Introduction
Pancreatic cancer (PC) is one of the most common
fatal cancer types (1). The overall
5-year survival rate is <5%; annually, almost half of patients
diagnosed with PC will succumb to the disease (2,3). At
present, the options for chemotherapy only minimally prolong the
life span of patients with PC (4–6). It is
urgent to investigate and validate the mechanisms of PC growth
(7,8). Extensive efforts have been made to
identify biomarkers for this disease. Research has indicated that
some miRNAs may exhibit variable expression levels within some
cancer tissues (9–11).
MicroRNAs (miRNAs/miRs) are endogenous short
non-coding RNAs, 19–25 nucleotides in length (11). miRNAs have been reported to serve an
important role in regulating the expression and function of
numerous genes and proteins (12).
Previous studies have demonstrated that miRNA may facilitate cancer
cell growth, invasion and migration via degradation of mRNA, by
pairing with bases in its 3′-untranslated region (UTR) (13,14).
Thus, miRNAs are considered to be key markers for the diagnosis and
investigation of PC (15). In recent
decades, a number of studies have been conducted to profile miRNA
expression within PC cells, in order to identify differential miRNA
expression between PC and adjacent normal tissues (16,17).
Numerous miRNAs have been identified, including miR-128, miR-21-5p,
miR-31-5p, miR-210, miR-217 and miR-375 (9,12,13,15,18–22);
however, the specific mechanisms of these identified miRNAs require
further investigation.
Double minute (MDM) family proteins are key
regulators for the onco-suppressor p53. Marine and Jochemsen
(23) identified that MDM4 was a p53
binding protein and knockdown of MDM4 could induce embryonic
lethality within mice. The utero stage death could be rescued by
deletion of the Trp53 gene.
In the present study, miR-128 expression levels
within PC cells were significantly decreased compared with adjacent
normal tissues, as confirmed by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis. Additionally, MDM4
may be associated with miR-128 expression and was demonstrated to
be a target of miR-128 via a dual luciferase assay. Furthermore,
the expression levels of p53 were upregulated by miR-128 by
targeting MDM4 to suppress tumor growth. The results of the present
study may contribute to the developments of PC treatment and
diagnosis.
Materials and methods
Patient recruitment and tissue sample
collection
A total of 30 patients (14 females and 16 males;
age, 22–63) diagnosed with PC were recruited from The Third
People's Hospital (Yancheng, China) from September 2015 to June
2017. In-hospital histories of patients were reviewed, including
the diagnosis, American journal of critical care (AJCC, version 8)
PC stage or metastasis (24). All PC
and adjacent noncancerous tissues were obtained intraoperatively
and fast frozen in liquid nitrogen, then stored at −80°C until
experimental analysis. The present study was approved by the
Institutional Review Board for Human Research of The Third People's
Hospital. All recruited patients provided written informed
consent.
Cell culture
Human pancreatic carcinoma cell line MIA PaCa-2 was
provided by the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in Dulbecco's
modified Eagle's medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2 in a humidified cell culture incubator (Sanyo
Electric Co., Ltd., Osaka, Japan).
Cell transfection
MIA PaCa-2 cells (1×105 cells/well) were
plated in 6-well plates and transfected with 100 nM miR-128 mimics
or scrambled sequence control (Shanghai GenePharma Co., Ltd.,
Shanghai, China) using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol, when MIA PaCa-2 cells reached 50–60%
confluence within the plate. The micmic sequence was:
5′-UAUAUGGCUUUAGAUACUGUGAA-3′. The scramble sequence was:
5′-UAUAUGGCUUUAGAUGACGCUGA-3′. Subsequently, cells washed out
following transfection 6 h and 2 ml Dulbecco's modified DMEM with
10% fetal bovine serum were added to each well. cells were
harvested with trypsin at 37°C with 5% CO2 for 5 min for
following experiments at certain time points after transfection,
including: 24, 48 and 72 h.
Cell proliferation assay
Cell proliferation was determined with a Cell
Counting kit (CCK)-8 (C0038; Beyotime Institute of Biotechnology,
Shanghai, China) according to the manufacturer's protocol. Briefly,
2×103 cells in the logarithmic growth phase were plated
in a 96-well plate and then were transfected with miR-128 mimics or
scramble control. These cells were used for the cell proliferation
assay at 24, 48 and 72 h by adding 20 µl CCK solution into each
well and the cells were further incubated for 0.5 h at 37°C with 5%
CO2 in the cell incubator. The number of living cells
was measured using a microplate reader at 450 nm wavelength.
Colony formation assay
MIA PaCa-2 cells transfected with miR-128 mimics or
scramble control vector were transferred into a 6-well plate (100
cells/well) and the medium was replaced after 6 h. Subsequently,
the cells were cultured for another 72 h at 37°C with 5%
CO2. When visible cell colonies formed, cells were
stained with 0.1% crystal violet (Sigma Aldrich; Merck KGaA,
Darmstadt, Germany) in DMEM for 15 min at room temperature and the
colony was observed under light microscope, (magnification, ×40;
Leica Microsystems, Inc., Buffalo Grove, IL, USA). The number of
colony formation in each group was normalized to that in the mock
vector (Shanghai GenePharma Co., Ltd.) transfection group.
