Introduction
Thyroid carcinoma (TC) is the most common type of
malignant endocrine tumor and its incidence has increased rapidly
in the past few decades (1,2). When treating TC patients with standard
therapies, including surgery, chemotherapy and radiotherapy, they
are likely to acquire a good prognosis; however, the prognosis for
patients with treatment resistance or tumor recurrence is poor
(3). TC is divided into
well-differentiated and undifferentiated types according to the
histopathological and clinical characteristics. The
well-differentiated types comprise papillary and follicular TC, and
affected patients have a good prognosis. However, anaplastic TC,
which are in the category of undifferentiated carcinoma, are
aggressive and usually lethal, while they are less common (4–6).
Although numerous studies have indicated that several important
molecular markers are associated with TCs, which are accountable
for the malignant transformation of thyroid cells, the biological
behavior of TC cells, as well as their progression (7), the detailed mechanisms remain to be
fully elucidated.
Sphingomyelin, cholesterol and other phospholipids
are important components of the cell membrane, act as bioactive
signaling molecules and have pivotal roles in TCs (8,9). Among
them, sphingosine-1-phosphate (S1P) is derived from sphingomyelin,
and not only functions as an intracellular second messengers to
regulate the cell cycle (10), but
also binds to the specific receptors on the cell surface to
regulate cell proliferation, apoptosis, invasion and migration
(5,10,11). In
addition, S1P also promotes cell survival in multiple malignancies
and simultaneously inhibits cell apoptosis, which is closely
associated with tumor formation (11,12).
Sphingosine kinase (SPHK) has a pivotal role in the
process of S1P synthesis. SPHK occurs in two isoforms in humans and
mice, SPHK1 and SPHK2. SPHK1 is predominantly located in the brain,
heart, lung, liver, spleen and hematopoietic immune system, and is
the most important molecule to regulate the generation of S1P
(9,13). Huang and Natarajan (14) reported that NIH 3T3 fibroblasts with
overexpression of SPHK1 acquired transformed phenotypes and that
tumorigenesis was promoted, which implied that SPHK1 has a marked
oncogenic activity. Numerous studies have also demonstrated that
the expression of SPHK1 was elevated in multiple human tumor types,
and was extensively involved in head and neck squamous cell
carcinoma (14), glioma (15), salivary gland carcinoma (16), prostate cancer (17), non-Hodgkin lymphomas (18), gastric cancer and papillary TC
(14–19). Furthermore, upregulation of SPHK1 in
certain cancer types is closely associated with a worse clinical
prognosis (20). Brünnert et
al (21) also indicated that
upregulation of SPHK1 expression induced epithelial growth factor
(EGF) receptor in gastric cancer via interaction with
lysophosphatidic acid (LPA) receptor. Subsequently, other studies
have reported that knockdown of SPHK1 inhibited the induction of
EGF in MCF7 cells and decreased the migration of 293 cells induced
by EGF (22). Taken together, these
studies implied that SPHK1/S1P may be a key regulator of tumor
invasion and metastasis. However, despite increasing evidence
demonstrating that SPHK1 is elevated in TC, the therapeutic
implications of SPHK1 and the associated molecular mechanisms have
remained largely elusive.
The present study first determined the expression of
SPHK1 in TC tissues and cell lines compared with that in paired
normal thyroid tissues and a normal thyroid cell line. Furthermore,
the effects of SPHK1 on the migration and invasion of TC cells were
assessed in vitro, and the association between the
expression of SPHK1 and the metastatic potential of TC cells was
explored in vivo. Subsequently, the underlying molecular
mechanisms were investigated. The present study provided an
experimental basis for the utilization of SPHK1 as a potential
therapeutic target in TC.
Materials and methods
Patients and samples
TC and adjacent normal tissues were collected from a
total of 53 patients who were diagnosed with TC and underwent
surgical resection at the Southwest Hospital of the Third Military
Medical University (Chongqing, China) between December 2015 and
December 2016. These patients had invasive tumors and the tumor
diameter was >2 cm. Samples were collected to ensure the safety
of patients. All collected tissue samples were snap-frozen and then
stored at −80°C. All of the patients provided informed consent and
the present study was approved by the medical ethics committee of
the Third Military Medical University (Chongqing, China).
Cell lines and reagents
The human TC cell lines SW579, TPC-1 and WRO, and
the normal thyroid cell line Nthy-ori 3–1 were obtained from the
American Type Culture Collection (Manassas, VA, USA), cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) and 100X
penicillin-streptomycin solution. The human K1 thyroid gland
papillary carcinoma cell line is a misidentified cell line that has
been reported to be a derivative of the GLAG-66 cell line of the
same cancer type (23), and was
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS and antibiotics.
The normal thyroid cell line Nthy-ori 3-1 was used as a normal
control. Cisplatin and doxorubicin were purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and dissolved in
dimethyl sulfoxide. S1P was purchased from Enzo Life Sciences
(Farmingdale, NY, USA). 5C, an inhibitor of SPHK1, was obtained
from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA). JTE013,
CAY10444 and TY52156 were purchased from MedChem Express (Monmouth
Junction, NJ, USA). LPA was obtained from Avanti Polar Lipids
(Alabaster, AL, USA). Anti-hairy and enhancer of split-1 (Hes1)
antibody (cat. no. ab49170) and anti-SPHK1 antibody (cat. no.
ab71700) were obtained from Abcam (Cambridge, UK), while rabbit
GAPDH monoclonal antibody (cat. no. 2118) was obtained from Cell
Signaling Technology Inc. (Danvers, MA, USA). Anti-Phospho-SPHK1
antibodies (cat. no. PAB16439) were purchased from Bio-Swamp Life
Science Lab (Wuhan, China). Notch1 intracellular domain (N1ICD)
antibody was obtained from EMD Millipore (Billerica, MA, USA).
Lentiviral transfection
The TC cell lines TPC-1 and WRO were seeded into
6-well plates at a density of 2×105 cells/well. One day
after adherence, cells were maintained in RPMI-1640 medium
containing 2% FBS. The siRNA was synthesized by Beyotime Institute
of Biotechnology (Haimen, China) with following sequences:
Lv-NC-siRNA: 5′-UUCUCCGAACGUGUCACGUTT-3′, Lv-SPHK1-siRNA#1: Sense
5′-GCAGCUUCCUUGAACCAUUTT-3′ and Lv-SPHK1-siRNA#2: Sense
5′-GUGCACCCAAACUACUUCUTT-3′. Transfection of siRNA was performed
with ViraPower™ (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's instructions (14). These cells were transfected with
Lv-NC-siRNA or Lv-SPHK1-siRNA at a multiplicity of infection of 20.
After 10 h of incubation, the medium was changed. The transfection
efficiency was determined as established by a previous study
(14) and cells were used for
further study.
Immunohistochemistry (IHC)
All TC tissue and normal adjacent samples were
submitted to the Department of Surgical Pathology of the Southwest
Hospital of the Third Military Medical University (Chongqing,
China) and examined by three experienced pathologists.
Paraffin-embedded blocks from each selected specimen were used for
IHC. Serial 4 mm sections were used for staining for SPHK1
(1:5,000) and p-SPHK1 (1:5,000). IHC were performed as previously
described (24).
