Introduction
Primary open angle glaucoma (POAG) is an optic
neuropathy in which high intraocular pressure (IOP) may result in
optic nerve damage, subsequently leading to peripheral or central
visual field loss (1). The increased
IOP in POAG is caused by an increase in aqueous outflow resistance
in the drainage pathways, rather than by excess secretion of
aqueous humor (2). In the classical
aqueous outflow pathway of normal eyes, 75% of the outflow
resistance is located in the trabecular meshwork (TM) and 25% is
located in Schlemm's canal (SC) (3).
Surgical treatment of POAG is currently mostly aimed
at decreasing the abnormal outflow resistance, with different
surgical approaches focused on different sites of the drainage
pathway. Selective laser trabeculoplasty (SLT) was first presented
by Latina and Park (4) in 1995. It
was designed to selectively target pigmented cells in the TM in
order to lower the resistance in the TM, while sparing adjacent
cells and tissues from thermal damage and maintaining the TM
architecture (5,6). It is an effective, non-invasive
IOP-lowering procedure for patients with POAG in whom the natural
aqueous outflow system is still intact. Certain studies have
revealed that SLT results in a >20% reduction in IOP (7,8).
However, SLT treatment is not uniformly effective in all eyes
(9,10). Identifying patients who are most
likely to benefit from the application of SLT is crucial.
SC is comprised of endothelial cells surrounded by
connective tissue, similar to a vein. In 1914, Salzmann first
described that blood reflux in SC was seen ongonioscopy after
applying gentle pressure on the globe of normal eyes (11). This blood reflux may reflect the
drainage resistance. Previous studies have indicated that, if blood
reflux into the canal is clearly seen through the TM of POAG
patients, better results may be achieved by canaloplasty (12,13).
However, to date, no method for the evaluation of the functional
status of SC prior to SLT has been described.
The aim of the present study was to investigate the
association between the blood reflux in SC observed during
gonioscopy and the decrease of IOP after SLT, in order to provide
clinical insight into its application for the prediction of SLT
outcomes.
Materials and methods
Subjects
The present study adhered to the tenets of the
Declaration of Helsinki. The study was approved by the
institutional ethics committees of Tongji Hospital, Huazhong
University of Science and Technology (Wuhan, China; Trial approval
no. K-2014-013). Written informed consent was obtained from each
patient prior to the start of the study.
This prospective cohort study sequentially recruited
patients from the ophthalmology clinics of Tongji Hospital
(Huazhong University of Science and Technology, Wuhan, China), from