Flow cytometry analysis of apoptotic
cells
Apoptosis was measured using Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI)-staining kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Briefly, 1×106 MIA PaCa-2 cells were plated in 100 mm
dishes (Corning Incorporated, Corning, NY, USA) and then
transfected with miR-128 mimics or scrambled control After 72 h
transfection, the cells were lysed and stained with Annexin V-FITC
and PI prior to analysis with a flow cytometer. This assay was
performed to sort and identify early apoptotic (Annexin
V-FITC+/PI-) cells, primary necrotic (Annexin V-FITC-/PI+) cells
and also late apoptotic (Annexin V-FITC+/PI+) cells (also known as
secondary necrotic cells). For each group, the experiment was
repeated in triplicate. Analyzation was performed using a FACScan
flow cytometer with CellQuest software version 5.1 (BD Biosciences,
Franklin Lakes, NJ, USA).
Predicted target analysis of
miR-128
miRecords (http://c1.accurascience.com/miRecords/) is an online
database for predicting target binding sites of miRNAs. It
integrates the results of numerous online miRNA target prediction
tools, including DIANAmicroT, miRanda, PicTar and TargetScan.
Predicted miR-128 targets were identified by miRecords
database.
3′UTR-luciferase reporter gene
assay
All vectors were purchased from Ambion (Thermo
Fisher Scientific, Inc.). Vectors carried the MDM4 sequence, which
contained the predicted miR-128 binding sites with wild-type or
mutant 3′UTR. MIA PaCa-2 cells were plated into 24-well plates
(1×104 cells/well) and transfected with MDM4 vector or
mutant vector using Lipofectamine® 2000 transfection
reagent (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. After 4 h, 50 nM of miR-128 mimics or
scramble control was transfected into the cells respectively. After
another 48 h, cells were lysed with 0.5% trypsin at 37°C containing
5% CO2 and luciferase activities were analyzed with a
dual luciferase assay kit (Promega Corporation, Madison, WI, USA)
along with the normalization to the control group treated with MDM4
wild-type and miR-128 scramble vectors.
RT-qPCR analysis
Total RNA was isolated from tumor and adjacent
tissues using a PicoPure™ RNA Isolation kit (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Then, 2 µg of RNA was utilized for cDNA synthesis using
SuperScript™ III first-strand synthesis system kit (Thermo Fisher
Scientific, Inc.). The reverse transcription reaction was performed
at 55°C for 30 min. Following reverse transcription reactions, qPCR
was performed with TaqMan™ gene expression master mix and
respective primers (Thermo Fisher Scientific, Inc.). The specific
PCR primer for miR-128 was as follows: forward,
5′-AACACTCCAGCTGGGTCACAGTGAACCGGTCT-3′ and reverse,
5′-CTCAACTGGTGTCGTGGA-3′. The primer for GAPDH was as follows:
Forward, 5′-AATGCATCCTGCACCACCAA-3′ and reverse,
5′-GTAGCCATATTCATTGTCATA-3′. The qPCR procedure was performed as
follows: For hold stage: Step 1: Heating from 25 to 95°C at a rate
of 1.6°C/sec, holding for 2 min at 50°C. Step 2: Heating from 50 to
95°C at a rate of 1.6°C/sec, holding for 10 min at 95°C. For qPCR
stage: Step 1: Initial denaturation at 95°C for 15 sec. Step 2:
Annealing extension at 60°C for 1 min. The temperature was reduced
from 95 to 60°C at the rate of 1.6°C/sec; the denaturation and
extension stages were repeated for 40 cycles. Cq was measured at
the PCR stage. Based on the RT-qPCR results of Cq number, the mRNA
expression levels were calculated using the 2−ΔΔCq
method (25) and normalized to an
internal reference control GAPDH.
Western blotting
MIA PaCa-2 cells were harvested 72 h
post-transfection with miR-128 mimics or mock, then total proteins
were extracted using 1X NuPAGE™ LDS sample buffer (Thermo Fisher
Scientific, Inc.). Protein concentration was quantified using a
Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific,
Inc.). A total of 30 µg of protein was loaded per lane and
electrophoresis was performed in 10% Tris-SDS gel. Following
electrophoresis, the proteins were transferred to polyvinylidene
fluoride membranes (Thermo Fisher Scientific, Inc.). For subsequent
blocking and antibody incubation, an iBind™ kit (Thermo Fisher
Scientific, Inc.) was used according to the manufacturer's
protocol. The prepared diluted primary antibodies, iBind™
Flex/iBind™ Flex FD solution, diluted secondary antibodies and
iBind™ Flex/iBind™ Flex FD solution were sequentially added to each
lane. Membranes were then incubated with primary antibodies
overnight at room temperature. Following this, membranes were
incubated with secondary antibodies for 1 h at room temperature.
and were rinsed in water prior to immunodetection. The primary
antibodies were as follows: Anti-p53 (cat. no. 2527, 1:300; Cell
Signaling Technology, Inc., Danvers, MA, USA), anti-MDM4 (cat. no.
sc-374147, 1:300; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-cleaved caspase-3 (cat. no. sc-98785, 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-β-actin (cat. no.