Animal study of in vivo metastasis
formation
A total of 15 healthy 4-week-old NOD/SCID mice
(male, 20±1 g) were purchased from the Animal Center of the Third
Military Medical University (Chongqing, China). These mice were
collected and maintained in a pathogen-free environment at the
animal facility of the Third Military Medical University
(Chongqing, China) according to the Care and Use or Experimental
Animals (Permission no. SYXK-PLA-20120031). All procedures for
animal experiments were approved by the Laboratory Animal Welfare
and Ethics Committee of the Third Military Medical University
(Chongqing, China; no. 20151117083) and were performed in
accordance with institutional guidelines. The mice were randomly
divided into a control group (which received an equal quantity of
normal saline to the siRNAs), a lentiviral vector expressing
negative control (NC) small interfering (si)RNA (Lv-NC-siRNA) and
an Lv-SPHK1-siRNA#1 group. For the in vivo metastasis assay,
2×105 TPC-1 cells transfected with Lv-NC-siRNA or
Lv-SPHK1-siRNA#1 or control treatment were mixed with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA) at a ratio of 1:1 and then
injected into the spleen of the NOD/SCID mice (under 80 mg/kg
pentobarbital IV. anesthesia and with the abdomen open according to
general procedures) (18). After 6
weeks, all animals were sacrificed by carbon dioxide euthanasia (no
more than 30% of the chamber volume/minute) and imaged using an
in vivo Imaging System (Bio-Real in vivo imaging
system; QuickView3000; Bio-Real Sciences; LABATECH GmbH; Salzburg,
Austria) to examine nodule tumor metastasis in different organs and
to assess the weight of tumors.
Measurement of SPHK1 activity
The activity of SPHK1 was measured with a commercial
SPHK1 Activity Assay kit (cat. no. KA0906; Abnova, Taipei, Taiwan)
according to the manufacturer's instructions.
Measurement of S1P
An S1P competitive ELISA kit (cat. no, K-1900,
Echelon Bioscience, Inc., Salt Lake City, UT, USA) was used for
detecting S1P levels. Cells were seeded onto 6-well plates at
105 cells/well without FBS and allowed to attach for 12
h. Subsequently, the cells were pre-treated with inhibitors for
another 15 min prior to stimulation with 10% FBS for 6 h. The
supernatant was collected for S1P analysis according to
manufacturer's instructions.
Cell viability assay
The Cell Counting Kit-8 (CCK-8) assay was used for
determining the viability of TC cells. TPC-1 and WRO cells were
seeded onto 96-well plates at a density of 1×104
cells/well (attached prior to the drug treatment) and then treated
with cisplatin or doxorubicin at 10 µM for 48 h. CCK-8 solution was
added into 96-well plates at the end of the experiment, followed by
incubation for 3 h at 37°C. The absorbance at 450 nm was read with
a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
and the cell viability relative to the control group was
calculated. The experiment was repeated 3 times following the first
experiment.
Western blot analysis
Treated TPC-1 TC cells were collected and lysed with
ultrasonication (VOSHIN96-II; Wuxi Voshin Instruments Co., Ltd.,
Wuxi, China) on ice for 30 min (rest for 5 sec every 5 sec),
followed by centrifugation of the lysate. The protein concentration
in the supernatant was determined using a BCA assay. For each
sample, 20 µg protein was loaded, subjected to 6% SDS-PAGE and
transferred onto PVDF membranes (cat. no. AR0136-04, Whuan Boster
Biological Technology ltd., Wuhan, China). Membranes were blocked
for 0.5 h at room temperature using TBST with 5% skimmed milk and
then incubated with Hes1 (1:5,000), N1ICD (1:5,000) and GAPDH
(1:10,000) antibody at 4°C for overnight. Subsequently, the
membranes were incubated with secondary antibody (horseradish
peroxidase conjugated Anti-Mouse Immunoglobulin G H&L; cat. no.
ab6789, Abcam) for 2 h at room temperature. The signals were
detected by enhanced chemiluminescence (Pierce; Thermo Fisher
Scientific, Inc.). GAPDH was regarded as an internal control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA was isolated with TRIzol (cat no.
12183555, Invitrogen; Thermo Fisher Scientific, Inc.) from the
tissues of TC patients and cell lines according to the
manufacturer's protocols. The concentration and integrity total RNA
was determined with a NanoDrop-1000 (Thermo Fisher Scientific,
Inc.) and by electrophoresis, respectively. A reverse transcription
kit (cat. no.: A5001, Promega Corp., Madison, WI, USA) was used for
synthesis of the first-strand complementary (c)DNA. Real-time qPCR
was used to amplify and quantify the cDNA of SPHK1 (forward primer,
5′-GGAGGAGGCAGAGATAAC-3′; reverse primer,
5′-TTAGCCCATTCACCACTTCA-3′), dickkopf-1 (DKK1; forward primer,
5′-CCTTGGATGGGTATTCCAGA-3′; reverse primer,
5′-CCTGAGGCACAGTCTGATGA-3′), Hes1 (forward primer,
5′-ACCTTCCAGTGGCTCCTC-3′; reverse primer,
5′-TTTAGTGTCCGTCAGAAGAGAG-3′) and Gli1 (forward primer,
5′-GCCATGAAACTTTCACCGTG-3′; reverse primer,
5′-TCTGGGGGGTTACCAAGTTA-3′) with ABI 7900HT Real-Time PCR System
(Applied Biosystems, Carlsbad, CA, USA) using One Step PrimeScript™
RT-PCR kit (cat no. RR064A; Takara Biotechnology Co., Ltd., Dalian,
China). GAPDH (forward primer, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′;
reverse primer, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′) mRNA was considered
as an internal control. The ∆∆Cq method was applied for
quantification following previous reports (25).
Migration and invasion assays
TC cells re-suspended in serum-free RPMI-1640 medium
were seeded into the upper chambers of a Transwell insert (8.0 µm,
EMD Millipore) at 3×104 cells/well. RPMI-1640 medium
containing 20% FBS was added to the lower chamber of 24-well plates
(Corning Inc., Corning, NY, USA). For the invasion assay, the upper
side of the filter was covered with Matrigel (BD Biosciences) and
1×105 cells per well were seeded onto the upper
chambers. After incubation for 24 h, cells on the upper side of the
membrane were removed and the filter membrane was fixed with 4%
paraformaldehyde and stained with 0.5% crystal violet (Beyotime
Institute of Biotechnology, Haimen, China). The numbers of cells
were counted in ten fields for each sample.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical significance was analyzed by SPSS version
16.0 statistical software (SPSS, Inc., Chicago, IL, USA). A
bivariate analysis was used to assess differences in multiple
groups (Student's t-test, one-way analysis of variance with
multiple comparisons by a post-hoc Tukey-honest significant
difference test for independent samples). A Kaplan-Meyer survival
analysis was also applied. Pearson's correlation analysis was
selected to determine the correlation of SPHK1 expression and
metastatic potential. P<0.05 was considered to indicate a
statistically significant difference.