March 2015 to November 2015. Patients with severe refractive medium
opacity, high intraocular pressure, dense trabecular pigmentation
preventing clear observation of blood reflux, severe systemic and
mental disease, or who had undergone any ocular surgery were
excluded.
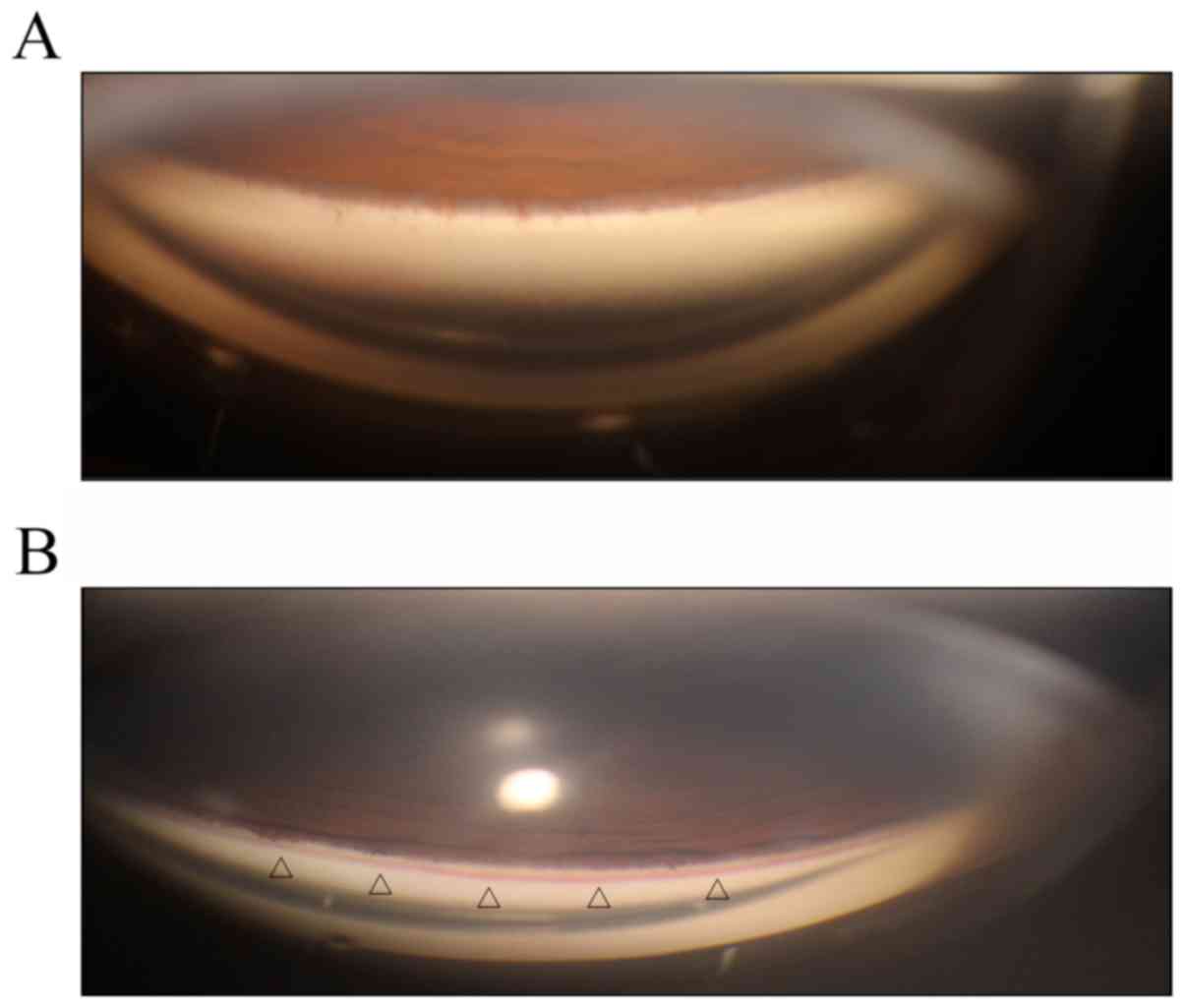
Surgical technique
All eyes underwent gonioscopy prior to SLT. During
gonioscopy, slight pressure was applied to the viewed quadrant to
raise the episcleral venous pressure and draw the blood back into
SC. Depending on the presence (positive group) or absence (negative
group) of blood reflux, patients were divided into two groups
(Fig. 1). The positive group was
comprised of 21 eyes and the negative group included 14 eyes. In
the positive group, the observation of blood reflux was recorded in
quadrants 1, 2, 3 and 4, based on the appearance of blood on the
trabeculum in each quadrant seen using the Haag-Streit-Goldmann
three-mirror lens.
To estimate the group size, a pilot study was
performed for measuring the IOP at 1 month after SLT in 10 eyes of
10 patients with uncontrolled POAG. The standard deviation of the
reduction of the IOP in this group was 3.90 mmHg. The present study
aimed to measure a difference in IOP of 4 mmHg among the groups. To
achieve a two-tailed α=0.05 and a statistical power of 80%, at
least 12 patients per group were required. Considering a compliance
rate of 90%, the present study eventually enrolled 35 eyes of 25
patients with POAG, including 21 eyes in the positive group and 14
eyes in the negative group. The sample size in the two groups was
suitable for the statistical equation and was acceptable according
to the requirements of the statistics. The essential information
and the baseline (pre-SLT) IOP of the patients are presented in
Table I.
 | Table I.Baseline data in all patients. |
Table I.
Baseline data in all patients.
| Parameter | Positive group
(n=21) | Negative group
(n=14) | P-value |
|---|
| Age (years) | 44.60±15.0 | 51.0±11.9 | 0.428 |
| Right eye n (%) | 7 (34) | 5 (36) | 0.889 |
| Left eye n (%) | 14 (66) | 9 (64) | / |
| Pre-SLT IOP
(mmHg) | 20.2±4.2 | 21.0±5.3 | 0.792 |
| Blood reflux
quadrants (N) |
2.2±0.8 | / | / |
All patients underwent a single 360° SLT session
that was performed by a single surgeon (HZ). After discussion, the
investigators agreed on a standardized laser technique to minimize
the variation in power, number and location of laser spots. An
initial laser energy of 0.6 mJ was applied and was then adjusted
until bubble formation was just visible. After SLT, the subjects
continued with the same IOP-lowering treatment, and were prescribed
Pranoprofen eye drops (Pranopulin, Senju Pharmaceutical Co., Ltd.,
Osaka, Japan) three times a day for 5 days (14).