7210, 1:1,000 Santa Cruz Biotechnology, Inc.). The secondary
antibody (horseradish peroxidase conjugated anti-rabbit; cat. no.
7074) was used at 1:3,000 and was obtained from Cell Signaling
Technology, Inc. The signal was detected with
SuperSigal® west femto maximum sensitivity substrate
(Thermo Fisher Scientific, Inc.), and the specific proteins were
detected with ChemiDoc™ imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), using Image Lab software version 4.0 (Bio-Rad
Laboratories, Inc.).
Statistical analysis
Statistical analysis between two groups was
performed with two-tailed Student's t-test and statistical
differences among multiple groups were analyzed by one-way analysis
of variance followed by Dunnett's test using GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant difference.
All experiments were performed in triplicate unless otherwise
stated and all data are presented as the mean ± standard
deviation.
Results
Expression of miR-128 in PC tissues
and normal adjacent tissues
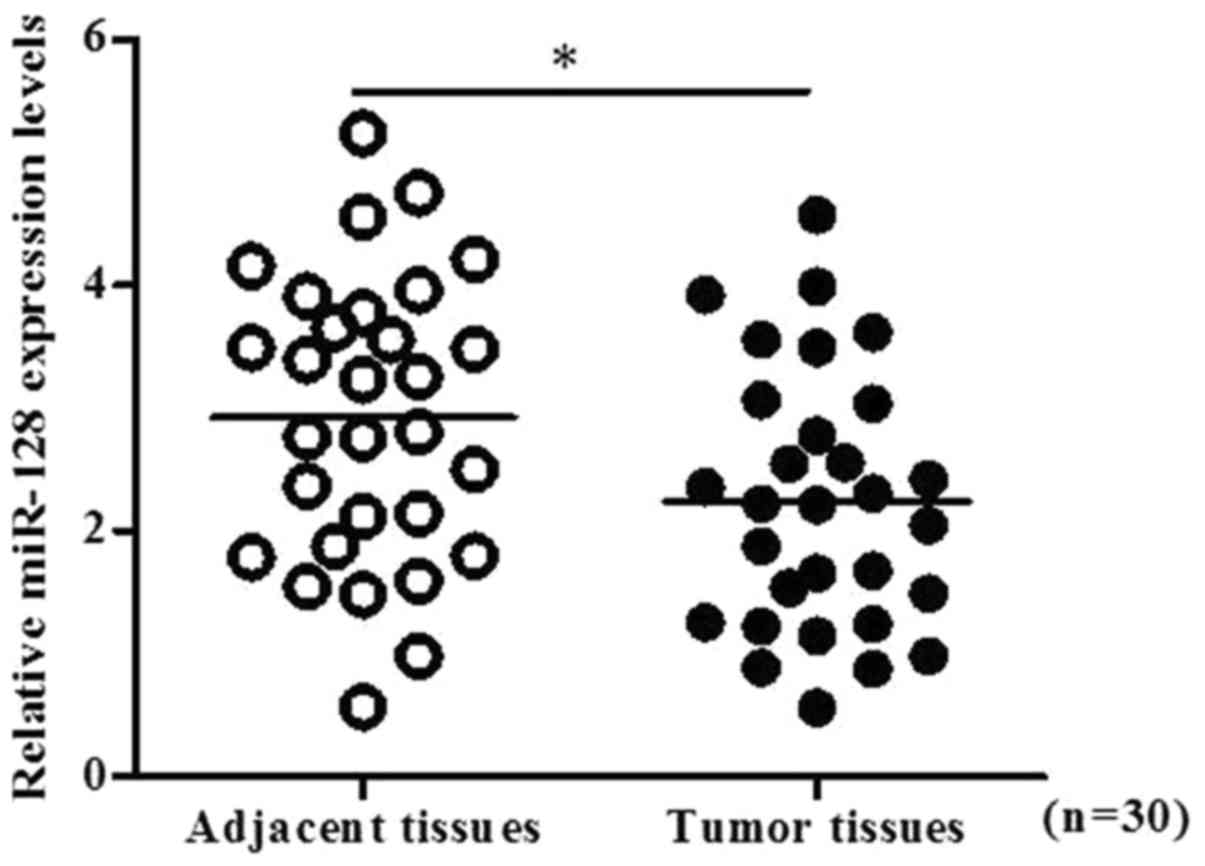
The expression of miR-128 within 30 pairs of PC