Results
SPHK1 is upregulated in human TC
tissues
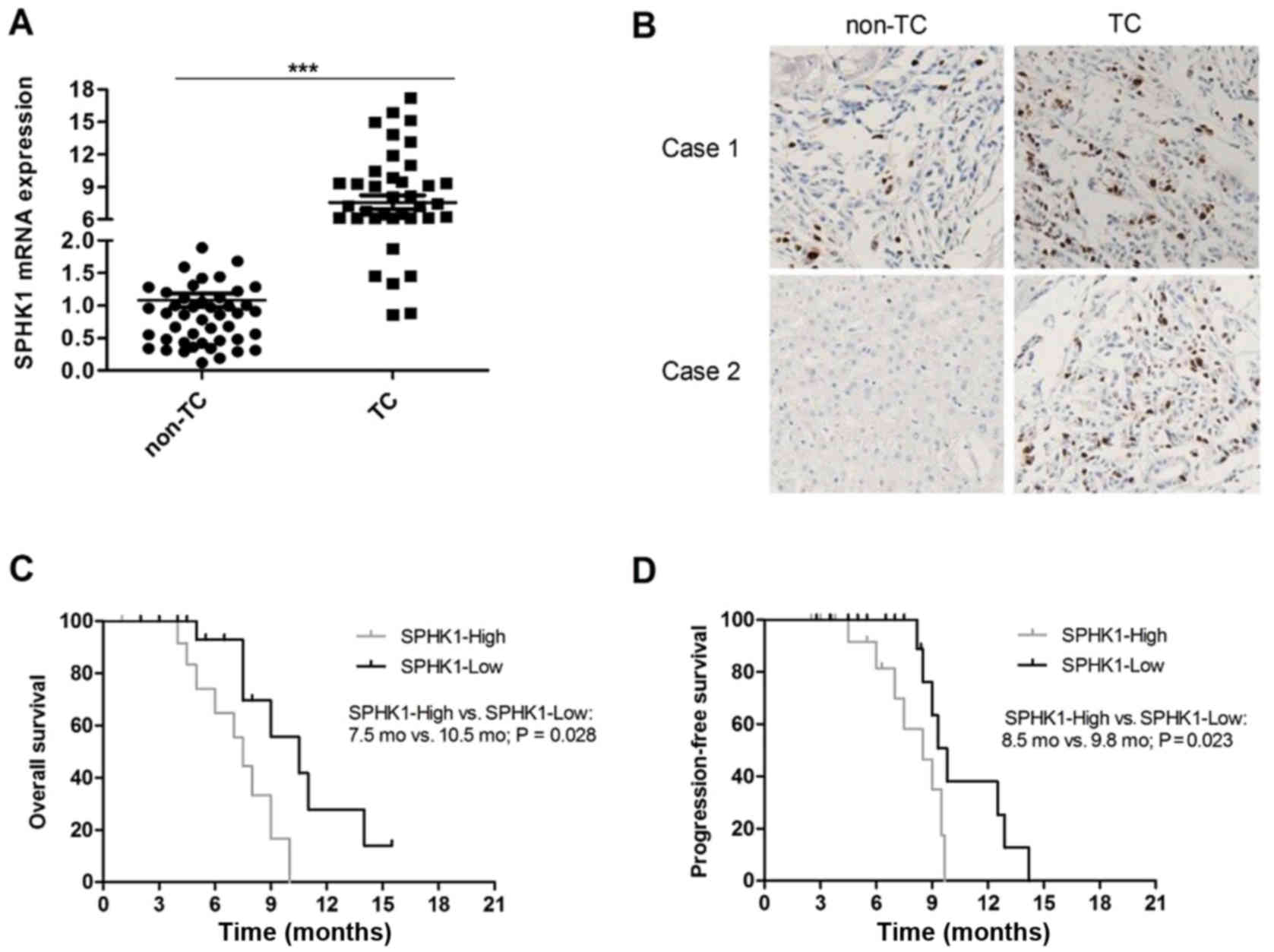
The expression of SPHK1 in human TC tissue samples
and adjacent normal thyroid (non-TC) tissue samples from 53
patients was determined by RT-qPCR. The results indicated that the
mRNA expression of SPHK1 was evidently elevated (at least 5-fold)
in human TC tissue samples compared with that in non-TC tissue
samples in 40 of 53 patients (75.47%; P<0.05, Fig. 1A). Furthermore, the protein levels of
SPHK1 were detected in TC tissue samples and non-TC tissue samples
by IHC. The protein levels of SPHK1 were obviously higher in TC
samples compared with those in non-TC samples and representative
images are presented in Fig. 1B. All
TC patients were divided into an SPHK1-high and an SPHK1-low group
according to the levels of SPHK1, and a Kaplan-Meier survival
analysis indicated that the level of SPHK1 was negatively
correlated with overall survival (OS; P=0.028; Fig. 1C) and progression-free survival (PFS;
P=0.023; Fig. 1D) of TC patients.
Taken together, the results indicated that high levels of SPHK1 are
correlated with a poorer survival and prognosis of TC patients.
Overexpression of SPHK1 in TC
patients, as well as S1P levels, are positively correlated with
high levels of phosphorylated (p)-SPHK1
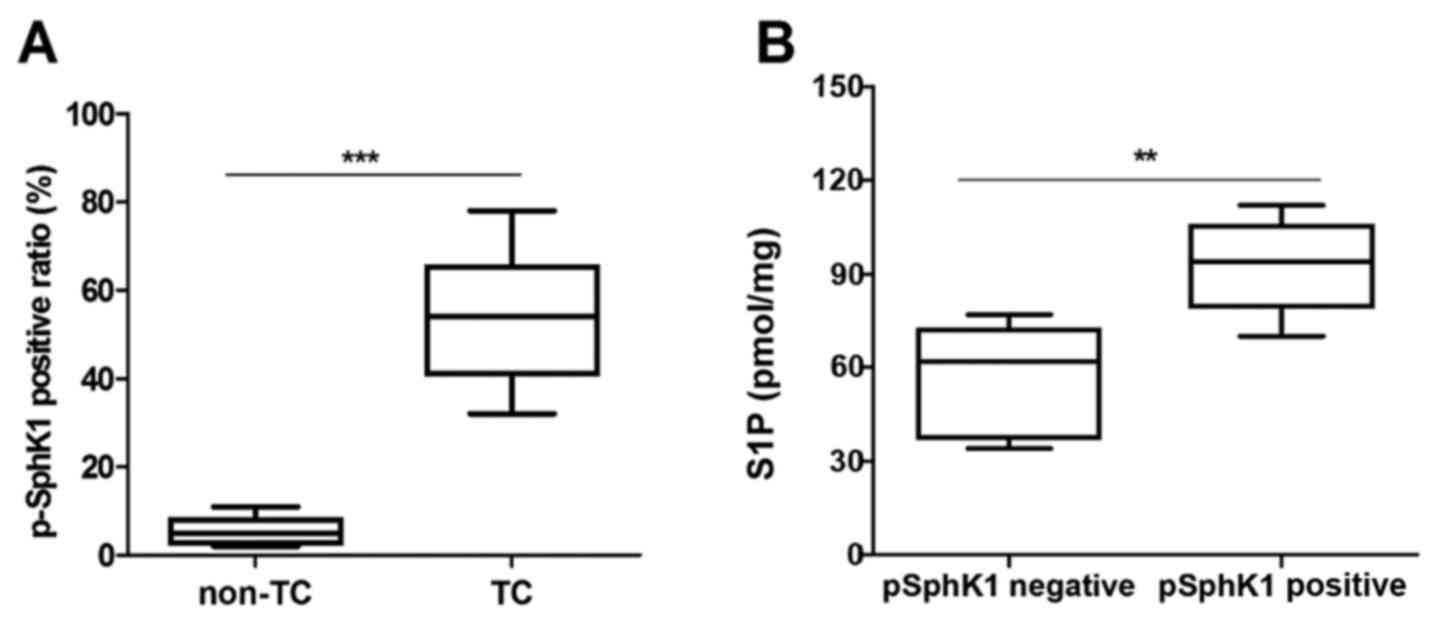
A previous study indicated that p-SPHK1 (Ser-225) is
associated with S1P export (15).
Thus, the present study further determined the levels of p-SPHK1 in
human TC tissue samples and non-TC tissue samples by IHC. It was
observed that TC tissue samples had higher p-SPHK1 levels compared
with non-TC tissue samples (Fig.
2A). In addition, the level of S1P export in cell supernatants
were evidently higher in p-SPHK1-positive tumors compared with that
in p-SPHK1-negative tumors. These results demonstrated that S1P
levels are obviously associated with p-SPHK1 levels in human TC
patients (Fig. 2B). Furthermore, the
association between p-SPHK1 levels and clinicopathological factors
(TNM stage) in human TC patients is presented in Table I.
 | Table I.Association between p-SPHK1 and
clinicopathological factors. |
Table I.