After the surgery, the IOP was measured at 1 and 2
weeks, and at 1, 3 and 6 months after SLT. All IOP measurements
were performed via non-contact tonometry by a single investigator
(JG) who was blinded to the specific group of the patients. The
measurements were taken at least three times in every eye and the
average was taken. Patients returned for follow-up at approximately
the same time of day (9–11 am) to minimize the effect of diurnal
IOP fluctuation. The number of medications was tapered according to
the level of IOP measured after SLT in order to maintain the target
IOP.
Statistical analysis
Statistical analysis was all performed using SPSS
version 19 (IBM Corp., Armonk, NY, USA) and values for the study
population were expressed as the mean ± standard deviation.
Intra-group and inter-group comparisons of the IOP were performed
using generalized estimation equations (GEE), which take the
correlation between right and left eye into consideration (15). Spearman's correlation was used to
evaluate the association between blood reflux quadrants and IOP
after SLT.
Results
SLT causes an obvious decrease in
IOP
The pre-operative mean IOP served as the baseline
IOP, and was 20.2±4.2 mmHg in the positive group and 21.0±5.3 mmHg
in the negative group (Table I).
Compared with the baseline, the positive and the negative group
exhibited a decrease in IOP at 1 and 2 weeks, and at 1 and 3 months
after the SLT. In the negative group, the IOP at 6 months after SLT
was not significantly different from that at baseline (P=0.066),
while the positive group still exhibited a significant decrease in
IOP compared with the baseline (P<0.001; Table II).
 | Table II.Data of IOP in the positive group and
the negative group at different time-points. |
Table II.
Data of IOP in the positive group and
the negative group at different time-points.
|
| Positive group | Negative group |
|---|
|
|
|
|
|---|
| Time-point | IOP (mmHg) | β (95%CI) | P-valuea | IOP (mmHg) | β (95%CI) | P-valuea |
|---|
| Pre | 20.2±4.2 | / | / | 21.0±5.3 | / | / |
| 1 w | 15.1±4.2 | −5.095
(−6.678,-3.513) | <0.001 | 16.1±3.5 | −4.929
(−7.140,-2.717) | <0.001 |
| 2 w | 14.8±3.3 | −5.462
(−7.411,-3.513) | <0.001 | 16.2±2.6 | −4.800
(−7.100,-2.500) | <0.001 |
| 1 m | 13.9±2.7 | −6.310
(−8.524,-4.095) | <0.001 | 16.8±3.6 | −4.236
(−6.909,-1.563) | 0.002 |
| 3 m | 13.7±2.1 | −6.538
(−8.458,-4.618) | <0.001 | 15.8±2.4 | −5.236
(−7.910,-2.562) | <0.001 |
| 6 m | 15.1±3.4 | −5.131
(−6.658,-3.603) | <0.001 | 17.8±3.0 | −3.309
(−6.843,-0.225) | 0.066 |
Blood reflux in SC is associated with
a greater decrease in IOP after SLT
The decrease in IOP was different between the two
groups. The two groups were also different in terms of age and
gender. When the age and gender were not adjusted, the positive
group only exhibited a significantly greater decrease in IOP
compared with that in the negative group at 1 and 3 months after
SLT (P=0.024 and 0.018, respectively). After those two variables
were adjusted by GEE, the positive group exhibited a greater
decrease in IOP after SLT at 1, 3 and 6 months (P=0.014, 0.013 and
0.045, respectively; Table
III).
 | Table III.Comparison between the IOP of the
positive and the negative group. |
Table III.
