Introduction
Patients with type 2 diabetes mellitus are at a
notably high risk for developing life-threatening cardiovascular
complications (1), and there is a
clear association between glycemic control and cardiovascular
diseases (2). Unlike vascular smooth
muscle cells, endothelial cells cannot regulate intrinsic glucose
levels, which can result in the accumulation of glucose and its
derivatives, leading to an array of metabolic disorders.
Furthermore, glucose toxicity can cause reduced cell viability and
increased senescence in endothelial cells via multiple signaling
pathways. The cardiovascular complications of diabetes have a close
association with the function of endothelial cells; however, the
underlying mechanism needs further investigation (3–5).
Therefore, endothelial cells are potential targets for preventing
the cardiovascular complications of diabetes.
Induced pluripotent stem cells (iPSCs) may be used
to treat a number of diseases, as they possess the potential for
self-renewal and multi-differentiation (6). It has previously been suggested that
transplanted iPSCs were able to inhibit vascular apoptosis and
fibrosis, thus improving cardiac function in diabetic rats
(7). However, little is known about
the mechanisms by which iPSCs, or factors released from these
cells, inhibit adverse cardiac remodeling (8). Despite their impressive therapeutic
ability, teratoma formation has been observed following
transplantation of iPSCs (9,10). iPSC-derived lineage cells have
avoided this issue; however, the derivatives may still suffer the
same difficulties as reported for adult stem cells, particularly in
cell survival, retention and coupling in damaged areas (11). Therefore, it is important to exploit
the powerful regenerative capacity of pluripotent stem cells while
circumventing the problems associated with cell
transplantation.
The discovery of cell-free components, including
exosomes, may provide a promising alternative for regenerative
medicine. Exosomes are small membrane vesicles that contain
membrane and cytosolic components, including proteins, lipids and
RNAs (12–14). They have an essential role in
intercellular communication via transporting this cargo to targeted
cells. Furthermore, the role of exosomes depends on their
components and cell origin. It has been reported that
adipose-derived mesenchymal stromal cells release exosomes that are
capable of promoting angiogenesis (15). In addition, embryonic stem
cell-derived exosomes have been shown to enhance cell proliferation
(16). As one of the most dynamic
cells with regenerative potential, iPSCs may also release exosomes,
suggesting further potential for disease treatment.
In the present study, human induced pluripotent stem
cell-derived exosomes (hiPSC-exo) were isolated and were
co-cultured with normal human umbilical vascular endothelial cells
(HUVECs) or HUVECs exposed to high glucose. Subsequently, the cell
viability, capacity to form capillary-like structures and cell
senescence was examined to demonstrate the role for hiPSC-exo in
endothelial cell growth.
Materials and methods
Cell culture
HiPSCs were induced at the Cardiovascular
Regenerative Engineering Laboratory (Shanghai, China), following a
previously described protocol (17)
and cultured in mTeSR (Stemcell Technologies, Inc., Vancouver,
Canada), which contains mTeSR1 basal medium (400 ml; cat. no.
05851) and mTeSR1 5X supplement (100 ml; cat. no. 05852). The
culture medium was replaced every day, and the cells were digested
with Accutase (cat. no. A6964; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and passaged at a 1:5 dilution every 3 days.
Following the culture of passaged cells for 48 h at 37°C in a 5%
CO2 and 95% air gas mixture atmosphere, the media was
changed. The media was then collected for exosome isolation
following a further incubation with cells at 37°C in a 5%
CO2 and 95% air gas mixture atmosphere for 24 h.
Primary HUVECs were isolateded from fresh umbilical
cord veins from 3 patients that had undergone normal pregnancy, 24
h following birth, from June 2015 to December of 2015 at the
Department of Obstetrics, The Obstetrics and Gynecology Hospital of
Fudan University (Shanghai, China). For the collection of HUVECS,
informed consent was obtained from all patients and ethical
approval was granted by the Experimental Animal and Ethics
Committee of the College of Basic Medical Sciences at Fudan
University (Shanghai, China). HUVECs were isolated by collagenase
digestion as previously described (18) and cultured in endothelial cell medium
(ScienCell Research Laboratories, Inc., San Diego, CA, USA)
supplemented with 5% fetal bovine serum (cat. no. 0025), 1%
endothelial cell growth supplement (cat. no. 1052) and 1%
penicillin/streptomycin solution (cat. no. 0503). Furthermore, the
culture medium was replaced every 2 days. When the HUVECs became
~80% confluent, cells were sub-cultured with 0.25% trypsin-EDTA and
phenol red 1X (cat. no. 25200072; Thermo Fisher Scientific Inc.,
Waltham, MA, USA), and were incubated at 37°C in an atmosphere
containing 5% CO2.
Exosome isolation
Exosomes from hiPSC were collected and purified
using ultracentrifugation. Briefly, timing began when hiPSCs were
70% confluent. After 48 h, 80 ml cell-conditioned medium was
collected and centrifuged at 3,000 × g for 30 min at 4°C and 10,000
× g for 30 min at 4°C to remove dead cells and cell debris. The
final supernatant was ultracentrifuged and exosomes were pelleted
at 100,000 × g for 70 min at 4°C. The pellet obtained was then
washed with PBS at 100,000 × g for 70 min at 4°C to eliminate
contaminating proteins. The purified exosome fraction was
re-suspended in PBS prior to further use.
HiPSC-exo labeling and uptake
assay
Exosomes were labeled using a PKH26 Red Fluorescent
Cell Linker kit (PKH26GL-1KT; Sigma-Aldrich; Merck KGaA) according
to the manufacturer's protocol. A total of 1 µl PKH26 was mixed
with 250 µl Diluent C (provided by the kit). HiPSC-exo with the
same volume was then added into the mixture and incubated at room
temperature for 5 min. Labeling was stopped by adding 500 µl 0.5%
bovine serum albumin followed by an incubation at room temperature
for 5 min. The exosomes were then centrifuged at 100,000 × g for 70
min at 4°C and re-suspended in PBS prior to the uptake assay. For
the uptake assay, HUVECs were seeded in 96-well plates and left to
proliferate for 24 h at 37°C in a 5% CO2 and 95% air gas
mixture. Following 24 h, 20, 50 µg/ml hiPSC-exo or the PBS control
were added into the dish and cultured for a further 24 h at 37°C in
a 5% CO2 and 95% air gas mixture. The following steps
were applied to the cells prior to observation under an inverted
phase contrast microscope at a magnification of ×20. The cells were
washed with PBS twice, fixed with freshly prepared 4%
paraformaldehyde in PBS at room temperature for 15 min, further
washed twice with PBS and incubated with 0.1% triton X-100/PBS at
room temperature for 15 min. Cells were subsequently washed twice
with PBS, stained with DAPI (1:5,000; cat. no. C1002; Beyotime,
Shanghai, China) for 5 min at room temperature and further washed
with PBS.