Association between p-SPHK1 and
clinicopathological factors.
|
| p-SPHK1 (n=53) |
|
|---|
|
|
|
|
|---|
| Factor | Negative (n,
%) | Positive (n,
%) | P-value |
|---|
| Age (years) |
|
| 0.591 |
|
>45 | 13 (25) | 8 (15) |
|
|
≤45 | 18 (34) | 14 (26) |
|
| Gender |
|
| 0.733 |
|
Male | 17 (32) | 14 (26) |
|
|
Female | 14 (26) | 8 (15) |
|
| Primary
tumora |
|
| 0.021 |
| T1 | 14 (26) | 6 (12) |
|
| T2, T3,
T4 | 17 (32) | 16 (30) |
|
| TNM stage |
|
| 0.008 |
| I and
II | 19 (36) | 9 (13) |
|
| III and
IV | 12 (26) | 13 (25) |
|
| N
classification |
|
| 0.841 |
| N0 | 13 (25) | 8 (15) |
|
| N1 | 18 (34) | 12 (26) |
|
Overexpression of SPHK1 in human TC
cells and their metastatic capacity is positively correlated with
SPHK1 levels
To further investigate the roles of SPHK1 in TC
progression, the expression of SPHK1 was detected in the human TC
cell lines SW579, K1, TPC-1 and WRO, compared with that in the
normal thyroid cell line Nthy-ori 3-1 as a control. The results
indicated that the mRNA levels of SPHK1 were significantly higher
(at least 2-fold) in the TC cell lines compared with those in the
normal thyroid cell line Nthy-ori 3-1 (Fig. 3A). In addition, the activity of SPHK1
was measured using an SPHK1 activity assay kit, which indicated
that SPHK1 activity was higher in TC cell lines than in Nthy-ori
3-1 (Fig. 3B). Furthermore, the TC
cell lines TPC-1 and K1 exhibited a more pronounced migration
ability than WRO, SW579 and Nthy-ori 3-1 (Fig. 3C). Further analysis suggested that
the migration ability of TC cells was positively correlated with
SPHK1 expression (Fig. 3D). Similar
results were also obtained regarding the invasion ability of TC
cells (Fig. 3E and F). Taken
together, these results demonstrated that the ability of TC cells
to form metastases was positively correlated with SPHK1 levels.
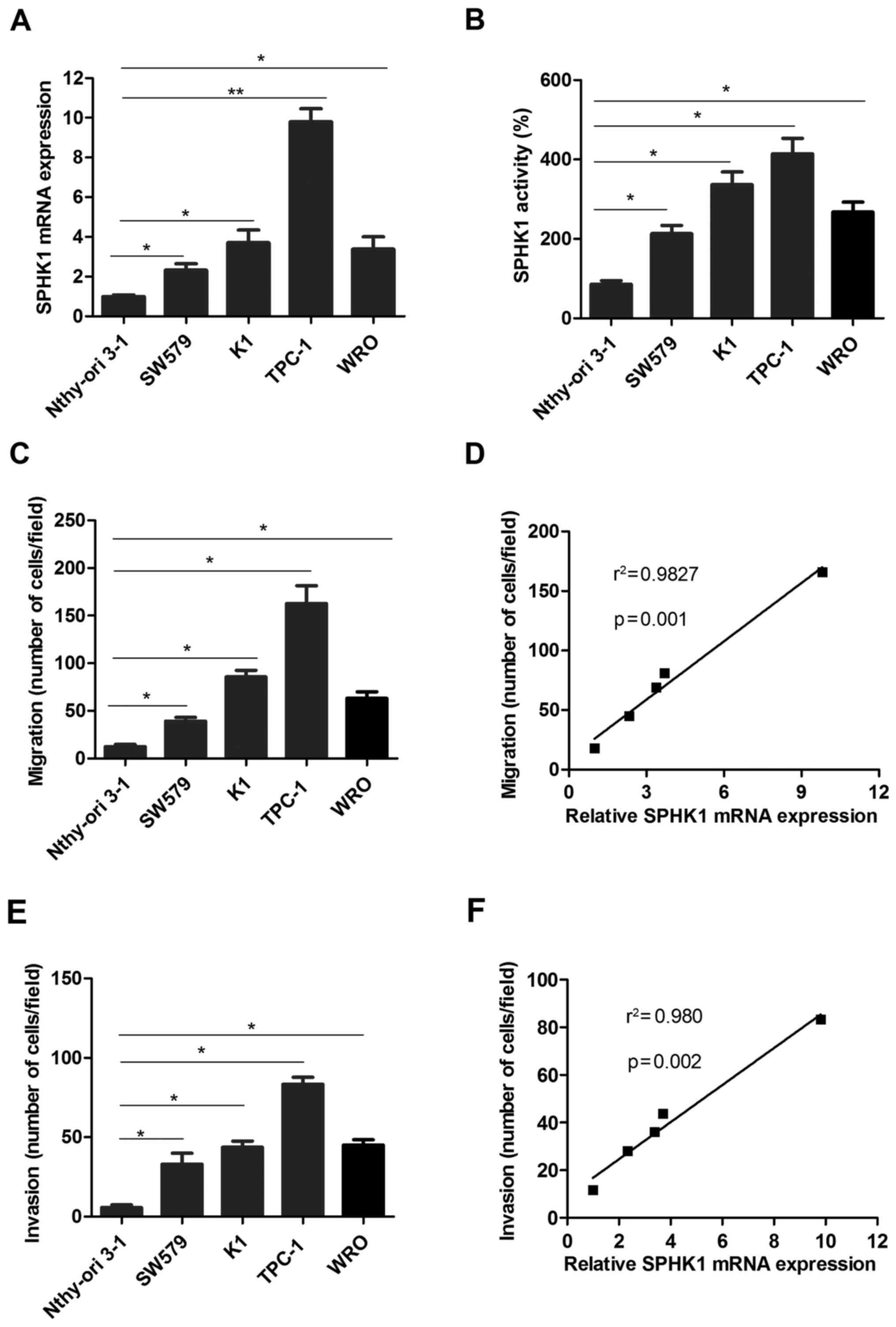 | Figure 3.SPHK1 levels in TC cell lines are
higher compared with those in normal thyroid cells and the
metastatic ability of TC cells is positively associated with SPHK1
expression. (A) SPHK1 mRNA expression was detected in TC cell lines
and the normal thyroid cell line Nthy-ori 3-1. (B) The activity of
SPHK1 was determined in TC cell lines and a normal thyroid cell
line. (C) The migration ability was determined in the TC cell lines
SW579, K1, TPC-1 and WRO, as well as the normal thyroid cell line
Nthy-ori 3-1 as a control. (D) Pearson's correlation analysis was