tissues and normal adjacent tissues was determined by RT-qPCR. The
results indicated that the levels of miR-128 were significantly
decreased within PC tissues compared with in normal adjacent
tissues (P<0.05; Fig. 1). In
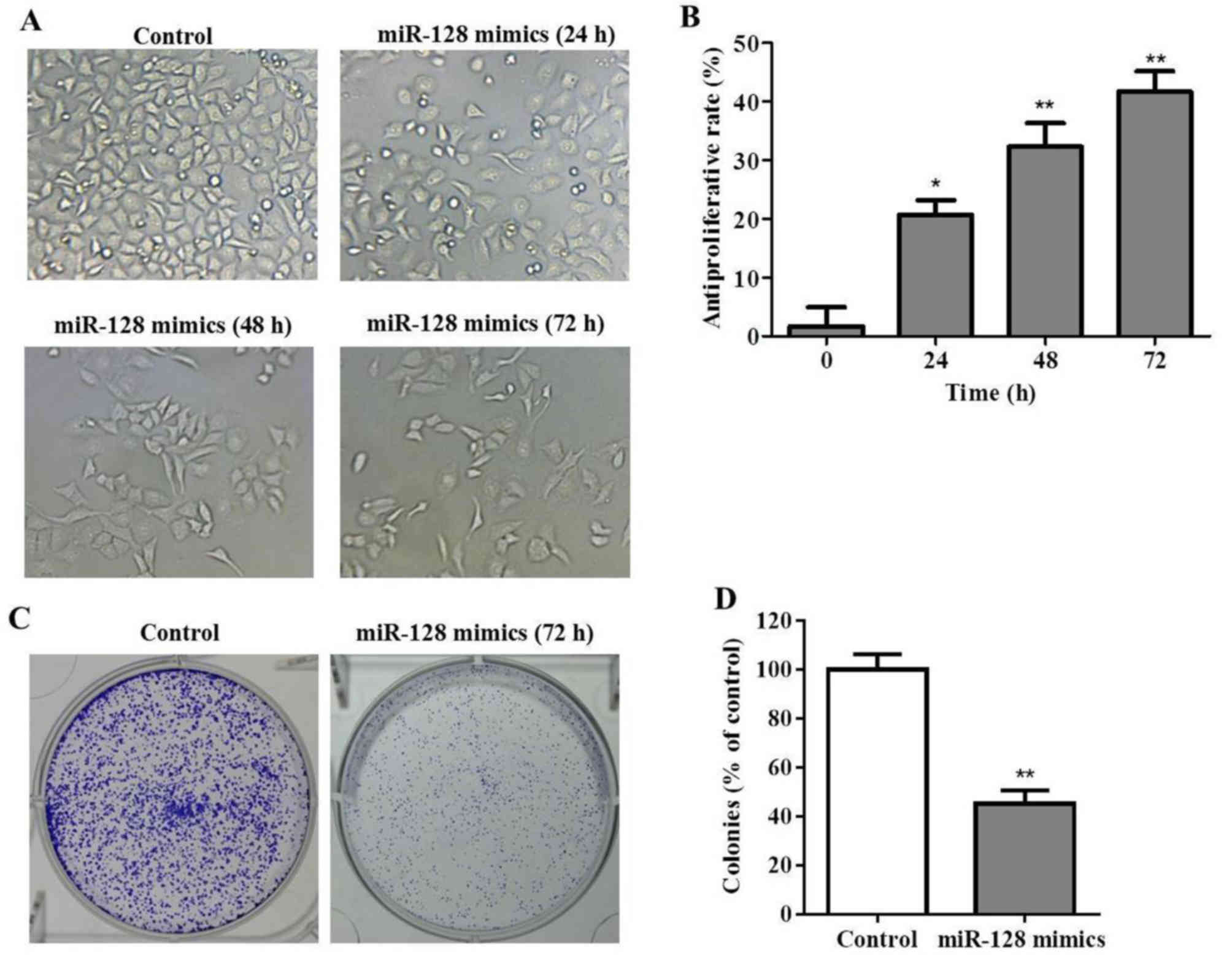
order to investigate the biological role of miR-128 in PC cells,
miR-128 mimics or mock vectors were transiently transfected into
MIA PaCa-2 cells. Subsequently, PC cell viability was determined
with a CCK-8 assay at various time points post-transfection. As
presented in Fig. 2A and B, miR-128
inhibited the growth of MIA PaCa-2 cells. The cell morphology and
density observed under the microscope, reduced cell proliferation
was observed within the miR-128 mimics group, particularly at 48
and 72 h post-transfection. In addition, cell morphologies were
round instead of epithelial indicating that miR-128 may not only
inhibit cell growth, but may also induce cell apoptosis.
Furthermore, the result of the colony formation assay indicated
that colony formation was significantly inhibited by miR-128 mimics
transfection (P<0.01; Fig. 2C and
D).
