammatory
processes, with the potential to interact with other risk factors,
as revealed in clinical case reports (37,38).
Given that uterine leiomyoma is the most common gynecological
disease, it is necessary to determine whether exposure to phthalate
is associated with leiomyoma risk. A previous study suggested a
possible association of increased urinary level of phthalate
metabolites with the risk of uterine leiomyoma (39). However, to the best of our knowledge,
no study on the effects of phthalate on human leiomyoma cells has
been reported.
In the present study, it was identified that in
vitro DEHP treatment leads to increased viability and elevated
PCNA and Bcl-2 expression in leiomyoma cells. PCNA localizes in the
nucleus of proliferating cells (40)
and serves as a cofactor for DNA replication and repair, and cell
cycle regulation (41,42). Bcl-2 family members are important
regulators of programmed cell death and act as inhibitors of
apoptosis (43–45). These findings suggest that DEHP may
cause an imbalance in cellular proliferation and apoptosis, which
drives the pathogenesis of uterine leiomyoma (46–48).
Furthermore, the present study revealed that HIF-1α
and COX-2 expression following DEHP treatment may elicit
inflammation. The current results indicated that DEHP exposure
increased the expression levels of HIF-1α and COX-2 after 48 h.
These findings suggest that chronic continuous exposure to DEHP may
be a critical factor for leiomyoma cells to experience persistently
enhanced inflammation. The results of this study indicate that
continual exposure to DEHP may induce HIF-1α and COX-2 expression
and may enhance proliferation ability, which is consistent with
previous similar studies (49,50).
Elevated expression of inflammatory proteins could elicit various
effects, including the promotion of tumor progression, by inducing
proliferation and resistance to apoptosis (51). HIF-1α and COX-2 expression was
indicated to be increased by DEHP treatment, suggesting that DEHP
may serve a critical function in inflammation of human leiomyoma
cells. Since the effects of DEHP following inhibition of
inflammation are not yet known, these effects of DEHP on human
leiomyoma cells should be studied in the future.
In conclusion, DEHP promoted cellular viability and
anti-apoptotic protein expression and induced HIF-1α and COX-2
expression in human leiomyoma cells. These results suggest that
DEHP may disrupt mechanisms underlying various processes in human
leiomyoma cells. Furthermore, the present study reveals a basic
mechanism of action of DEHP in human leiomyoma cells. Further
research on the effects of various endocrine disruptors on the
pathogenesis of uterine leiomyoma during early development may
reveal strategies to prevent this disease. Further in vivo
study will be necessary to confirm these findings, since the
current results were based solely on an in vitro model.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Research
Foundation of Korea grant (grant no. 2017R1D1A1B03034223) funded by
the Korean government.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
JHK conceived and designed the experiments,
performed the experiments, analyzed the data and wrote the
paper.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Falcone T and Walters MD: Hysterectomy for
benign disease. Obstet Gynecol. 111:753–767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parker WH: Etiology, symptomatology, and
diagnosis of uterine myomas. Fertil Steril. 87:725–736. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sell SM, Tullis C, Stracner D, Song CY and
Gewin J: Minimal interval defined on 7q in uterine leiomyoma.
Cancer Genet Cytogenet. 157:67–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rose DP, Goldman M, Connolly JM and Strong
LE: High-fiber diet reduces serum estrogen concentrations in
premenopausal women. Am J Clin Nutr. 54:520–525. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prentice R, Thompson D, Clifford C,
Gorbach S, Goldin B and Byar D: Dietary fat reduction and plasma
estradiol concentration in healthy postmenopausal women. The
women's health trial study group. J Natl Cancer Inst. 82:129–134.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Greenberg MD and Kazamel TI: Medical and
socioeconomic impact of uterine fibroids. Obstet Gynecol Clin North
Am. 22:625–636. 1995.PubMed/NCBI
|
|
7
|
Wang F, Chen J, Wang L, Ma Y and Mayinuer
N: CYP1A1 genetic polymorphisms and uterine leiomyoma risk: A
meta-analysis. Int J Clin Exp Med. 8:3590–3594. 2015.PubMed/NCBI
|
|
8
|
Markey CM, Rubin BS, Soto AM and
Sonnenschein C: Endocrine disruptors: From Wingspread to
environmental developmental biology. J Steroid Biochem Mol Biol.