used to determine the correlation between SPHK1 expression and
migration ability of TC cells (r2=0.9827, P=0.001). (E)
The invasion ability was determined in the TC cell lines SW579, K1,
TPC-1 and WRO, and the normal thyroid cell line Nthy-ori 3-1 as a
control. (F) Pearson's correlation analysis was used to determine
the correlation between SPHK1 expression and invasion ability of TC
cells (r2=0.980, P=0.002). Values are expressed as the
mean ± standard deviation. *P<0.05; **P<0.01. SPHK1,
sphingosine kinase 1; TC, thyroid carcinoma. |
Silencing of SPHK1 expression impairs
the migration and invasion ability of TC cells, and reduces
metastasis of TPC-1 cells in NOD/SCID mice
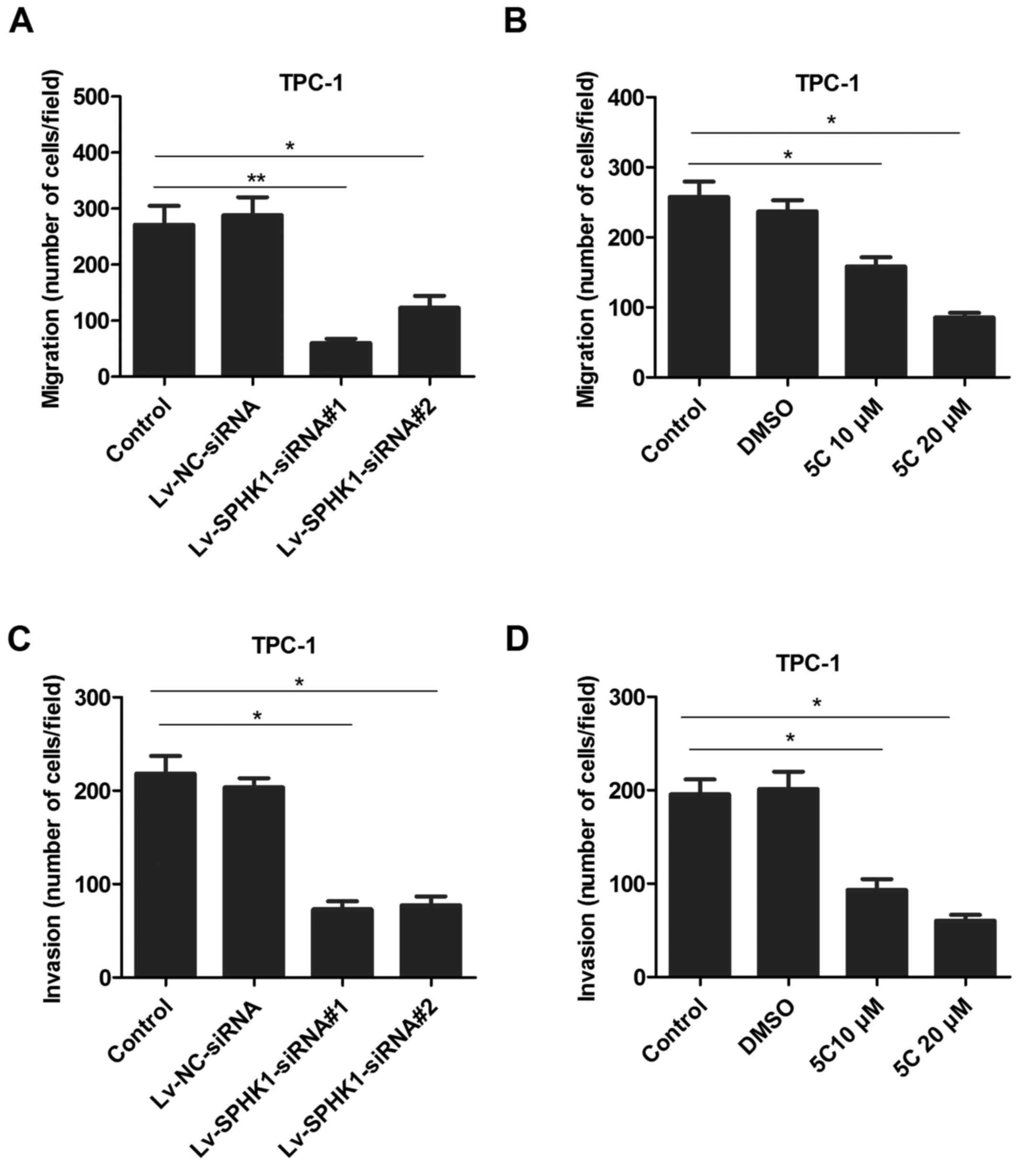
To determine whether SPHK1 levels are associated
with the ability of TC cells migrate and invade, a TPC-1 cell model
was selected for further investigation. TPC-1 cells were
transfected with lentivirus expressing SPHK1 siRNAs
(Lv-SPHK1-siRNA#1 and Lv-SPHK1-siRNA #2; Fig. 4A) or treated with the SPHK1 inhibitor
5C (10 or 20 µM; Fig. 4B) for 24 h;
subsequently, a Transwell assay was used to determine the migratory
ability. The results indicated that the migration ability was
markedly lower in the Lv-SPHK1-siRNA and SPHK1 inhibitor groups
compared with that in the control group (Fig. 4A and B). A similar trend was also
observed regarding the invasion ability of TC cells (Fig. 4C and D).
Intrasplenic injection was then performed as a valid
method (18) for assessing the
metastatic ability of TPC-1 cells in vivo. Injection of
control, Lv-NC-siRNA or Lv-SPHK1-siRNA#1 cells into the spleen of
NOD/SCID mice was performed and mice in the control and
Lv-NC-siRNA#1 groups developed metastatic tumors after 6 weeks.
Tumors were detected in the liver (5/5 in the control and
Lv-NC-siRNA group), colon (3/5 in the control and 4/5 in the
Lv-NC-siRNA group), lung (4/5 in the control and 3/5 in the
Lv-NC-siRNA group), spleen (3/5 in the control group and 2/5 in the
Lv-NC-siRNA group) and lymph nodes (4/5 in the control group and
Lv-NC-siRNA group). By contrast, the mice in the Lv-SPHK1-siRNA#1
group exhibited significantly fewer metastatic nodules in these
organs (P<0.05; Table II). Taken
together, these results demonstrated that the metastatic capacity
of TC cells was positively correlated with SPHK1 expression.
 | Table II.Metastatic capacity of SPHK1 cells
in vivo. |
Table II.
Metastatic capacity of SPHK1 cells
in vivo.