Transmission electron microscopy
Transmission electron microscopy images of hiPSC-exo
were obtained using an FEI Tecnai G2 Spirit twin transmission
electron microscope (FEI; Thermo Fisher Scientific, Inc.) operating
at 80 kV. Briefly, 10 µl hiPSC-exo sample was loaded onto a
formvar-carbon coated copper grid for 2 min and excess sample was
absorbed with filter paper by gently touching the edge of the grid
and removed. Next, uranyl acetate was used to stain the grid for 1
min at room temperature and the excess liquid was absorbed using
filter paper. The copper grid was then dried under an incandescent
lamp.
Nanoparticle tracking analysis
Exosome size and concentration analysis was
performed using Nanoparticle Tracking Analyzer (version, 3.1;
Build, 3.1.54; Malvern Instruments, Ltd., Worcestershire, UK). For
the measurement, 10 µl hiPSC-exo sample was diluted in 1 ml of
PBS.
Cell counting assay
A cell counting kit-8 (CCK-8; cat. no. CK04; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was used to identify
the effect of hiPSC-exo on the viability of HUVECs. For this assay,
the same initial number (1×103) of HUVECs was seeded in
96-well plates and allowed to proliferate for 24 h at 37°C, in a 5%
CO2 and 95% air gas mixture. The cells were then treated
with different conditions [normal glucose (5 mM); normal glucose +
hiPSC-exo (10, 20 or 50 µg/ml); high glucose (33 mM) and high
glucose + hiPSC-exo (10, 20 or 50 µg/ml)] for a further 48 h at
37°C in a 5% CO2 and 95% air gas mixture. The culture
media were aspirated and CCK-8 mixed media (CCK-8: Dulbecco's
modified Eagle's medium, 1:10) was added to the different groups.
After 2 h, the absorbance was measured at 450 nm using a
spectrophotometer microplate reader. The assays were performed in
sextuplicate and each experiment was repeated five times.
Capillary-like structure formation
assay
A capillary-like structure formation assay was
performed to identify the functional role of hiPSC-exo on
endothelial cells. Briefly, HUVECs (1×104 cells) were
cultured in 24-well plates at 37°C in a 5% CO2 and 95%
air gas mixture for 24 h and subjected to different treatments
(normal glucose (5 mM), normal glucose + hiPSC-exo (20 µg/ml), high
glucose (33 mM) and high glucose + hiPSC-exo (20 µg/ml) for another
48 h at 37°C, in a 5% CO2 and 95% air gas mixture. After
48 h, HUVECs were trypsinized and cultured in 24-well plates
(1×105 cells/well) coated with 200 µl Matrigel matrix
(cat. no. 356234; BD Biosciences, Franklin Lakes, NJ, USA). Tube
length was quantified after 6 h by calculating the mean length in
five random microscopic fields with an inverted phase contrast
microscope at magnification, ×10. ImageJ software (version, 1.42q)
was then used to analyze the data (National Institutes of Health,
Bethesda, MD, USA). Each experiment was repeated three times.
Senescence-associated staining
Senescence-associated staining was applied to
demonstrate that hiPSC-exo could inhibit cell senescence in HUVECs.
Briefly, HUVECs (1×104 cells) were cultured in 24-well
plates at 37°C with 5% CO2 for 24 h and treated with
normal glucose (5 mM), normal glucose + hiPSC-exo (20 µg/ml), high
glucose (33 mM) and high glucose + hiPSC-exo (20 µg/ml) for a
further 48 h at 37°C, in a 5% CO2 and 95% air gas
mixture. Subsequently, senescence-associated β-galactosidase
(SA-β-gal) staining was performed using an SA-β-gal staining kit
(cat. no. K320-250; BioVision, Inc., Milpitas, CA, USA) according
to the manufacturer's instructions. HUVECs were washed three times
with PBS and fixed for 15 min at room temperature with fixative
solution. After incubation with staining solution overnight at
37°C, senescence was quantified by calculating mean proportion of
senescent cells in five random microscopic fields using an inverted
phase contrast microscope at magnification, ×20 and ImageJ
software. Each experiment was repeated three times.
Western blot analysis
hiPSCs and hiPSC-exo were lysed using
radioimmunoprecipitation assay buffer (cat. no. 89901; Thermo
Fisher Scientific, Inc.) for 30 min on ice, followed by a
centrifugation at a speed of 12,000 × g for 20 min at 4°C. The
concentration of the protein was determined via bicinchoninic acid
assay (Pierce BCA Protein Assay Reagent A; cat. no. 23227; Thermo
Fisher Scientific, Inc.). A total of 20 µg protein was mixed with
5X SDS loading buffer and loaded onto 10% SDS-PAGE. The separated
protein bands in the gel were transferred onto a polyvinylidene
difluoride (PVDF) membrane. The membrane was then blocked with 5%
non-fat milk in PBS with 0.1% Tween-20 (PBST) at room temperature
for 2 h. PVDF membranes were then incubated with primary antibodies
at 4°C overnight and then washed with PBST. Secondary antibodies
were added to each blot, and incubated at room temperature for 2 h;
PVDF membranes were then washed with PBST and incubated with Super
Signal™ West Pico (cat. no. 34580; Thermo Fisher
Scientific, Inc.) and observed using a Western blot visualizer
(Tanon 5500; Tanon Science & Technology Co., Ltd., Shanghai,
China). Primary antibodies used for exosome identification were
goat anti-Alix (N-20; 1:1,000; cat. no. sc-49267; Santa Cruz
Biotechnology Inc., Dallas, TX) and anti-cluster of differentiation
(CD63; rabbit IgG; 1:1,000; cat. no. EXOAB-CD63A-1; System
Biosciences Inc., Palo Alto, CA). Secondary antibodies used were
horseradish peroxidase (HRP)-conjugated donkey anti-goat IgG
(1:2,000; cat. no. 705-035-003; Jackson ImmunoResearch Laboratories
Inc., West Grove, PA) and HRP-conjugated donkey anti-rabbit IgG
(1:2,000; cat. no. 711-005-152; Jackson ImmunoResearch Laboratories
Inc.). The experiment was repeated three times.