83:235–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu L, Moore AB and Dixon D: Receptor
tyrosine Kinases and their hormonal regulation in uterine
leiomyoma. Semin Reprod Med. 28:250–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharpe RM, Fisher JS, Millar MM, Jobling S
and Sumpter JP: Gestational and lactational exposure of rats to
xenoestrogens results in reduced testicular size and sperm
production. Environ Health Perspect. 103:1136–1143. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin Y, Wei J, Li Y, Chen J, Zhou Z, Song
L, Wei Z, Lv Z, Chen X, Xia W and Xu S: Developmental exposure to
di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to
long-term adverse effects on glucose homeostasis in the rat. Am J
Physiol Endocrinol Metab. 301:E527–E538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tomaszewski KE, Heindel SW, Jenkins WL and
Melnick RL: Induction of peroxisomal acyl CoA oxidase activity and
lipid peroxidation in primary rat hepatocyte cultures. Toxicology.
17:49–60. 1990. View Article : Google Scholar
|
|
13
|
Halden RU: Plastics and health risks. Annu
Rev Public Health. 31:179–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ha M, Guan X, Wei L, Li P, Yang M and Liu
C: Di-(2-ethylhexyl) phthalate inhibits testosterone level through
disturbed hypothalamic-pituitary-testis axis and ERK-mediated
5α-Reductase 2. Sci Total Environ. 563–564:566–575. 2016.
View Article : Google Scholar
|
|
15
|
Tseng IL, Yang YF, Yu CW, Li WH and Liao
VH: Phthalates induce neurotoxicity affecting locomotor and
thermotactic behaviors and AFD neurons through oxidative stress in
Caenorhabditis elegans. PLoS One. 8:e826572013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caldwell JC: DEHP: Genotoxicity and
potential carcinogenic mechanisms-a review. Mutat Res. 751:82–157.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McKee RH, Butala JH, David RM and Gans G:
NTP center for the evaluation of risks to human reproduction
reports on phthalates: Addressing the data gaps. Reprod Toxicol.
18:1–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoyer PB: Reproductive toxicology: Current
and future directions. Biochem Pharmacol. 62:1557–1564. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci
USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maxwell PH, Wiesener MS, Chang GW,
Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER and
Ratcliffe PJ: The tumour suppressor protein VHL targets
hypoxia-inducible factors for oxygen-dependent proteolysis. Nature.
399:271–275. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris AL: Hypoxia-a key regulatory factor
in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Csiki I, Yanagisawa K, Haruki N, Nadaf S,
Morrow JD, Johnson DH and Carbone DP: Thioredoxin-1 modulates
transcription of cyclooxygenase-2 via hypoxia-inducible
factor-1alpha in non-small cell lung cancer. Cancer Res.
66:143–150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao Y and Prescott SM: Many actions of
cyclooxygenase-2 in cellular dynamics and in cancer. J Cell
Physiol. 190:279–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygenases in inflammation, cancer, and development.
Oncogene. 18:7908–7916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Williams C, Shattuck-Brandt RL and DuBois
RN: The role of COX-2 in intestinal cancer. Ann N Y Acad Sci.
889:72–83. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dempke W, Rie C, Grothey A and Schmoll HJ:
Cyclooxygenase-2: A novel target for cancer chemotherapy? J Cancer
Res Clin Oncol. 127:411–417. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singer CA, Baker KJ, McCaffrey A, AuCoin
DP, Dechert MA and Gerthoffer WT: p38MAPK and NF-kappaB mediate
COX-2 expression in human airway myocytes. Am J Physiol Lung Cell
Mol Physiol. 2855:L1087–L1098. 2003. View Article : Google Scholar
|
|
28
|
Swartz CD, Afshari CA, Yu L, Hall KE and
Dixon D: Estrogen-induced changes in IGF-I, Myb family and MAP
kinase pathway genes in human uterine leiomyoma and normal uterine
smooth muscle cell lines. Mol Hum Reprod. 11:441–450. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Colón I, Caro D, Bourdony CJ and Rosario
O: Identification of phthalate esters in the serum of young Puerto
Rican girls with premature breast development. Environ Health
Perspect. 108:895–900. 2000. View Article : Google Scholar
|
|
30
|
Durmaz E, Ozmert EN, Erkekoglu P, Giray B,
Derman O, Hincal F and Yurdakök K: Plasma phthalate levels in
pubertal gynecomastia. Pediatrics. 125:e122–e129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wittassek M and Angerer J: Phthalates:
Metabolism and exposure. Int J Androl. 31:131–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen FP and Chien MH: Lower concentrations
of phthalates induce proliferation inhuman breast cancer cells.