| Group | Liver | Colon | Lung | Spleen | Lymph nodes | P-value |
|---|
| Control | 5/5 | 3.5 | 4/5 | 3/5 | 4/5 |
|
| Lv-NC-siRNA | 5/5 | 4/5 | 3/5 | 2/5 | 4/5 | 0.021 |
|
lV-SPHK1-siRNA#1 | 2/5 | 1/5 | 1/5 | 0/5 | 1/5 |
|
Inhibition of SPHK1 expression
sensitizes TC cells to cisplatin and doxorubicin
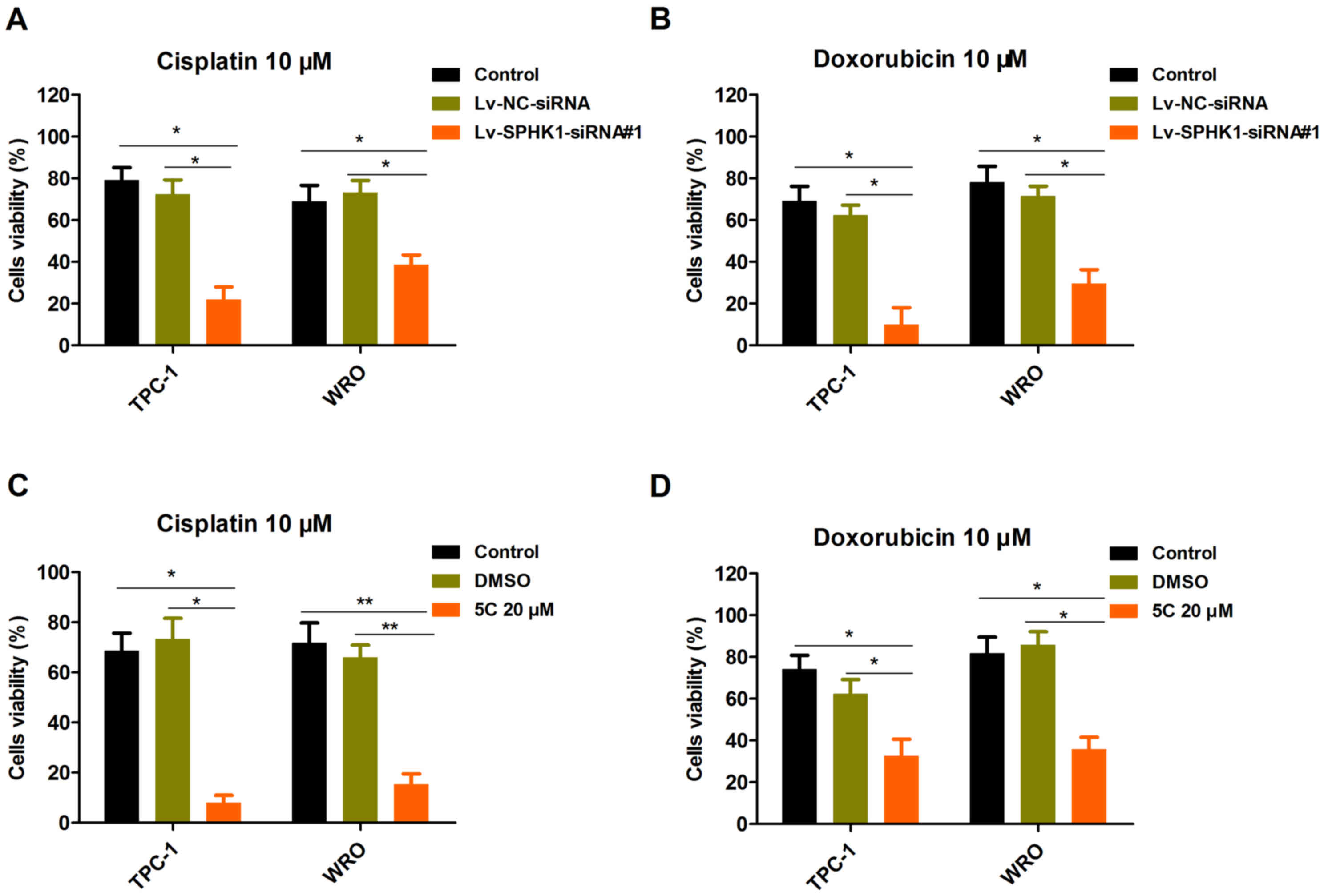
The present study investigated the effects of
silencing SPHK1 expression on the viability of TC cell lines. TPC-1
and WRO cells were selected for further study. TPC-1 and WRO cells
were transfected with Lv-NC-siRNA or Lv-SPHK1-siRNA#1 for 24 h,
followed by treatment with 10 µM cisplatin (Fig. 5A) or 10 µM doxorubicin (Fig. 5B) for 48 h. The CCK-8 assay
demonstrated that the cell viability in the Lv-SPHK1-siRNA#1 group
was obviously decreased compared with that in the control or
Lv-NC-siRNA group (Fig. 5A and B). A
similar result was also obtained in TPC-1 and WRO cells treated
with SPHK1 inhibitor 5C (Fig. 5C and
D). In brief, these results suggested that induction of
antiproliferative effects is a major mechanism by which silencing
of SPHK1 expression modulates the sensitivity of TC cells to
cisplatin and doxorubicin.
SPHK1 increases S1P levels and
activates the Notch signaling pathway via S1P receptor 3
(S1PR3)
It has been reported that S1PR has an important role
in the regulation of cell survival by SPHK1 (15). Thus, the present study mainly focused
on Wnt, Hedgehog and Notch signaling as candidate downstream
pathways of S1PR. The present results indicated that Hes1 was
downregulated after TPC-1 cells were treated with Lv-SPHK1-siRNA#1
(Fig. 6A) or SPHK1 inhibitor 5C
(Fig. 6B) for 24 h. These results
suggest that the Notch signaling pathway was repressed by silencing
of SPHK1 expression. By contrast, stimulation with S1P obviously
upregulated the expression of Hes1 in TPC-1 cells rather than LPA
(Fig. 6C). Furthermore, the protein
expression of Hes1 was also downregulated in TPC-1 cells
transfected with Lv-SPHK1-siRNA#1 (Fig.
6D). However, DKK1 and Gli1 were not significantly affected
(Fig. 6A and B). In addition, the
induction of Hes1 expression by S1P was abrogated by S1PR3
antagonists TY52156 (2 µM) and CAY10444 (5 µM), but not by S1PR2
antagonist JTE013 (2 µM) (Fig. 6E).
These results indicated that there was a reduction, but not an
abrogation as for the other antagonists. To further investigate
whether S1P activates the Notch signaling pathway, the cleavage of
Notch in TPC-1 cells was also detected. The expression of N1ICD was
significantly upregulated in TPC-1 cells treated with S1P, while
overexpression of N1ICD was performed as a positive control
(Fig. 6F). Collectively, these
results suggested that SPHK1 induces the activation of the Notch
signaling pathway and that SPHK1 mediates S1P-induced Notch
signaling pathway activation via S1PR3.
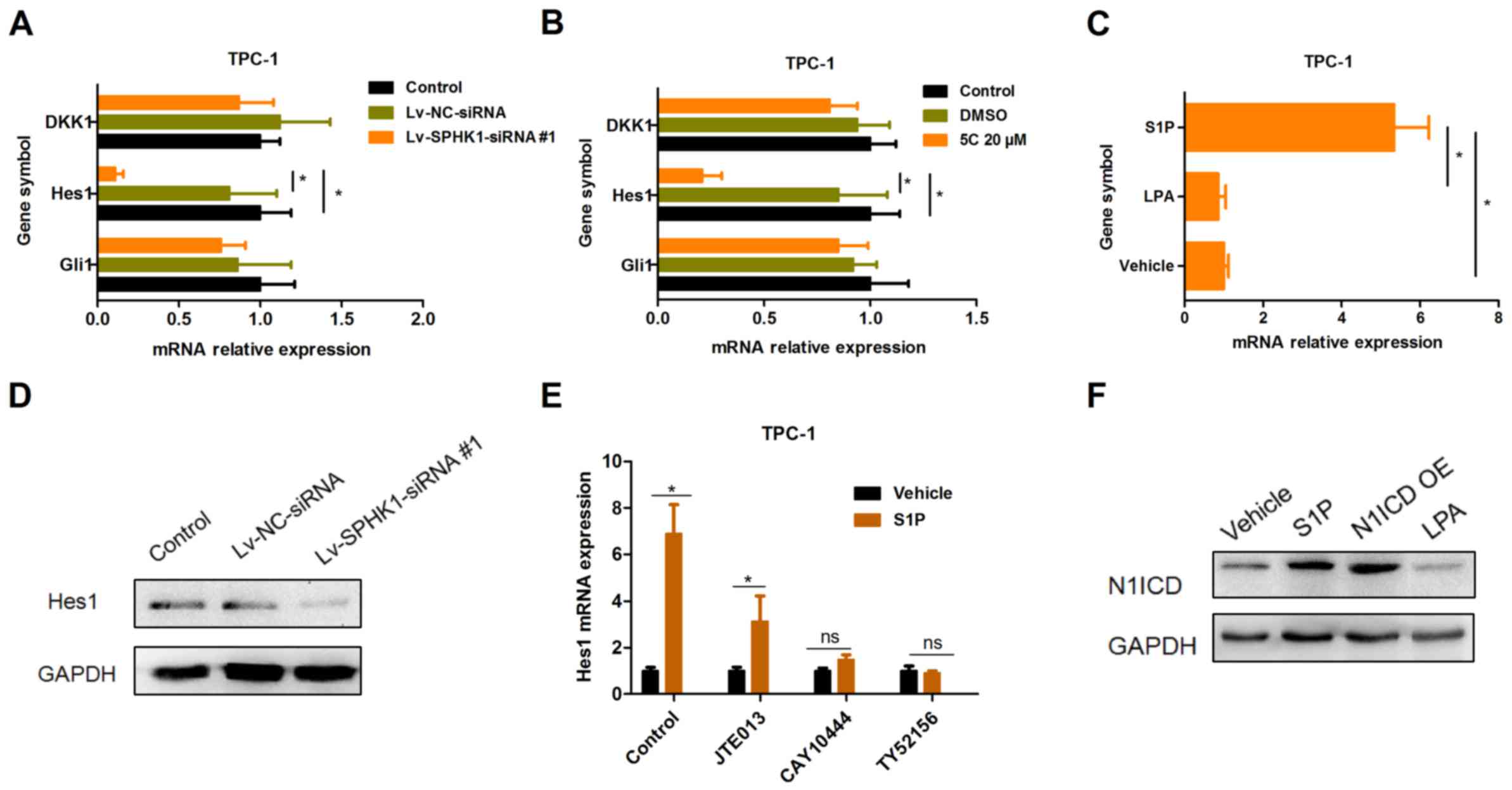 | Figure 6.SPHK1 causes an increase in S1P
levels and activates the Notch signaling pathway via S1PR3. (A and
B) TPC-1 cells were (A) transfected with Lv-NC-siRNA or
Lv-SPHK1-siRNA#1 at an MOI of 20 for 24 h, or (B) treated with DMSO
or 20 µM 5C for 24 h, and the expression of DKK1, Hes1 and Gli1 was
determined by an RT-qPCR assay. (C) TPC-1 cells were treated with
S1P (300 nM) and LPA (300 nM) for 24 h, and the expression of Hes1
was determined by RT-qPCR. (D) TPC-1 cells were transfected with
Lv-NC-siRNA or Lv-SPHK1-siRNA#1 at an MOI of 20 for 72 h, and
western blot analysis was used to determine the protein expression
of Hes1. (E) Effects of JTE013 (2 µM), CAY10444 (5 µM) or TY52156
(2 µM) on S1P-induced Hes1 expression. Values are expressed as the
mean ± standard deviation. *P<0.05. (F) Effects of S1P, LPA or
N1ICD OE on N1ICD expression; GAPDH was used as a control. N1ICD
OE, Notch1 intracellular domain overexpression vector;
Lv-SPHK1-siRNA, lentiviral vector expressing small interfering RNA
targeted to SPHK1; ns, no significance; NC, negative control; TC,
thyroid carcinoma; MOI, multiplicity of infection; DMSO, dimethyl
sulfoxide; DKK1, dickkopf 1; Hes1, hairy and enhancer of split-1;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; S1P, sphingosine-1-phosphate; LPA, lysophosphatidic
acid. |
Discussion
In contrast to the anti-proliferative effects of
ceramide and sphingosine, S1P regulates cell proliferation,
migration and survival, promotes angiogenesis and has a pivotal
role in the pathogenesis of inflammation as well as chemotaxis of
lymphocytes (26). As a pivotal
enzyme for S1P production in vivo, SPHK1 activated by
extracellular receptor agonist mainly has a role in regulating
extracellular S1P levels (27,28). In
addition, numerous studies have indicated that inhibition of SPHK1