Statistical analysis
Statistical analysis was performed using one-way
analysis of variance and a post-hoc Bonferroni test. P<0.05 was
considered to indicate a statistically significant difference. Data
are presented as the mean ± standard error of the mean, unless
otherwise stated. Furthermore, statistical analysis was performed
using GraphPad Prism 6.07 (GraphPad Software, Inc., La Jolla, CA)
and SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Identification of exosomes derived
from hiPSC
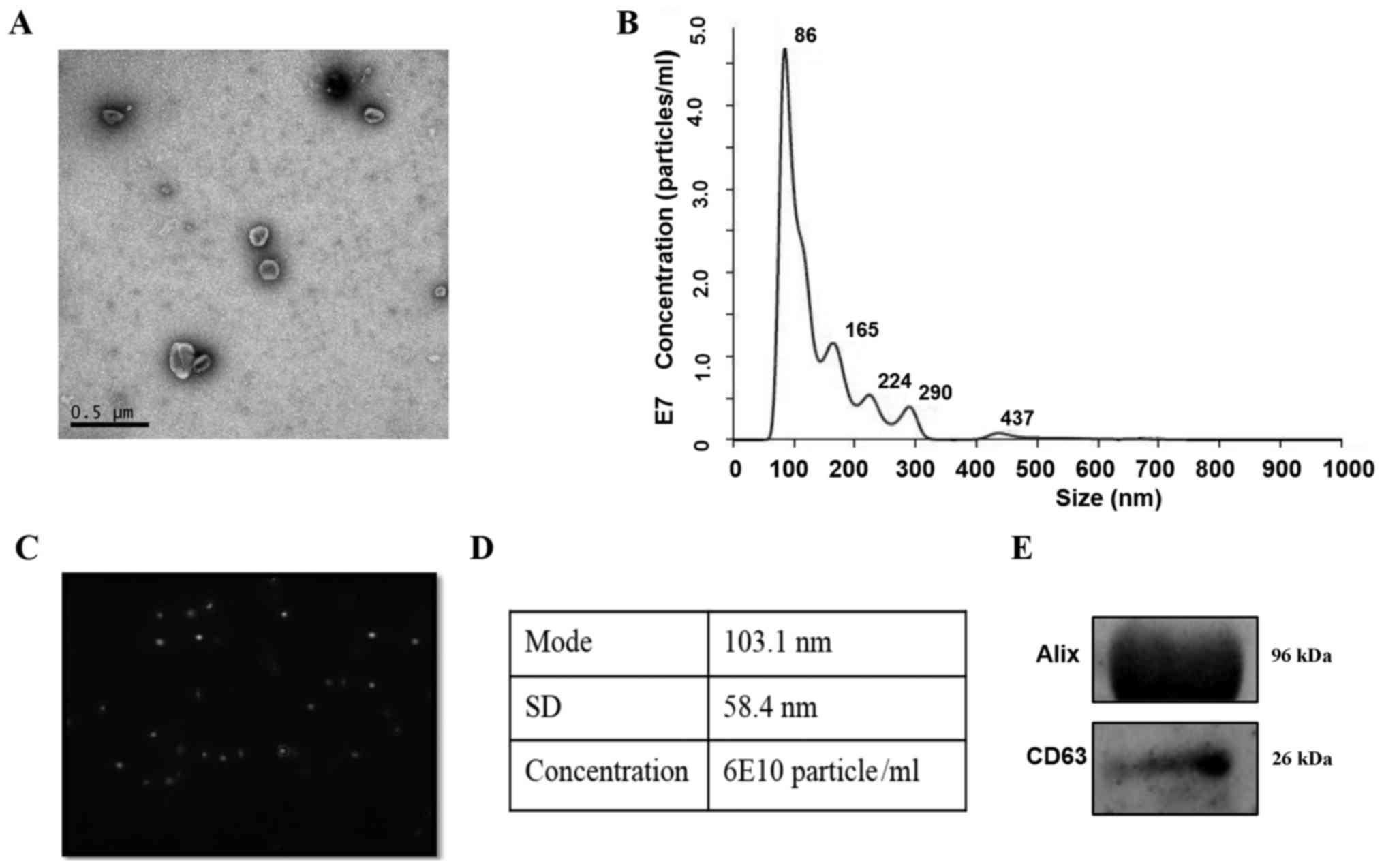
A transmission electron microscopic observation of
hiPSC-exo showed the presence of spherical vesicles with a typical
cup-shape (Fig. 1A). Upon conducting
nanoparticle tracking analysis, the concentration/size graph and
particle pictorial diagram revealed a homogeneous population of
exosomes ranging between 50 and 150 nm (Fig. 1B and C). The mode size of hiPSC-exo
was 103.1 nm, and the total concentration of particles with a
diameter between 30 and 150 nm was 6×1010 particles/ml
(Fig. 1D). Additionally, hiPSC-exo
expressed the exosomal marker proteins Alix and CD63 (Fig. 1E).