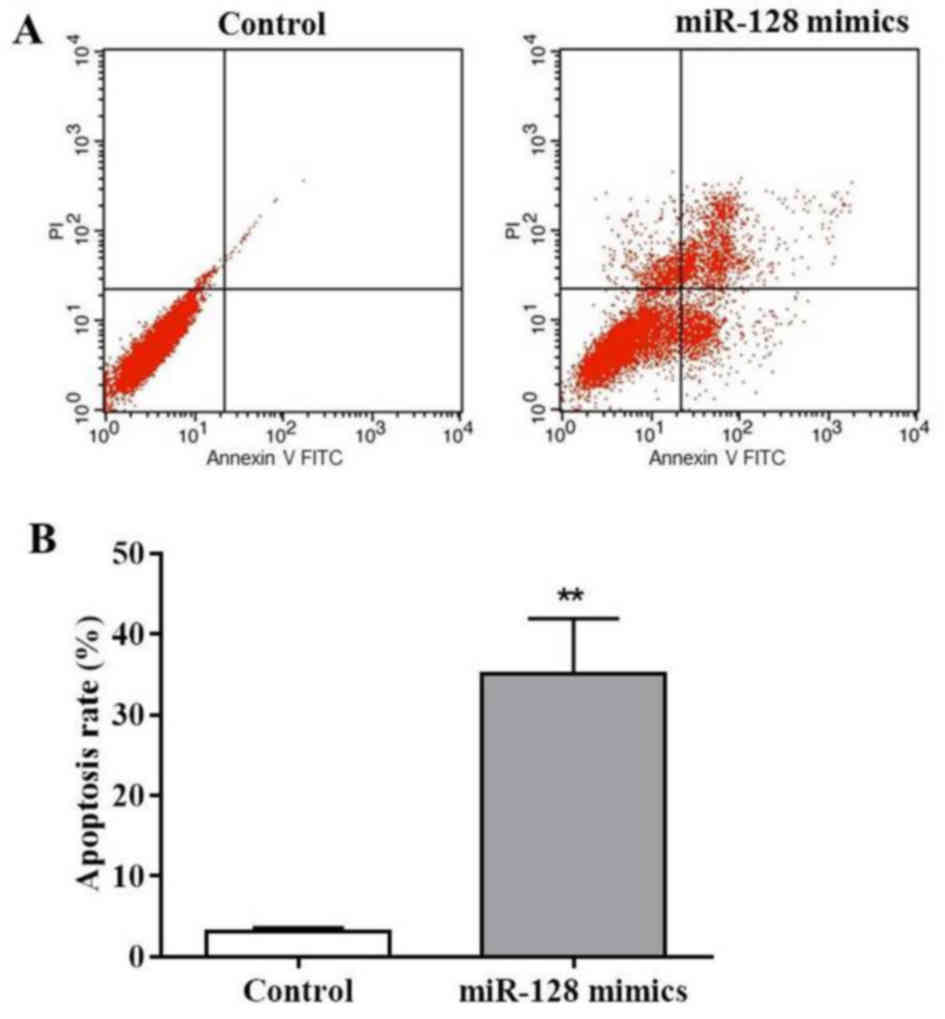
miR-128 induced apoptosis in PC
cells
As observed in Fig.
2A, MIA PaCa-2 cells revealed apoptotic characteristics
following transfection with miR-128 mimics. Thus, flow cytometry
analysis was performed to investigate the proportion of apoptotic
cells in the miR-128 mimics-transfected cells using Annexin V-FITC
and PI staining. The results of the present study demonstrated that
miR-128 mimics markedly increased the early apoptotic cell number
(Annexin V-FITC+/PI-) and late apoptotic cell number (Annexin
V-FITC+/PI+; Fig. 3A). The apoptotic
rate following miR-128 mimics transfection was 32.1%, which was
significantly increased compared with in the control (P<0.01;
Fig. 3B). These results demonstrated
that miR-128 may induce MIA PaCa-2 cell apoptosis.
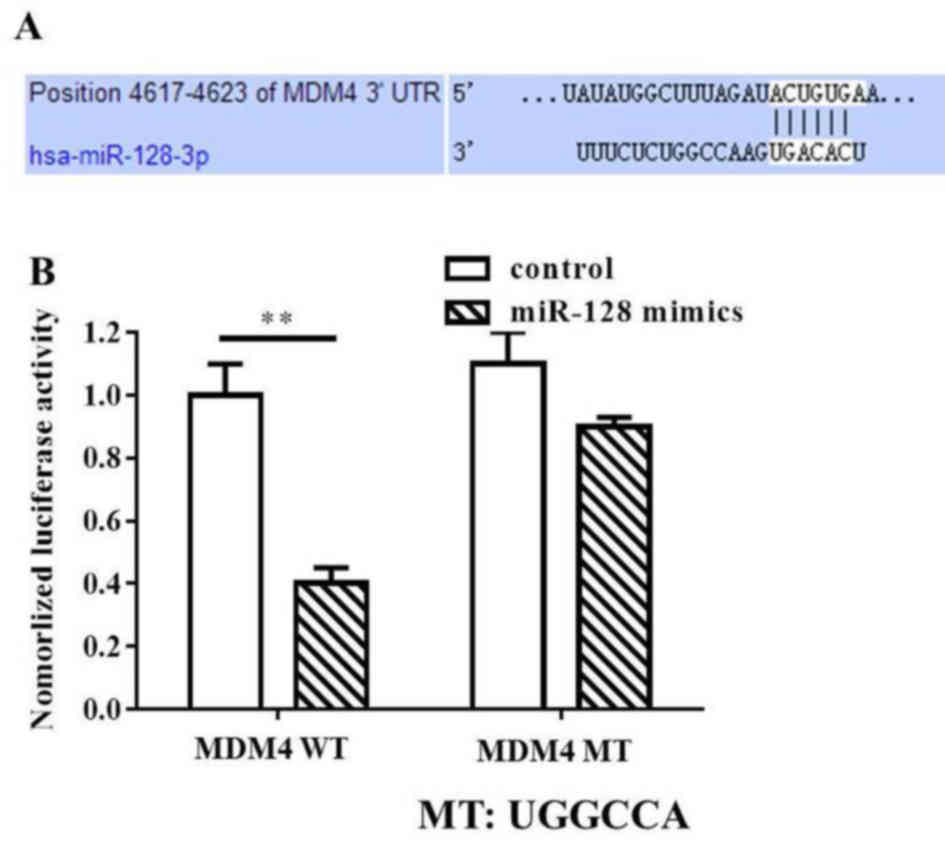
miR-128 directly targets MDM4
To investigate the underlying mechanisms of
miR-128-mediated inhibition of MIA PaCa-2 cell growth and apoptosis
induction, bioinformatics analysis was performed to identify the
targets of miR-128 in humans. MDM4 was of particular interest among
all the potential targets due to the high prediction scores in
TargetScan (http://www.targetscan.org/vert_61/), and microRNA.org (http://www.microrna.org/microrna/) online
bioinformatics methods. Therefore, the following experiments were
performed to confirm whether MDM4 was a direct target of miR-128.