Comparison between the IOP of the
positive and the negative group.
|
| Unadjusted | Adjusted |
|---|
|
|
|
|
|---|
| Time-point | β (95%CI) | P-value | β (95%CI) | P-value |
|---|
| Pre | −0.762
(−4.862,3.338) | 0.716 | −0.890
(−4.837,3.056) | 0.659 |
| 1 w | −0.929
(−3.753,1.895) | 0.519 | −1.148
(−3.665,1.369) | 0.371 |
| 2 w | −1.424
(−3.585,0.737) | 0.197 | −1.589
(−3.696,0.518) | 0.139 |
| 1 m | −2.836
(−5.290,-0.382) | 0.024 | −2.909
(−5.238,-0.580) | 0.014 |
| 3 m | −2.064
(−3.777,-0.351) | 0.018 | −2.149
(−3.836,-0.462) | 0.013 |
| 6 m | −2.625
(−5.484,0.234) | 0.072 | −2.863
(−5.667,-0.059) | 0.045 |
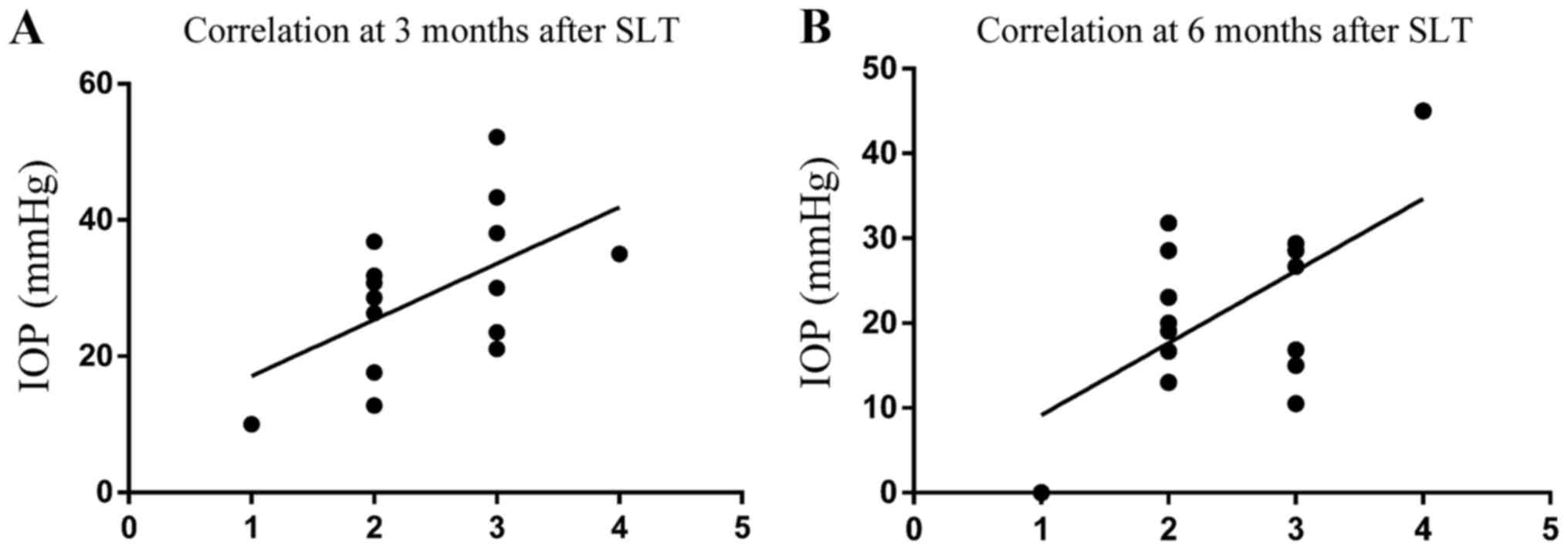
Positive correlation between the
number of quadrants with blood reflux and IOP decrease after
SLT
After 360° SLT, the reflux-positive group clearly
presented with a more marked reduction in IOP than the negative
group. To further investigate this, a correlation analysis was used
to analyze the association between the extent of decrease in IOP
and the number of quadrants in which blood reflux appeared in SC.
(Fig. 2). At 3 and 6 months, a
strongly positive correlation between the number of quadrants with
blood reflux in SC and the percentage decrease in IOP was
identified (P=0.037 and 0.020, respectively).
Discussion
In the present prospective observational study, POAG
patients with blood reflux in SC exhibited a greater decrease in
IOP after SLT and the number of quadrants with blood reflux was
positively correlated with the decrease.
IOP is regarded as an important factor in the
progression of POAG (16). Almost
70% of the aqueous hum or outflow occurs via the anterior chamber
to the TM, SC and collector channels, entering the systemic venous
circulation through the episcleral veins (17). Studies on the aqueous outflow
pathways have revealed that, in humans, 75% of the resistance to
the aqueous humor outflow is localized to the TM, and 25% to SC,
the outer wall of SC or the tissue surrounding SC (18,19). As
an abnormal increase of the outflow resistance may cause an
increase in IOP, the development of a novel surgical treatment for
glaucoma must involve the search for how to reduce IOP, and more
specifically, to reduce the outflow resistance in glaucoma.