impairs tumor cell proliferation (29,30).
Zhang et al (31) have
reported that inhibition of SPHK1 expression significantly
decreased the migration capability of hepatocellular carcinoma
cells. The present study indicated that SPHK1 expression was
markedly upregulated in TC compared with those in normal thyroid
tissues and was closely associated with a poorer OS and PFS in
patients with TC. These results were consistent with those of
previous studies and imply that SPHK1 may be closely associated
with the occurrence, progression and development of TC.
Activation of SPHK1 is a crucial factor for cell
proliferation, transformation, metastasis and chemoresistance
(23). It was reported to be
upregulated in multiple types of solid tumor and to be closely
correlated with poor prognosis (17). The observations of the present study
were in line with the above in patients with TC who poorly
responded to doxorubicin treatment (3). Furthermore, the present results
indicated that the cytotoxic effects of chemotherapeutic drugs on
TC cells were promoted by siRNA-mediated knockdown or
pharmacological inhibition of SPHK1 in vitro. A previous
study indicated that inhibition of SPHK1 activity by treatment with
safingol markedly potentiated epigallocatechin gallate-induced
apoptotic activity in vitro and in vivo (32). The results of the present study were
consistent with several studies on other cancer types (33–35). In
addition, inhibition of SPHK1 was demonstrated to sensitize TC
cells to chemotherapeutic drugs, including 5-fluorouracil,
docetaxel, doxorubicin and cisplatin. Taken together, SPHK1
inhibitors, alone or in combination with conventional anti-cancer
drugs, have been demonstrated to have marked anti-tumor
effects.
Furthermore, the results of the present study not
only demonstrated that SPHK1 expression was markedly upregulated in
TC tissues and cell lines, but also indicated that the upregulation
of SPHK1 has a significant role in the invasion and metastasis of
TC cells. A previous study indicated that SPHK1-5C (as utilized in
the present study), a chemotherapeutic drug, significantly
inhibited the serum-induced growth and survival of TC cells via the
extracellular signal-regulated kinase/AKT pathway (36). However, the downstream mechanisms of
the activated SPHK1/S1P pathway that promote the invasion and
migration of TC cells have remained to be elucidated. The results
of the present study indicated that the Notch target gene Hes1 was
evidently upregulated in TPC-1 cells following stimulation with S1P
and S1P-induced Hes1 expression was abrogated by S1PR3 antagonists,
which implied that activation of the SPHK1/S1P pathway may promote
the invasion and migration of TC cells. Furthermore, it was
demonstrated that inhibition of SPHK1 expression interferes with
the S1P/Notch signaling pathway and that it impairs the migration
and invasion of TC cells in vitro as well as the metastatic
capacity of TC in an in vivo metastasis model. Accordingly,
the development of SPHK1-targeted therapeutics to improve the
prognosis of patients with TC should be further investigated.
Acknowledgements
TC tissue and normal adjacent samples were
immunostained by the Department of Surgical Pathology, Southwest
Hospital of the Third Military Medical University (Chongqing,
China).
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ analyzed and interpreted the patient data. ZZ and
JM performed the experiments, including the immunohistological
staining. BH was responsible for the patient sample collection. ZZ
and BH drafted the manuscript. YZ performed the statistical
analysis. SW designed this study. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All patients provided informed consent and the
present study was approved by the medical Ethics Committee of the
Third Military Medical University (Chongqing, China). All
procedures for animal experiments were approved by the Laboratory
Animal Welfare and Ethics Committee of the Third Military Medical
University (Chongqing, China; no. 20151117083) and were performed
in accordance with institutional guidelines.
Consent for publication
Not applicable.
Competing interests
All authors have no conflict of interest to
declare.
References
|
1
|
Schmidt D and Kuwert T: Hybrid molecular
imaging in differentiated thyroid carcinoma. Front Horm Res.
45:37–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grewal RK, Ho A and Schöder H: Novel
approaches to thyroid cancer treatment and response assessment.
Semin Nucl Med. 46:109–118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nixon IJ: Well-differentiated thyroid
cancer-are you overtreating your patients? Endokrynol Pol.