In the present study, wild-type and mutant MDM4-3′-UTR expression
plasmids were constructed, which were fused with a luciferase
reporter gene (Fig. 4A). The results
indicated that the miR-128 mimics significantly inhibited the
luciferase activity of cells transfected with wild-type MDM4
(P<0.01). However, no significant variations between the control
and miR-128 mimics treatment were observed with mutant MDM4
(Fig. 4B). Thus, the findings of the
present study suggested that miR-128 may directly target MDM4.
miR-128 induces apoptosis in PC cells
via upregulation of p53 expression
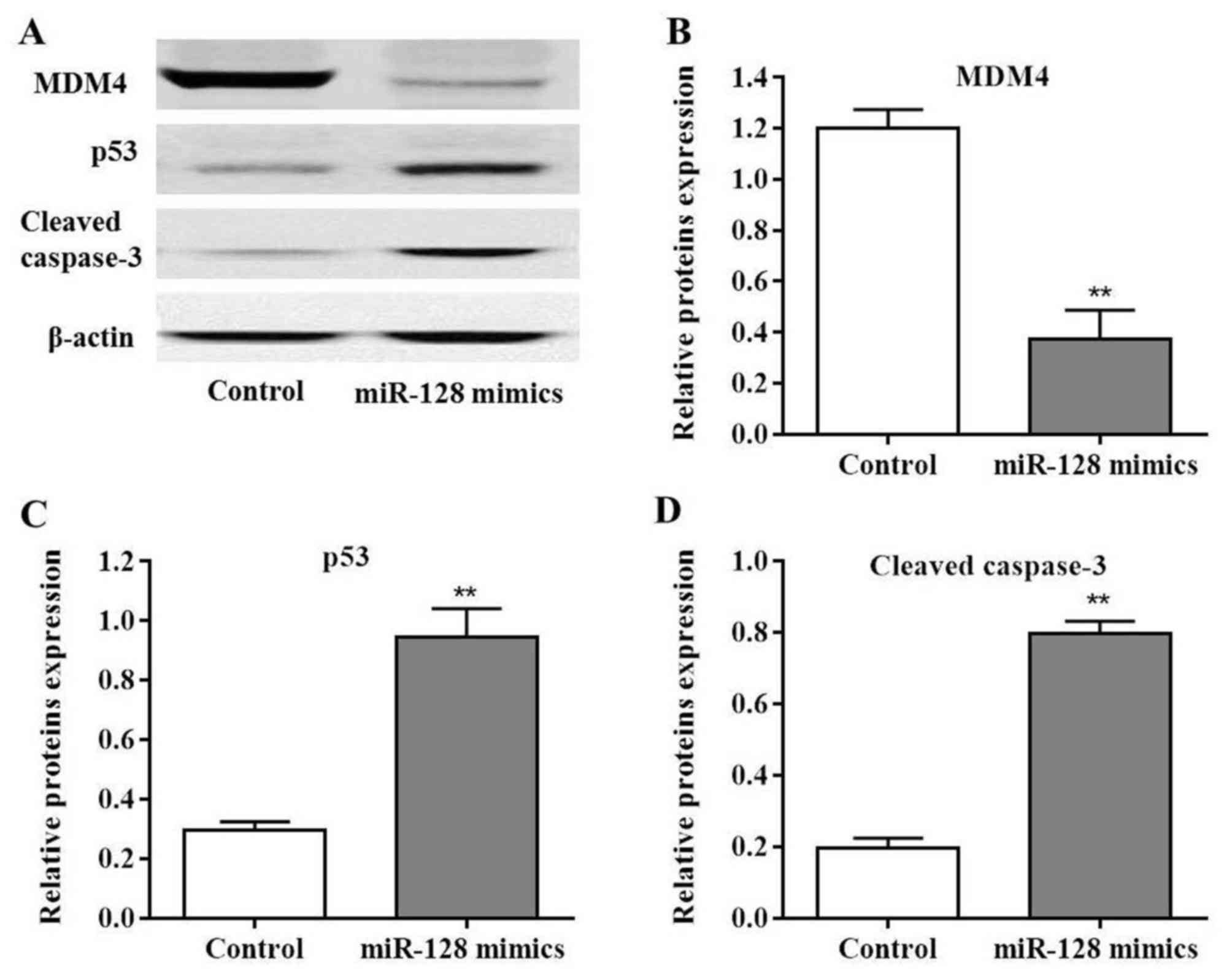
As MDM4 was considered to be the target of miR-128,
MDM4 protein expression was measured in PC cells following
transfection with miR-128 mimics. MDM4 levels were significantly
decreased post-transfection compared with the control (P<0.01;
Fig. 5A and B). Additionally,
miR-128 may induce MIA PaCa-2 cell apoptosis; in order to further
determine the possible mechanisms involved in this process,
molecules were selected that have been reported to be involved in
the caspase signaling pathway. The results of the present study
indicated that the expression levels of p53 and cleaved caspase-3
protein were significantly upregulated in MIA PaCa-2 cells
post-transfection with miR-128 mimics compared with the control
(P<0.01; Fig. 5C and D).