Numerous surgeries are available that target the
sites of the outflow resistance, including trabeculectomy,
viscocanalostomy, argon laser trabeculoplasty and SLT (5). Surgeries designed to incise or remove
the abnormal TM in glaucoma, e.g., trabeculectomy, address the
abnormal resistance in the TM, while other surgeries target SC by
unroofing or expanding the canal, e.g., viscocanalostomy (20,21). SLT
is based on the principle of selective thermolysis, in which laser
energy applied to the TM selectively targets pigmented cells
without causing significant collateral thermal damage (6). In the present study, SLT resulted in a
20.1–30.4% reduction in IOP. Thus, the effect exhibited a great
variation in patients with POAG. Numerous factors have been
investigated, but none of them has been identified as a significant
predictor of successful treatment, including age, sex, race,
glaucoma type, TM pigmentation, angle grade, lens status and
central corneal thickness (22). As
the SLT mainly reduces the resistance located in the TM, it is
suspected that the resistance originating from SC may be an
important factor for determining the difference between the
positive and the negative group.
Blood is ordinarily visible in the SC by gonioscopy.
The three most common reasons are ocular hypotony, high episcleral
venous pressure and compression of the episcleral veins by the
examiner's goniolens (23,24). Grieshaber (25) observed blood reflux into SC in eyes
with POAG by using provocative gonioscopy, and identified a
negative correlation between blood reflux and IOP, with a lower IOP
inducing better filling of SC. Furthermore, Gong and Francis
(26) demonstrated that impaired and
interrupted blood reflux into SC was consistent with a decrease in
the number of quadrants with fluorescein egress into the episcleral
veins via collector channels, and that blockage of the collector
channel ostia occurred in patients with POAG. It is reasonable to
assume that prompt reflux of blood into the canal and good
episcleral filling reflects a patent canal and healthy collector
channels, and suggests that the major problem of outflow resistance
lies in the TM.
Although blood regurgitation occurs in the reverse
direction of the natural aqueous humour passage, it may indicate
that the canal is not collapsed, or at least that there are no
adhesions present between the outer and inner wall. This also
indicates that it is unlikely that resistance to aqueous outflow is
present in the distal outflow facility beyond the SC. This allows
the blood to fill the canal, which may obviously occur through the
TM (the site that most likely contributes to aqueous outflow
resistance). Consequently, a significant reduction in IOP is
achieved after SLT, particularly in eyes with complete
circumferential filling. The present study analysed the association
between the number of quadrants in which blood reflux appeared and
the decrease in IOP. The clear correlation between the quadrants
with blood reflux in SC and the percentage decrease in IOP strongly
supports this assumption. However, the present study had certain
limitations. A total of 35 eyes from 25 POAG patients who presented
at our department between March 2015 and November 2015 were
included. This time window was short and the sample size was small.
The drugs used by the patients prior to and after the treatment
were recorded as part of the study, but it was not possible to
perform a quantitative analysis of the association between the drug
intake and the reduction of IOP after SLT. These factors require
further study.
In conclusion, the present study indicated that
blood reflux in SC had a favourable effect on the IOP after SLT and
the number of blood reflux quadrants was inversely correlated with
the post-SLT IOP. Blood reflux in SC may provide an indication of
the functional status of the SC and the distal outflow facility. As
the eyes with positive blood reflux had a more persistent
significant IOP reduction and higher success rates after SLT, this
diagnostic method may help to identify patients who may benefit the
most from SLT.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81770921).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JG and SA performed the experiments. YZ and HZ
conceived the study.
Ethical approval and consent to
participate
The study was approved by the institutional ethics
committees of Tongji Hospital, Huazhong University of Science and
Technology (Wuhan, China; Trial approval number, K-2014-013).
Informed consent was obtained from each patient prior to the start
of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Quigley HA: Glaucoma. Lancet.
377:1367–1377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tamm ER and Fuchshofer R: What increases
outflow resistance in primary open-angle glaucoma? Surv Ophthalmol.