Climacteric. 17:377–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martinasso G, Maggiora M, Trombetta A,
Canuto RA and Muzio G: Effects of di(2-ethylhexyl)phthalate, a
widely used peroxisome proliferator and plasticizer, oncell growth
in the human keratinocyte cell line NCTC 2544. J Toxic Environ
Health A. 69:353–365. 2006. View Article : Google Scholar
|
|
34
|
Chen X, Qin Q, Zhang W, Zhang Y, Zheng H,
Liu C, Yang Y, Xiong W and Yuan J: Activation of thepi3k-akt-mtor
signaling pathway promotes dehp-induced hep3b cell proliferation.
Food Chem Toxicol. 59:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu H, Zheng J, Xiao X, Zheng S, Dong K,
Liu J and Wang Y: Environmental endocrine disruptors promote
invasion and metastasis of SK-N-SH human neuroblastoma cells. Oncol
Rep. 23:129–139. 2010.PubMed/NCBI
|
|
36
|
Jepsen KF, Abildtrup A and Larsen ST:
Monophthalates promote IL-6 and IL-8production in the human
epithelial cell line A549. Toxicol In Vitro. 18:265–269. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Burmeister A, Assi LK, Ferro CJ, Hughes
RG, Barnett AH, Bellary S, Cockwell P, Pratt G and Hutchison CA:
The relationship between high-sensitivity CRP and polyclonal free
light chains as markers of inflammation in chronic disease. Int J
Lab Hematol. 36:415–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ferguson KK, Loch-Caruso R and Meeker JD:
Urinary phthalatemetabolites inrelation to biomarkers of
inflammation and oxidative stress: NHANES 1999–2006. Environ Res.
111:718–726. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang PC, Tsai EM, Li WF, Liao PC, Chung
MC, Wang YH and Wang SL: Associationbetween phthalate exposure and
glutathione S-transferase M1 polymorphism in adenomyosis, leiomyoma
and endometriosis. Hum Reprod. 25:986–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Palomba S, Orio F Jr, Russo T, Falbo A,
Tolino A, Lombardi G, Cimini V and Zullo F: Antiproliferativeand
proapoptotic effects of raloxifene on uterine leiomyomas in
postmenopausal women. Fertil Steril. 84:154–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsurimoto T: PCNA, a multifunctional ring
on DNA. Biochim Biophys Acta. 1443:23–39. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Majka J and Burgers PM: The PCNA-RFC
families of DNA clamps and clamp loaders. Prog Nucleic Acid Res Mol
Biol. 78:227–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Petros AM, Olejniczak ET and Fesik SW:
Structural biology of the Bcl-2 family of proteins. Biochim Biophys
Acta. 1644:83–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sorenson CM: Bcl-2 family members and
disease. Biochim Biophys Acta. 1644:169–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: Roles in cell survival andoncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Burroughs KD, Fuchs-Young R, Davis B and
Walker CL: Altered hormonalresponsiveness of proliferation and
apoptosis during myometrial maturation and the development of
uterine leiomyomas in the rat. Biol Reprod. 63:1322–1330. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Martel KM, Ko AC, Christman GM and
Stribley JM: Apoptosis in human uterine leiomyomas. Semin Reprod
Med. 22:91–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Maruo T, Ohara N, Wang J and Matsuo H: Sex
steroidal regulation of uterine leiomyoma growth and apoptosis. Hum
Reprod Update. 10:207–220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kao AP, Wang KH, Long CY, Chai CY, Tsai
CF, Hsieh TH, Hsu CY, Chang CC, Lee JN and Tsai EM: Interleukin-1β
induces cyclooxygenase-2 expression and promotes theinvasive
ability of human mesenchymal stem cells derived from
ovarianendometrioma. Fertil Steril. 96:678–684.e1. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dairkee SH, Seok J, Champion S, Sayeed A,
Mindrinos M, Xiao W, Davis RW and Goodson WH: Biphenol A induces a
profile of tumor aggressiveness in high-risk cellsfrom breast
cancer patients. Cancer Res. 68:2076–2080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang KH, Kao AP, Chang CC, Lee JN, Chai
CY, Hou MF, Liu CM and Tsai EM: Modulation of tumorigenesis and
oestrogen receptor-alpha expression by cell culture conditions in a
stem cell-derived breast epithelial cell line. Biol Cell.
102:159–172. 2010. View Article : Google Scholar : PubMed/NCBI
|