Collectively, these results indicated that miR-128 promoted MIA
PaCa-2 cell apoptosis via the p53 and caspase-3 dependent
pathway.
Discussion
The role of miR-128 has been discussed in various
types of cancer as its expression may be suppressed at the mRNA
level (10,15). It has also been reported that miR-128
is decreased in PC and may inhibit PC cell proliferation (26); however, further investigation is
required to understand the mechanisms of miR-128 in the regulation
of PC cell growth (10,27,28).
The present study clarified one of the biological
roles of miR-128 in regulating PC cell growth. Compared with in
adjacent normal tissue, miR-128 expression levels were
significantly downregulated in pancreatic cancer tissues.
Additionally, miR-128 may significantly inhibit MIA PaCa-2 cell
growth in functional experiments. miR-128 was indicated to induce
cell apoptosis, as demonstrated by flow cytometry analysis. To the
best of our knowledge, the present study is the first to report the
induction of cell apoptosis by miR-128 in PC.
In the present study, MDM4 was identified as the
direct target of miR-128 using bioinformatics analysis (29). miR-128 may bind to the MDM4 3′UTR via
base pairing. Based on this knowledge, the dual luciferase assay
demonstrated that restored miR-128 expression levels could decrease
luciferase activity, whereas no alterations were observed in the
luciferase activity with mutant MDM4 3′UTR. In addition, analysis
of protein expression levels indicated that miR-128 decreased MDM4
protein expression. Furthermore, along with decreased MDM4 protein,
significant increases in p53 and cleaved caspase-3 protein
expression levels were observed. The results of the present study
suggested that miR-128 may regulate the expression of MDM4 by
directly binding to the 3′UTR of MDM4. Therefore, the increased p53
expression promoted the caspase-3 dependent cell apoptosis
program.
In conclusion, the results of the present study
demonstrate that miR-128 may inhibit the proliferation of
pancreatic cancer cells by targeting MDM4 to induce cell apoptosis.
These findings add to the current understanding of miR-128 in
pancreatic cancer cells and may have potential applications in the
development of treatment strategies for pancreatic cancer. However,
the detailed mechanism of MDM4 in the pathogenesis of pancreatic
cancer requires further investigation.
Acknowledgements
The authors would like to thank Dr Hongli Wang (The
Third People's Hospital, Yancheng, China) for bioinformatics
analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HH managed the experimental design, tissue
collection, the execution of experiments and was the major
contributor in developing the first draft of the manuscript. LW
analyzed and interpreted the patient data. JX reviewed and approved
the final version of the manuscript. AW reviewed and approved the
final draft of the manuscript prior to submission.
Ethics approval and consent to
participate
Ethical approval for the present study was received
from the Clinical Research and Ethics Committee at The Third
People's Hospital (Yancheng, China).
Consent for publication
All patients provided written informed consent for
the publication of all associated data in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vaz J, Ansari D, Sasor A and Andersson R:
SPARC: A potential prognostic and therapeutic target in pancreatic
cancer. Pancreas. 44:1024–1035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spadi R, Brusa F, Ponzetti A, Chiappino I,
Birocco N, Ciuffreda L and Satolli MA: Current therapeutic
strategies for advanced pancreatic cancer: A review for clinicians.
World J Clin Oncol. 7:27–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin QJ, Yang F, Jin C and Fu DL: Current
status and progress of pancreatic cancer in China. World J
Gastroenterol. 21:7988–8003. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Puleo F, Maréchal R, Demetter P, Bali MA,
Calomme A, Closset J, Bachet JB, Deviere J and Van Laethem JL: New
challenges in perioperative management of pancreatic cancer. World
J Gastroenterol. 21:2281–2293. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis JL, Pandalai PK, Ripley RT, Langan
RC and Avital I: Expanding surgical treatment of pancreatic cancer:
The role of regional chemotherapy. Pancreas. 41:678–684. 2014.
View Article : Google Scholar
|
|
6
|
Rachagani S, Macha MA, Heimann N,
Seshacharyulu P, Haridas D, Chugh S and Batra SK: Clinical
implications of miRNAs in the pathogenesis, diagnosis and therapy
of pancreatic cancer. Adv Drug Deliv Rev. 81:16–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tempero MA, Arnoletti JP, Behrman SW,
Ben-Josef E, Benson AB III, Casper ES, Cohen SJ, Czito B, Ellenhorn
JD, Hawkins WG, et al: Pancreatic Adenocarcinoma, version 2.2012:
Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw.