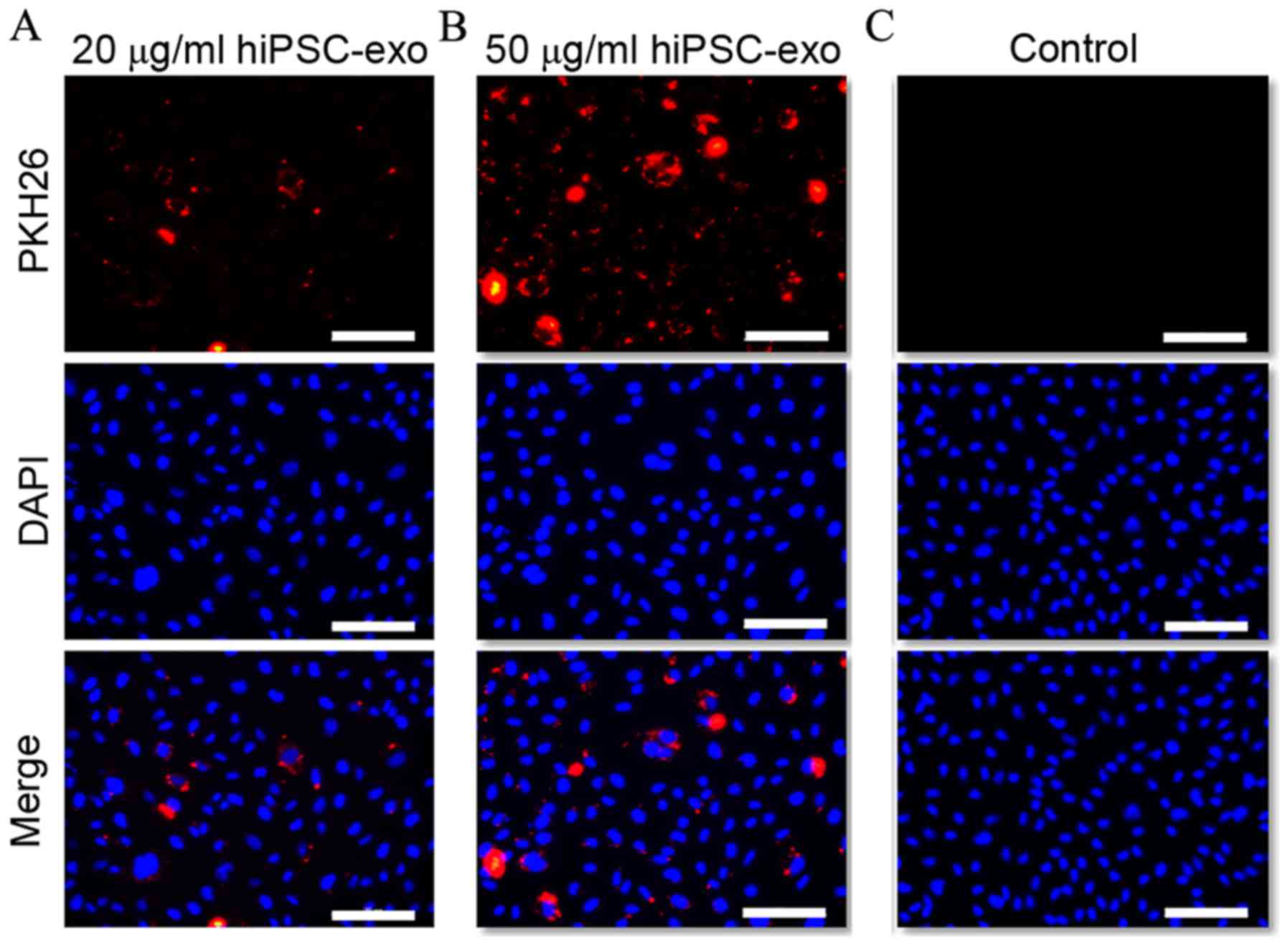
Uptake of hiPSC-exo by HUVECs
In order to function, hiPSC-exo requires the ability
to be endocytosed by target cells. Previous research has
demonstrated that exosomes express adhesion molecules that may be
associated with the adherence of exosomes to cells; however, the
cellular and molecular basis for specific targeting to acceptor
cells remains to be elucidated. For example, exosomes released by
the human intestinal epithelial cell line T84 could be endocytosed
by dendritic cells (DCs), but not B or T lymphocytes (19). The present study therefore tested if
hiPSC-exo could be taken up by HUVECs. It was demonstrated in
Fig. 2A and B that when HUVECs were
treated with PKH26 labeled hiPSC-exo (20 and 50 µg/ml) exosomes
were endocytosed by cells in a concentration-dependent manner. By
contrast, the control group, which was subjected to the same
procedure, did not show any intracellular fluorescence as shown in
Fig. 2C.
hiPSC-exo reversed high
glucose-induced decreased cell viability
Fig. 2 demonstrated
that hiPSC-exo may be endocytosed by HUVECs. It was then
investigated if endocytosed hiPSC-exo could influence the fate of
HUVECs. In CCK-8 assay the amount of formazan dye in cells is
directly proportional to the number of living cells. In the present
study, HUVECs were treated with normal (5.5 mM) or high (33 mM)
concentrations of glucose, in combination with different
concentrations (0, 10, 20 or 50 µg/ml) of hiPSC-exo. After 48 h
treatment, the OD value of the eight groups was measured. Compared
with the control group [normal concentration of glucose (5.5 mM)],
the OD value for high glucose (33 mM) treated HUVECs was
significantly decreased (Fig. 3).
Furthermore, exosomes had no statistically significant effect on
normal HUVECs. HiPSC-exo significantly reversed the harmful effect
of high glucose. However, the effects between hiPSC-exo 20 and 50
µg/ml did not differ significantly (Fig.
3). Thus, a concentration of 20 µg/ml was selected for use in
subsequent experiments.