67:60–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frampton JE: Lenvatinib: A review in
refractory thyroid cancer. Target Oncol. 11:115–122. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Williams D: Thyroid growth and cancer. Eur
Thyroid J. 4:164–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmid D, Ricci C, Behrens G and Leitzmann
MF: Adiposity and risk of thyroid cancer: A systematic review and
meta-analysis. Obes Rev. 16:1042–1054. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bae YJ, Schaab M and Kratzsch J:
Calcitonin as biomarker for the medullary thyroid carcinoma. Recent
Results Cancer Res. 204:117–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patmanathan SN, Wang W, Yap LF, Herr DR
and Paterson IC: Mechanisms of sphingosine 1-phosphate receptor
signalling in cancer. Cell Signal. 34:66–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farez MF and Correale J: Sphingosine
1-phosphate signaling in astrocytes: Implications for progressive
multiple sclerosis. J Neurol Sci. 361:60–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guerrero M, Urbano M and Roberts E:
Sphingosine 1-phosphate receptor 1 agonists: A patent review
(2013–2015). Expert Opin Ther Pat. 26:455–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwong E, Li Y, Hylemon PB and Zhou H: Bile
acids and sphingosine-1-phosphate receptor 2 in hepatic lipid
metabolism. Acta Pharm Sin B. 5:151–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanllehí P, Abad JL, Casas J and Delgado
A: Inhibitors of sphingosine-1-phosphate metabolism (sphingosine
kinases and sphingosine-1-phosphate lyase). Chem Phys Lipids.
197:69–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furuya H, Shimizu Y, Tamashiro PM, Iino K,
Bielawski J, Chan OTM, Pagano I and Kawamori T: Sphingosine kinase
1 expression enhances colon tumor growth. J Transl Med. 15:1202017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang LS and Natarajan V: Sphingolipids in
pulmonary fibrosis. Adv Biol Regul. 57:55–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu PH, Chen MB, Liu YY, Wu MH, Li WT, Wei
MX, Liu CY and Qin SK: Identification of sphingosine kinase 1
(SphK1) as a primary target of icaritin in hepatocellular carcinoma
cells. Oncotarget. 8:22800–22810. 2017.PubMed/NCBI
|
|
16
|
Li S, Zhou Y, Zheng X, Wu X, Liang Y, Wang
S and Zhang Y: Sphk1 promotes breast epithelial cell proliferation
via NF-κB-p65-mediated cyclin D1 expression. Oncotarget.
7:80579–80585. 2016.PubMed/NCBI
|
|
17
|
Fan Z, Jiang H, Wang Z and Qu J:
Atorvastatin partially inhibits the epithelial-mesenchymal
transition in A549 cells induced by TGF-β1 by attenuating the
upregulation of SphK1. Oncol Rep. 36:1016–1022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang W, Xie Z, Cui W, Guo Y, Xu L, Wu J
and Guan H: Comprehensive gene and microRNA expression profiling
reveals a role for miRNAs in the oncogenic roles of SphK1 in
papillary thyroid cancer. J Cancer Res Clin Oncol. 143:601–611.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guan H, Liu L, Cai J, Liu J, Ye C, Li M
and Li Y: Sphingosine kinase 1 is overexpressed and promotes
proliferation in human thyroid cancer. Mol Endocrinol.
25:1858–1866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murakami M, Ichihara M, Sobue S, Kikuchi
R, Ito H, Kimura A, Iwasaki T, Takagi A, Kojima T, Takahashi M, et
al: RET signaling-induced SPHK1 gene expression plays a role in
both GDNF-induced differentiation and MEN2-type oncogenesis. J
Neurochem. 102:1585–1594. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brünnert D, Sztachelska M, Bornkessel F,
Treder N, Wolczynski S, Goyal P and Zygmunt M: Lysophosphatidic
acid and sphingosine 1-phosphate metabolic pathways and their
receptors are differentially regulated during decidualization of
human endometrial stromal cells. Mol Hum Reprod. 20:1016–1025.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Datta A, Loo SY, Huang B, Wong L, Tan SS,
Tan TZ, Lee SC, Thiery JP, Lim YC, Yong WP, et al: SPHK1 regulates
proliferation and survival responses in triple-negative breast
cancer. Oncotarget. 5:5920–5933. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ribeiro FR, Meireles AM, Rocha AS and
Teixeira MR: Conventional and molecular cytogenetics of human
non-medullary thyroid carcinoma: Characterization of eight cell
line models and review of the literature on clinical samples. BMC
Cancer. 8:3712008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamura T, Matsuyama N, Kirino M, Kasai
M, Kiyohara S and Ikenaga T: Distribution, innervation, and
cellular organization of taste buds in the sea catfish, plotosus
japonicus. Brain Behav Evol. 89:209–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Selvam Panneer S, De Palma RM, Oaks JJ,
Oleinik N, Peterson YK, Stahelin RV, Skordalakes E, Ponnusamy S,
Garrett-Mayer E, Smith CD and Ogretmen B: Binding of the
sphingolipid S1P to hTERT stabilizes telomerase at the nuclear
periphery by allosterically mimicking protein phosphorylation. Sci
Signal. 8:ra582015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu H, Ma Y, He HW, Zhao WL and Shao RG:
SPHK1 (sphingosine kinase 1) induces epithelial-mesenchymal
transition by promoting the autophagy-linked lysosomal degradation
of CDH1/E-cadherin in hepatoma cells. Autophagy. 13:900–913. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sukocheva O, Wadham C, Gamble J and Xia P:
Sphingosine-1-phosphate receptor 1 transmits estrogens' effects in
endothelial cells. Steroids. 104:237–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Y, Dong B, Huang J, Kong W, Xue W, Zhu
Y, Zhang J and Huang Y: Sphingosine kinase 1 is overexpressed and
promotes adrenocortical carcinoma progression. Oncotarget.
7:3233–3244. 2016.PubMed/NCBI
|
|
30
|
Okada T, Ding G, Sonoda H, Kajimoto T,
Haga Y, Khosrowbeygi A, Gao S, Miwa N, Jahangeer S and Nakamura S:
Involvement of N-terminal-extended form of sphingosine kinase 2 in
serum-dependent regulation of cell proliferation and apoptosis. J
Biol Chem. 280:36318–36325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z, Yan Z, Yuan Z, Sun Y, He H and
Mai C: SPHK1 inhibitor suppresses cell proliferation and invasion
associated with the inhibition of NF-κB pathway in hepatocellular
carcinoma. Tumour Biol. 36:1503–1509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsukamoto S, Huang Y, Kumazoe M, Lesnick
C, Yamada S, Ueda N, Suzuki T, Yamashita S, Kim YH, Fujimura Y, et
al: Sphingosine kinase-1 protects multiple myeloma from apoptosis
driven by cancer-specific inhibition of RTKs. Mol Cancer Ther.
14:2303–2312. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu H, Duan P, Zhu H and Rao D: miR-613
inhibits bladder cancer proliferation and migration through
targeting SphK1. Am J Transl Res. 9:1213–1221. 2017.PubMed/NCBI
|
|
34
|
Zhang S, Deng Z, Yao C, Huang P, Zhang Y,
Cao S and Li X: AT7867 inhibits human colorectal cancer cells via
AKT-Dependent and AKT-Independent mechanisms. PLoS One.
12:e01695852017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li W, Li J, Wang Y, Zhang K, Li N, Tian Z,
Ni B, Wang H and Ruan Z: Sphingosine kinase 1 is a potential
therapeutic target for nasopharyngeal carcinoma. Oncotarget.
7:80586–80598. 2016.PubMed/NCBI
|
|
36
|
Zhu P, Liao LY, Zhao TT, Mo XM, Chen GG
and Liu ZM: GPER/ERK&;AKT/NF-κB pathway is involved in
cadmium-induced proliferation, invasion and migration of
GPER-positive thyroid cancer cells. Mol Cell Endocrinol. 442:68–80.
2017. View Article : Google Scholar : PubMed/NCBI
|