10:703–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herman JM, Wild AT, Wang H, Tran PT, Chang
KJ, Taylor GE, Donehower RC, Pawlik TM, Ziegler MA, Cai H, et al:
Randomized phase III multi-institutional study of TNFerade biologic
with fluorouracil and radiotherapy for locally advanced pancreatic
cancer: Final results. J Clin Oncol. 31:886–894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones S, Zhang X, Parsons DW, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et
al: Core signaling pathways in human pancreatic cancers revealed by
global genomic analyses. Science. 321:1801–1806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hao J, Zhang S, Zhou Y, Hu X and Shao C:
MicroRNA 483-3p suppresses the expression of DPC4/Smad4 in
pancreatic cancer. FEBS Lett. 585:207–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan S, Ansarullah DK, Kumar D, Jaggi M
and Chauhan SC: Targeting microRNAs in pancreatic cancer:
Microplayers in the big game. Cancer Res. 73:6541–6547. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu J, Li A, Hong SM, Hruban RH and Giggins
M: MicroRNA alterations of pancreatic intraepithelial neoplasias.
Clin Cancer Res. 18:981–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ali S, Saleh H, Sethi S, Sarkar FH and
Philip PA: MicroRNA profiling of diagnostic needle aspirates from
patients with pancreatic cancer. Br J Cancer. 107:1354–1360. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang J, Liu J, Chen-Xiao K, Zhang X, Lee
WN, Go VL and Xiao GG: Advance in microRNA as a potential biomarker
for early detection of pancreatic cancer. Biomark Res. 4:202016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma MZ, Kong X, Weng MZ, Cheng K, Gong W,
Quan ZW and Peng CH: Candidate microRNA biomarkers of pancreatic
ductal adenocarcinoma: Meta-analysis, experimental validation and
clinical significance. J Exp Clin Cancer Res. 32:712013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He H, Hao SJ, Yao L, Yang F, Di Y, Li J,
Jiang YJ, Jin C and Fu DL: MicroRNA-218 inhibits cell invasion and
migration of pancreatic cancer via regulating ROBO1. Cancer Biol
Ther. 15:1333–1339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Hao J, Xie F, Hu X, Liu C, Tong
J, Zhou J, Wu J and Shao C: Downregulation of miR-132 by promoter
methylation contributes to pancreatic cancer development.
Carcinogenesis. 32:1183–1189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Munding JB, Adai AT, Maghnouj A, Urbanik
A, Zöllner H, Liffers ST, Chromik AM, Uhl W, Szafranska-Schwarzbach
AE, Tannapfel A and Hahn SA: Global microRNA expression profiling
of microdissected tissues identifies miR-135b as a novel biomarker
for pancreatic ductal adenocarcinoma. Int J Cancer. 131:E86–E95.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao C, Zhang J, Zhang S, Yu D, Chen Y,
Liu Q, Shi M, Ni C and Zhu M: Diagnostic and biological
significance of microRNA-192 in pancreatic ductal adenocarcinoma.
Oncology Rep. 30:276–284. 2013. View Article : Google Scholar
|
|
21
|
Zhang J, Zhao CY, Zhang SH, Yu DH, Chen Y,
Liu QH, Shi M, Ni CR and Zhu MH: Upregulation of miR-194
contributes to tumor growth and progression in pancreatic ductal
adenocarcinoma. Oncol Re. 31:1157–1164. 2014. View Article : Google Scholar
|
|
22
|
Abue M, Yokoyama M, Shibuya R, Tamai K,
Yamaguchi K, Sato I, Tanaka N, Hamada S, Shimosegawa T, Sugamura K
and Satoh K: Circulating miR-483-3p and miR-21 is highly expressed
in plasma of pancreatic cancer. Int J Oncol. 46:539–547. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marine JC and Jochemsen AG: Mdmx as an
essential regulator of p53 activity. Biochem Biolphys Res Commun.
331:750–760. 2005. View Article : Google Scholar
|
|
24
|
Gospodarowicz MK, Brierley JD and
Wittekind C: TNM Classification of Malignant Tumours. 2017.
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delata Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner
MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ and Schmittgen
TD: Expression profiling identifies microRNA signature in
pancreatic cancer. Int J Cancer. 120:1046–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Szafranska AE, Davison TS, John J, Cannon
T, Sipos B, Maghnouj A, Labourier E and Hahn SA: MicroRNA
expression alterations are linked to tumorigenesis and
non-neoplastic processes in pancreatic ductal adenocarcinoma.
Oncogene. 26:4442–4452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Li M, Wang H, Fisher WE, Lin PH,
Yao Q and Chen C: Profiling of 95 microRNAs in pancreatic cancer
cell lines and surgical specimens by real-time PCR analysis. World
J Surg. 33:698–709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee KH, Lee JK, Choi DW, Do IG, Sohn I,
Jang KT, Jung SH, Heo JS, Choi SH and Lee KT: Postoperative
prognosis prediction of pancreatic cancer with seven microRNAs.
Pancreas. 44:764–768. 2015. View Article : Google Scholar : PubMed/NCBI
|