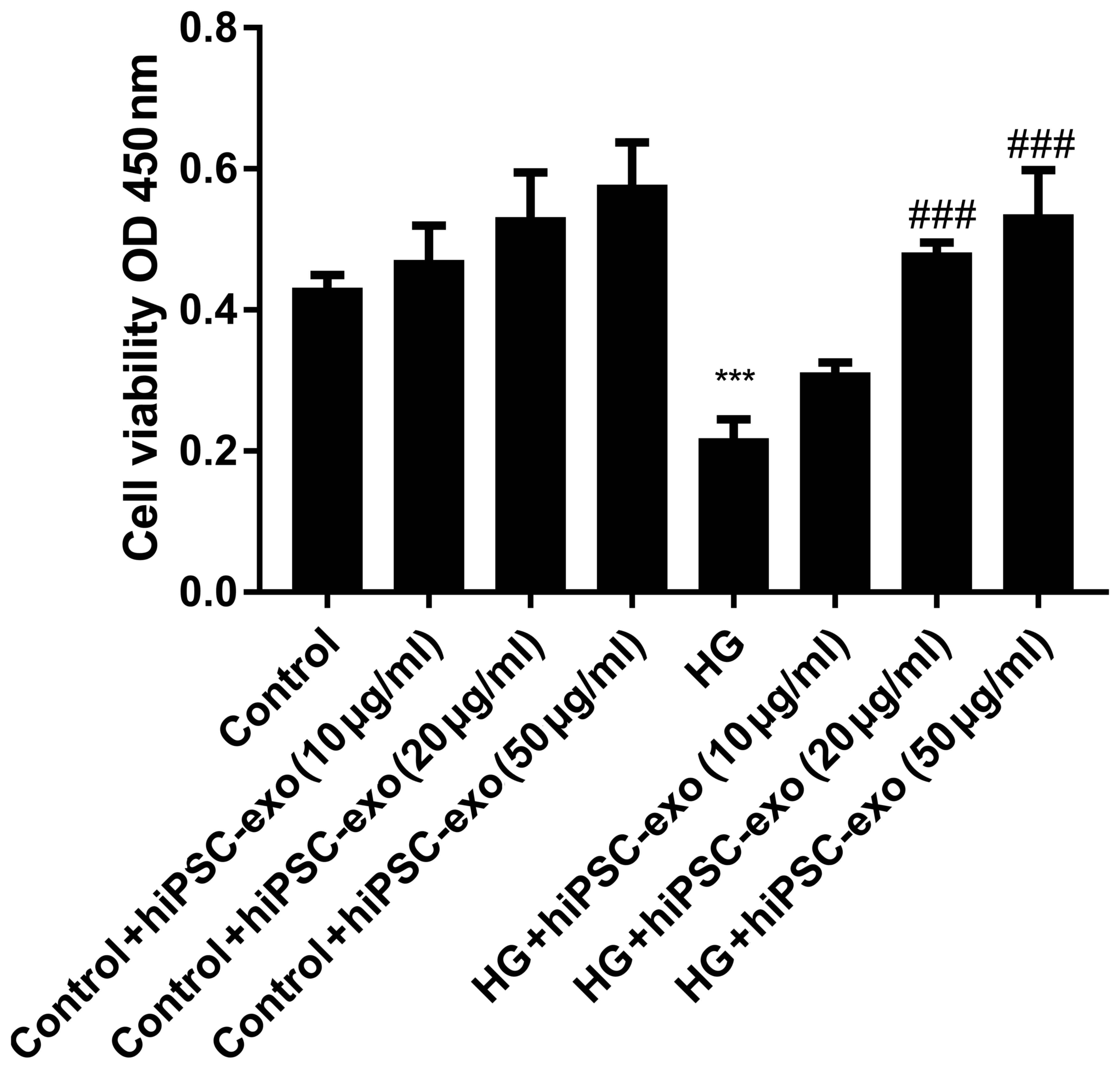 | Figure 3.hiPSC-exo ameliorated the high
glucose-induced decrease in cell viability in a dose-dependent
manner. Cell counting kit-8 assays were performed to evaluate cell
viability. HUVECs (1×103) were seeded in 96-well plates.
The cell viability was assessed by measuring the OD value of HUVECs
after 48 h of treatment. Control, normal glucose (5.5 mM); HG, high
glucose (33 mM); hiPSC-exo (10, 20 or 50 µg/ml). Control vs. HG,
***P<0.0001, n=5; HG vs. HG + hiPSC-exo (20 µg/ml),
###P<0.0001, n=5; HG vs. HG + hiPSC-exo (50 µg/ml),
###P<0.0001, n=5. hiPSC-exo, human induced
pluripotent stem cell-derived exosomes; HUVECs, human umbilical
vascular endothelial cells; OD, optical density; HG, high
glucose. |
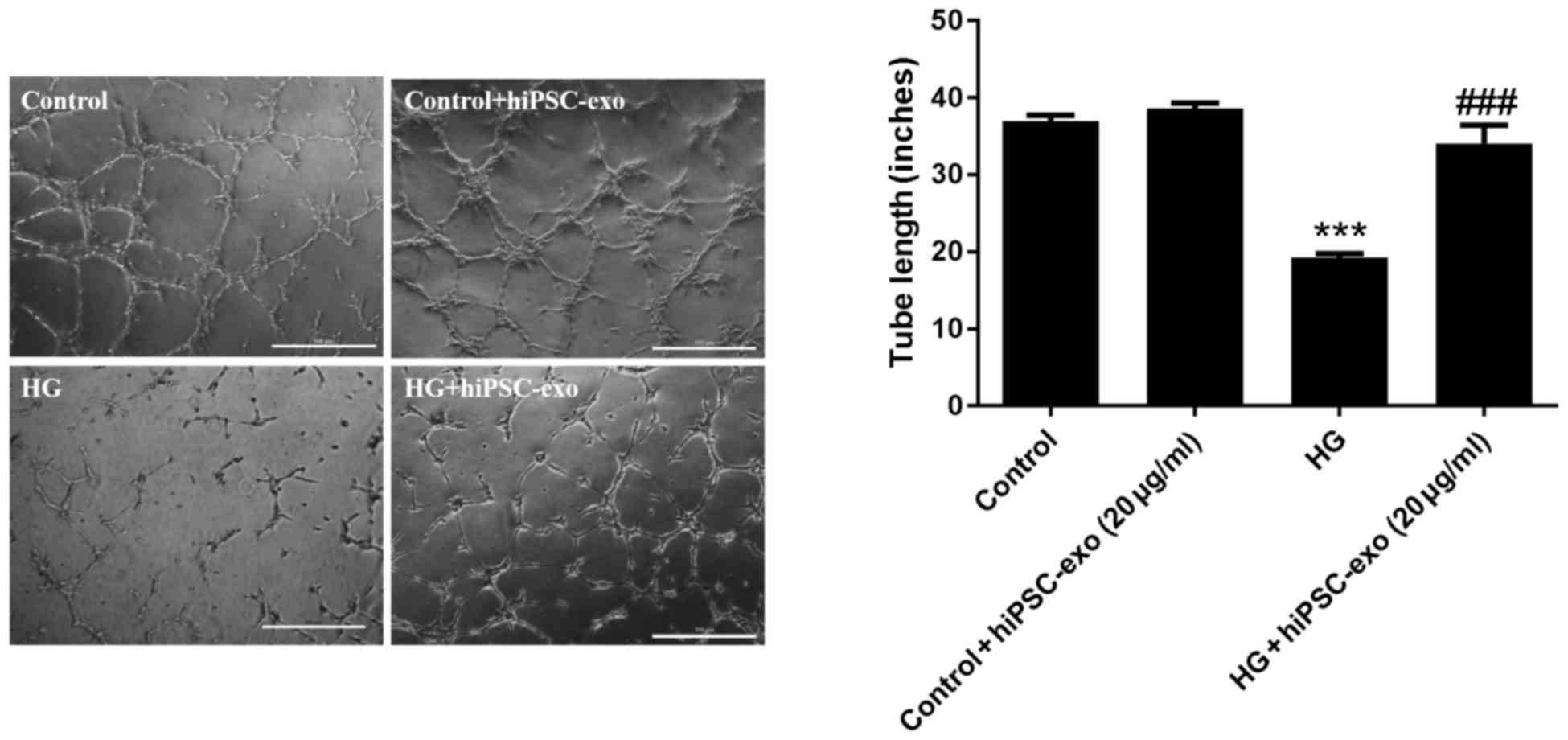
hiPSC-exo reversed high
glucose-induced decreases in capillary-like structure
formation
Following treatment with normal glucose, normal
glucose + hiPSC-exo, high glucose or high glucose + hiPSC-exo,
HUVECs were trypsinized and cultured on Matrigel to assess in
vitro capillary-like structure formation. The results
demonstrated that high glucose significantly decreased
capillary-like structure formation in HUVECs, whereas hiPSC-exo
reversed this effect. However, hiPSC-exo had a minimal effects on
normal HUVECs (Fig. 4).