52 Suppl 2:S101–S104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bill A and Svedbergh B: Scanning electron
microscopic studies of the trabecular meshwork and the canal of
Schlemm-an attempt to localize the main resistance to outflow of
aqueous humor in man. Acta Ophthalmol (Copenh). 50:295–320. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Latina MA and Park C: Selective targeting
of trabecular meshwork cells: In vitro studies of pulsed and CW
laser interactions. Exp Eye Res. 60:359–371. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson DH and Johnson M: How does
nonpenetrating glaucoma surgery work? Aqueous outflow resistance
and glaucoma surgery. J Glaucoma. 10:55–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leahy KE and White AJ: Selective laser
trabeculoplasty: Current perspectives. Clin Ophthalmol. 9:833–841.
2015.PubMed/NCBI
|
|
7
|
Waisbourd M and Katz LJ: Selective laser
trabeculoplasty as a first-line therapy: A review. Can J
Ophthalmol. 49:519–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Keyser M, De Belder M, De Belder S and
De Groot V: Where does selective laser trabeculoplasty stand now? A
review. Eye Vis (Lond). 3:102016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong MO, Lee JW, Choy BN, Chan JC and Lai
JS: Systematic review and meta-analysis on the efficacy of
selective laser trabeculoplasty in open-angle glaucoma. Surv
Ophthalmol. 60:36–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McAlinden C: Selective laser
trabeculoplasty (SLT) vs. other treatment modalities for glaucoma:
Systematic review. Eye (Lond). 28:249–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kronfeld PC: Further gonioscopic studies
on the canal of Schlemm. Arch Ophthal. 41:393–405. 1949. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grieshaber MC: Channelography and
mechanism of action in canaloplasty. Ophthalmologe. 112:319–324.
2015.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grieshaber MC, Pienaar A, Olivier J and
Stegmann R: Clinical evaluation of the aqueous outflow system in
primary open-angle glaucoma for canaloplasty. Invest Ophthalmol Vis
Sci. 51:1498–1504. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Francis BA, Loewen N, Hong B, Dustin L,
Kaplowitz K, Kinast R, Bacharach J, Radhakrishnan S, Iwach A,
Rudavska L, et al: Repeatability of selective laser trabeculoplasty
for open-angle glaucoma. BMC Ophthalmol. 16:1282016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li M, Song Y, Zhao Y, Yan X and Zhang H:
Influence of exercise on the structure of the anterior chamber of
the eye. Acta Ophthalmol. 96:e247–e253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coleman AL and Miglior S: Risk factors for
glaucoma onset and progression. Surv Ophthalmol. 53 Suppl 1:S3–S10.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhartiya S, Ichhpujani P and Shaarawy T:
Surgery on the trabecular meshwork: Histopathological evidence. J
Curr Glaucoma Pract. 9:51–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ethier CR, Coloma FM, Sit AJ and Johnson
M: Two pore types in the inner-wall endothelium of Schlemm's canal.
Invest Ophthalmol Vis Sci. 39:2041–2048. 1998.PubMed/NCBI
|
|
19
|
Johnson MC and Kamm RD: The role of
Schlemm's canal in aqueous outflow from the human eye. Invest
Ophthalmol Vis Sci. 24:320–305. 1983.PubMed/NCBI
|
|
20
|
Weinreb RN, Leung CK, Crowston JG,
Medeiros FA, Friedman DS, Wiggs JL and Martin KR: Primary
open-angle glaucoma. Nat Rev Dis Primers. 2:160672016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee DA and Higginbotham EJ: Glaucoma and
its treatment: A review. Am J Health Syst Pharm. 62:691–699.
2005.PubMed/NCBI
|
|
22
|
Kennedy JB, SooHoo JR, Kahook MY and
Seibold LK: Selective laser trabeculoplasty: An update. Asia Pac J
Ophthalmol (Phila). 5:63–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phelps CD, Asseff CF, Weisman RL, Podos SM
and Becker B: Blood reflux into Schlemm's canal. Arch Ophthalmol.
88:625–631. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Phelps CD: Arterial anastomosis with
Schlemm's canal: A rare cause of secondary open-angle glaucoma.
Trans Am Ophthalmol Soc. 83:304–315. 1985.PubMed/NCBI
|
|
25
|
Grieshaber MC: Ab externo Schlemm's canal
surgery: Viscocanalostomy and canaloplasty. Dev Ophthalmol.
50:109–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gong H and Francis A: Schlemm's Canal and
Collector Channels as Therapeutic TargetsSurgical Innovations in
Glaucoma. Samples JR and Ahmed IIK: Springer; New York, NY: pp.
3–25. 2014, View Article : Google Scholar
|