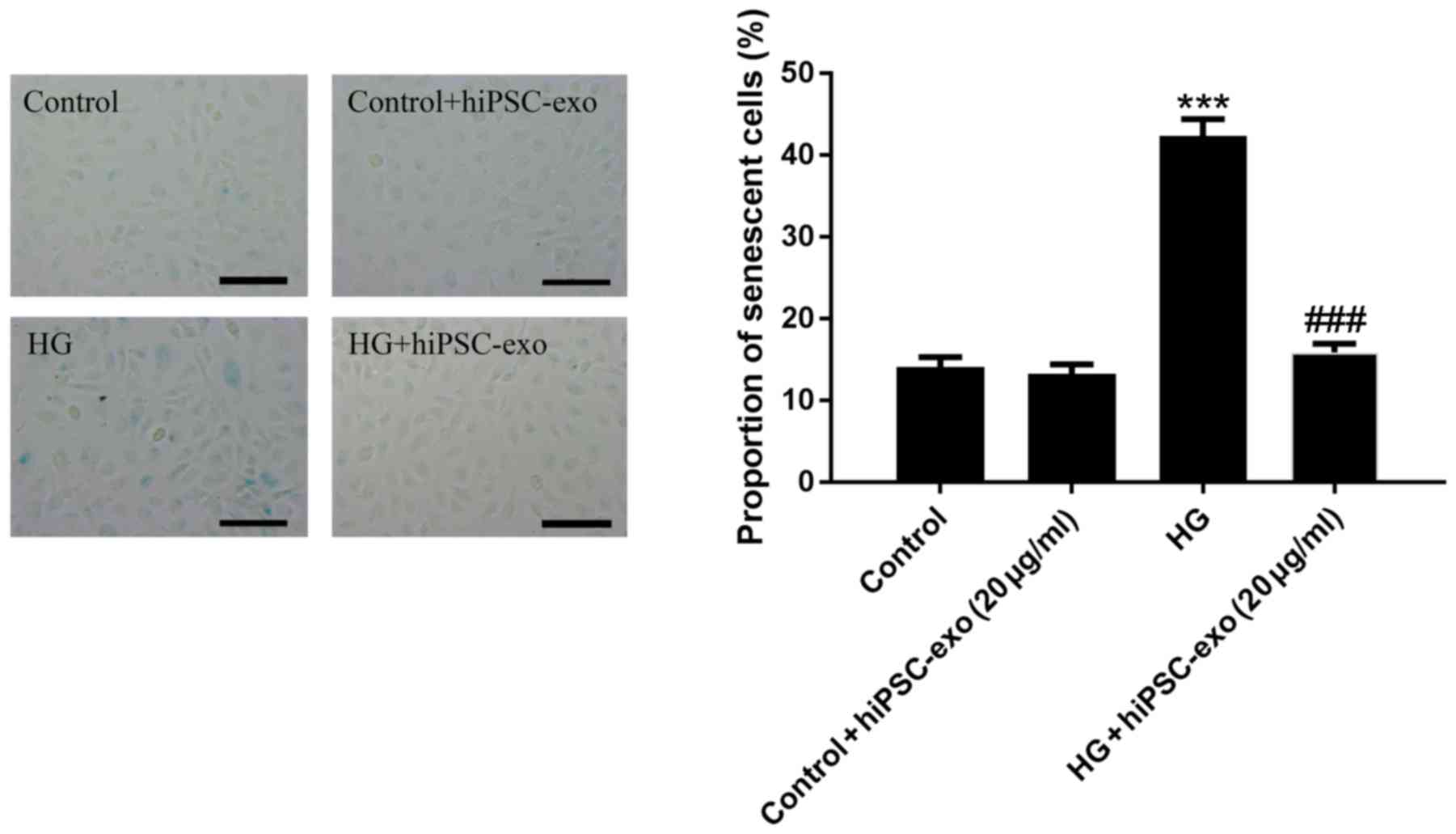
Anti-senescence effect of hiPSC-exo in
high glucose-injured HUVECs
To verify the effects of hiPSC-exo on cell
senescence, a senescence detection assay was performed on HUVECs
after being treated with different conditions (normal glucose,
normal glucose + hiPSC-exo, high glucose and high glucose +
hiPSC-exo). The cells of the control group were transparent and
plump whereas the cells of the high glucose group showed a
flattened and enlarged morphology (Fig.
5). hiPSC-exo significantly protected HUVECs against cellular
senescence induced by high glucose (Fig.
5). Furthermore, there was no statistically significant
difference between the normal glucose group and either group
treated with hiPSC-exo. Collectively, the results showed that
hiPSC-exo are readily absorbed by target cells, wherein they can
modulate cell viability and survival.
Discussion
To the best of our knowledge, this is the first
report demonstrating that exosomes derived from hiPSCs are able to
protect HUVECs from high glucose in vitro. In the present
study, HUVECs were found to be capable of absorbing hiPSC-exo at a
high efficiency. The mechanism of exosome uptake by targeted cells
has long been studied. The existing model suggests that exosomes
express adhesion molecules to adhere to cells (20); however, the cellular and molecular
basis for their specific targeting to acceptor cells remains to be
elucidated. Barrès et al (21) demonstrated previously that galectin-5
was bound to the surface of rat reticulocyte exosomes and modulated
vesicle uptake by macrophages. In the immune system, it has been
demonstrated that T cells were able to recruit major
histocompatibility complex class II-containing DC exosomes, and
that this recruitment was dependent on leukocyte
function-associated antigen-1 (22).
The function of exosomes in physiological and
pathological conditions depends on their cellular origin and
contents. As one of the most dynamic types of cell, hiPSCs have the
capacity of self-renewal and multi-differentiation, thus they exert
a therapeutic effect when used to treat various diseases, including
myocardial infarction (23,24). The role of exosomes derived from
hiPSCs was explored to examine their therapeutic effects. The
results of the present study demonstrated that hiPSC-exo could
promote cell viability and enhance tube formation, and inhibit cell
senescence in HUVECs injured by high glucose. A number of previous
studies have demonstrated that exosomes from different types of
cells exert different effects. Bang et al (25) revealed that cardiac fibroblasts
secreted exosomes to mediate cardiomyocyte hypertrophy, suggesting
that this is a potential therapeutic target. Intravenous
administration of cell-free mesenchymal stromal cells
(MSCs)-generated exosomes improved functional recovery and enhanced
neurite remodeling, neurogenesis and angiogenesis following stroke
in rats (26), suggesting that
exosomes may be important in cell therapy. A previous study by Li
et al (27) demonstrated that
exosomes derived from liver nonparenchymal cells mediated the
cell-to-cell transmission of interferon type I-α-induced antiviral
activity. However, not all types of exosomes mediate
cell-protective effects on target cells. Currently, the role of
exosomes in tumors is being accepted, particularly with regard to
tumor metastasis. The observations of the recent study by Zhang
et al (28), demonstrated the
dynamic and reciprocal cross-talk between tumor cells and the
metastatic niche. They found that exosomes prepared the
microenvironment of the target organ of metastasis for tumor cell
colonization. Another study also demonstrated that tumor exosome
integrins determine organotropic metastasis. It also revealed that
exosomes from mouse and human lung-, liver- and brain-tropic tumor
cells preferentially fuse with resident cells at the predicted
destination (29).
In recent years, the incidence of diabetes mellitus
has increased significantly, and its chronic vascular complications
continue to affect patients' lives (29–31).
Although the underlying specific mechanism is unclear, a growing
body of research has indicated that the intracellular
overproduction of reactive oxygen species (ROS), caused by
hyperglycemia, is the common mechanism of vascular complications of
diabetes mellitus. In a previous study by Kiritoshi et al
(30), it was suggested that
hyperglycemia increases mitochondrial ROS production, resulting in
nuclear factor-κB activation, cyclooxygenase (COX)-2 mRNA
induction, COX-2 protein production and prostaglandin E2 synthesis.
Yu et al (31) previously
determined that a dynamic change in mitochondrial morphology in
high glucose conditions contributed to the overproduction of ROS.
The present study indicated that mitochondrial fission/fusion
machinery may be a previously unrecognized target to control acute
and chronic production of ROS in hyperglycemia-associated
disorders. Therefore, it is vital to understand the specific
mechanisms of ROS overproduction and resulting vascular
complications of diabetes mellitus, and to find novel strategies
and drugs to treat this problem. This is necessary to prevent or
reduce the dysfunction of endothelial cells, which are important in
maintaining vascular function. A number of studies have
demonstrated the therapeutic effects of exosomes from different
types of cells on normal and injured endothelial cells. Conigliaro
et al (32) found that
exosomes released by CD90+ cancer cells modulated endothelial
cells, promoting an angiogenic phenotype and cell-to-cell adhesion.
When rats with traumatic brain injury were treated with MSC-derived
exosomes, Zhang et al (33)
found that newly generated endothelial cells in the lesion boundary
zone were significantly increased with a parallel reduction in
neuroinflammation.
Therefore, the present study may offer a novel
strategy for maintaining the normal function of endothelial cells
during the vascular complications of diabetes mellitus.
Acknowledgements
The abstract was presented at a meeting of the 13th
Congress of the International Society of Heart Research (ISHR)
Chinese Section, which took place on September 21–25, 2016, Wuhan,
China. The present study was supported by International Cooperation
and Exchanges (81220108002 to S. Chen), Great Research Plan Program
(91539120 to S. Chen), and General Program (81470260 to M. Xiang)
of the National Natural Science Foundation of China, and the
National Key R&D Program of China (2016YFC1305101 to S.
Chen).
Glossary
Abbreviations
Abbreviations:
|
hiPSC-exo
|
human induced pluripotent stem
cell-derived exosomes
|
|
HUVECs
|
human umbilical vascular endothelial
cells
|
References
|
1
|
Boyle PJ: Diabetes mellitus and
macrovascular disease: Mechanisms and mediators. Am J Med. 120 9
Suppl 2:S12–S17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun KX, Liu ZK, Cao YY, Juan J, Xiang X,
Yang C, Huang SP, Liu XF, Li N, Tang X, et al: Relationship between
brachial-ankle pulse wave velocity and glycemic control of type 2
diabetes mellitus patients in Beijing community population. Beijing
Da Xue Xue Bao Yi Xue Ban. 47:431–436. 2015.(In Chinese).
PubMed/NCBI
|
|
3
|
Hermans MP: Diabetes and the endothelium.
Acta Clin Belg. 62:97–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bürrig KF: The endothelium of advanced
arteriosclerotic plaques in humans. Arterioscler Thromb.
11:1678–1689. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minamino T, Miyauchi H, Yoshida T, Ishida
Y, Yoshida H and Komuro I: Endothelial cell senescence in human
atherosclerosis: Role of telomere in endothelial dysfunction.
Circulation. 105:1541–1544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Funakoshi S, Miki K, Takaki T, Okubo C,
Hatani T, Chonabayashi K, Nishikawa M, Takei I, Oishi A, Narita M,
et al: Enhanced engraftment, proliferation, and therapeutic
potential in heart using optimized human iPSC-derived
cardiomyocytes. Sci Rep. 6:191112016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neel S and Singla DK: Induced pluripotent
stem (iPS) cells inhibit apoptosis and fibrosis in
streptozotocin-induced diabetic rats. Mol Pharm. 8:2350–2357. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan B and Singla DK: Transplanted induced
pluripotent stem cells mitigate oxidative stress and improve
cardiac function through the Akt cell survival pathway in diabetic
cardiomyopathy. Mol Pharm. 10:3425–3432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blin G, Nury D, Stefanovic S, Neri T,
Guillevic O, Brinon B, Bellamy V, Rücker-Martin C, Barbry P, Bel A,
et al: A purified population of multipotent cardiovascular
progenitors derived from primate pluripotent stem cells engrafts in
postmyocardial infarcted nonhuman primates. J Clin Invest.
120:1125–1139. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caspi O, Huber I, Kehat I, Habib M, Arbel
G, Gepstein A, Yankelson L, Aronson D, Beyar R and Gepstein L:
Transplantation of human embryonic stem cell-derived cardiomyocytes
improves myocardial performance in infarcted rat hearts. J Am Coll
Cardiol. 50:1884–1893. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan M, Nickoloff E, Abramova T, Johnson
J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN,
Benedict C, et al: Embryonic stem cell-derived exosomes promote
endogenous repair mechanisms and enhance cardiac function following
myocardial infarction. Circ Res. 117:52–64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnstone RM, Adam M, Hammond JR, Orr L
and Turbide C: Vesicle formation during reticulocyte maturation.
Association of plasma membrane activities with released vesicles
(exosomes). J Biol Chem. 262:9412–9420. 1987.PubMed/NCBI
|
|
13
|
Raposo G, Nijman HW, Stoorvogel W,
Liejendekker R, Harding CV, Melief CJ and Geuze HJ: B lymphocytes
secrete antigen-presenting vesicles. J Exp Med. 183:1161–1172.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pascucci L, Alessandri G, Dall'Aglio C,
Mercati F, Coliolo P, Bazzucchi C, Dante S, Petrini S, Curina G and
Ceccarelli P: Membrane vesicles mediate pro-angiogenic activity of
equine adipose-derived mesenchymal stromal cells. Vet J.
202:361–366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jeong D, Jo W, Yoon J, Kim J, Gianchandani
S, Gho YS and Park J: Nanovesicles engineered from ES cells for
enhanced cell proliferation. Biomaterials. 35:9302–9310. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun N, Panetta NJ, Gupta DM, Wilson KD,
Lee A, Jia F, Hu S, Cherry AM, Robbins RC, Longaker MT and Wu JC:
Feeder-free derivation of induced pluripotent stem cells from adult
human adipose stem cells. Proc Natl Acad Sci USA. 106:15720–15725.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang L, Yin M, Wei X, Liu J, Wang X, Niu
C, Kang X, Xu J, Zhou Z, Sun S, et al: Bach1 represses
Wnt/β-catenin signaling and angiogenesis. Circ Res. 117:364–375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mallegol J, Van Niel G, Lebreton C,
Lepelletier Y, Candalh C, Dugave C, Heath JK, Raposo G,
Cerf-Bensussan N and Heyman M: T84-intestinal epithelial exosomes
bear MHC class II/peptide complexes potentiating antigen
presentation by dendritic cells. Gastroenterology. 132:1866–1876.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang N, Sun B, Gupta A, Rempel H and
Pulliam L: Monocyte exosomes induce adhesion molecules and
cytokines via activation of NF-κB in endothelial cells. FASEB J.
30:3097–3106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barrès C, Blanc L, Bette-Bobillo P, André
S, Mamoun R, Gabius HJ and Vidal M: Galectin-5 is bound onto the
surface of rat reticulocyte exosomes and modulates vesicle uptake
by macrophages. Blood. 115:696–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoen Nolte-'t EN, Buschow SI, Anderton SM,
Stoorvogel W and Wauben MH: Activated T cells recruit exosomes
secreted by dendritic cells via LFA-1. Blood. 113:1977–1981. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang G, Shi W, Hu X, Zhang J, Gong Z, Guo
X, Ren Z and Zeng F: Therapeutic effects of induced pluripotent
stem cells in chimeric mice with β-thalassemia. Haematologica.
99:1304–1311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wendel JS, Ye L, Tao R, Zhang J, Zhang J,
Kamp TJ and Tranquillo RT: Functional effects of a
tissue-engineered cardiac patch from human induced pluripotent stem
cell-derived cardiomyocytes in a rat infarct model. Stem Cells
Transl Med. 4:1324–1332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bang C, Batkai S, Dangwal S, Gupta SK,
Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, et al:
Cardiac fibroblast-derived microRNA passenger strand-enriched
exosomes mediate cardiomyocyte hypertrophy. J Clin Invest.
124:2136–2146. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG and
Chopp M: Systemic administration of exosomes released from
mesenchymal stromal cells promote functional recovery and
neurovascular plasticity after stroke in rats. J Cereb Blood Flow
Metab. 33:1711–1715. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H,
Liu J, Pan T, Chen J, Wu M, et al: Exosomes mediate the
cell-to-cell transmission of IFN-α-induced antiviral activity. Nat
Immunol. 14:793–803. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang
Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al:
Microenvironment-induced PTEN loss by exosomal microRNA primes
brain metastasis outgrowth. Nature. 527:100–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoshino A, Costa-Silva B, Shen TL,
Rodrigues G, Hashimoto A, Mark Tesic M, Molina H, Kohsaka S, Di
Giannatale A, Ceder S, et al: Tumour exosome integrins determine
organotropic metastasis. Nature. 527:329–335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiritoshi S, Nishikawa T, Sonoda K,
Kukidome D, Senokuchi T, Matsuo T, Matsumura T, Tokunaga H,
Brownlee M and Araki E: Reactive oxygen species from mitochondria
induce cyclooxygenase-2 gene expression in human mesangial cells:
Potential role in diabetic nephropathy. Diabetes. 52:2570–2577.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu T, Robotham JL and Yoon Y: Increased
production of reactive oxygen species in hyperglycemic conditions
requires dynamic change of mitochondrial morphology. Proc Natl Acad
Sci USA. 103:2653–2658. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Conigliaro A, Costa V, Lo Dico A, Saieva
L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M,
et al: CD90+ liver cancer cells modulate endothelial cell phenotype
through the release of exosomes containing H19 lncRNA. Mol Cancer.
14:1552015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Chopp M, Meng Y, Katakowski M,
Xin H, Mahmood A and Xiong Y: Effect of exosomes derived from
multipluripotent mesenchymal stromal cells on functional recovery
and neurovascular plasticity in rats after traumatic brain injury.
J Neurosurg. 122:856–867. 2015. View Article : Google Scholar : PubMed/NCBI
|